层状双氢氧化物(LDH)是一类具有水镁石型层状结构的纳米材料,其主层板由部分二价阳离子被三价阳离子取代形成正电荷层,层间通过阴离子静电平衡维持电中性[13,14],这种独特结构赋予其双向离子交换特性:既可通过层间阴离子置换负载缓蚀剂,又能通过主层板阳离子释放金属缓蚀离子[15]。Zheludkevich等[16]制备了装载有钒酸根离子的水滑石(LDH)纳米容器(MgAl-LDH、ZnAl-LDH),Li等[17]探究了装载有钼酸根缓蚀剂的ZnAl-LDH对低碳钢的腐蚀行为的影响,Alibakhshi等[18]将不同的阴离子缓蚀剂(硝酸盐、钼酸盐和磷酸盐)装入ZnAl-LDH,研究表明所制备的纳米容器在腐蚀介质中具有显著的缓控释放及缓蚀能力。基于此,本研究拟构建含LDH纳米容器的智能响应硅烷涂层:通过LDH纳米容器与入侵Cl-的响应置换来捕获固定Cl-,并同步释放阴离子缓蚀剂,同时借助主层板Ce3+的缓释效应实现双缓蚀机制协同防护,从而提升碳钢的主动防腐能力。
本研究使用具有缓蚀功能的Ce3+部分替换Al3+,用一步共沉淀法装载无机缓蚀剂NO
1 实验方法
1.1 实验材料
九水合硝酸铝、六水合硝酸铈、氢氧化钠、正硅酸四乙酯(TEOS)、(3-缩水甘油丙氧基)三甲氧基硅烷(GPTMS)、冰乙酸和亚硝酸钠均购自上海麦克林生化科技有限公司,六水合硝酸锌和氨水购自西陇科学股份有限公司。所有化学品均原样使用,没有进一步处理。去离子水为实验室自制。金属基材为30 mm × 30 mm × 2 mm的Q235碳钢,采用80、180、600、1000目的砂纸对金属基底进行打磨处理,以去除其表面损伤、油污、氧化层,然后用乙醇超声洗涤,烘干备用。
1.2 负载无机缓蚀剂的ZnAlCe-LDH的制备
采用一步共沉淀法来合成插层无机缓蚀剂的ZnAlCe-LDH缓蚀剂材料。先取7.437 g Zn(NO3)2·6H2O、4.689 g Al(NO3)3·9H2O和0.904 g Ce(NO3)3·6H2O以摩尔比(Zn2+∶Al3+ = 2∶1;Al3+∶Ce3+ = 6∶1)的比例配制25 mL的混合盐溶液(溶液A),配制25 mL 1.6 mol/L的NaOH溶液(溶液B)。然后,将25 mL溶液A以缓慢均匀的速率通过滴定管滴加到反应容器的无机缓蚀剂溶液C中(25 mL 0.25 mol/L NaNO2溶液)。同时滴加溶液B来保持反应过程中pH恒定为10±0.2。反应保持600 r/min的转速,使共沉淀反应能够剧烈发生。反应过程中始终使用pH计来检测pH变化,使pH的变化保持在10±0.2的范围内波动。保持通有N2,且反应过程中使用的所有水都是煮沸后的去离子水。共沉淀滴定完成后,将混合物溶液在室温下保持通氮气搅拌1 h,然后密封放置到65 ℃下陈化24 h,陈化后的产物使用去离子水以5000 r/min的转速进行离心洗涤,至少洗涤3次,然后冻干,获得无机缓蚀剂(NO
1.3 掺杂LDH的溶胶凝胶涂层的制备
首先,取3 mL GPTMS、1 mL TEOS、40 mL无水乙醇、13 mL去离子水置于干燥洁净的100 mL烧杯中,然后将烧杯放置在磁力搅拌器上以550 r/min的速度,50 ℃的水浴进行溶胶凝胶的水解缩合反应。在反应进行30 min后,加入1 mL 0.3 mol/L乙酸,加速水解,在水解1 h后加入1 mL 0.5 mol/L氨水,以促进缩合反应的发生,继续加热2 h后,停止搅拌和加热,陈化12 h,获得硅烷溶液。
取合成的LDH-NO2⁻型缓蚀剂填料和去离子水,配置不同浓度的LDH水溶液(1 mg/mL,2.5 mg/mL,10 mg/mL),在振荡器上振荡1~2 min,超声5 min,使水滑石分散均匀。然后取1 mL LDH水溶液加入4 mL硅烷溶液中,超声5 min后,在400 r/min搅拌10 min,制得掺杂LDH-NO
图1
图1
水滑石及掺杂LDH-NO
Fig.1
Schematic illustration of the preparation of layered double hydroxide (LDH) and silane-based sol-gel coatings modified with NO
1.4 表征
采用扫描电镜(SEM,Axia ChemisEM HiVac)和场发射扫描显微镜(FE-SEM,Zeiss Sigma 300)对合成的水滑石、制备的涂层和浸泡实验后碳钢表面形貌进行了表征,并用EDS分析了表面元素的分布。采用Fourier变换红外光谱仪(FT-IR,Perkin-Elmer Spectrum Two)分析不同水滑石的化学成分的差别,使用KBr微球在450~4000 cm-1的范围内进行测试。采用X射线衍射仪(XRD,Ultima IV),在5~80°范围内,以10 (°)/min的扫描速率对水滑石的晶体结构进行了测定。在35~800 ℃的N2气氛下,以10 ℃/min的升温速率(TG/DTG,TG209F1 Libr)对不同水滑石的热稳定性进行了分析。使用X射线光电子能谱仪(XPS,ESCALAB Qxi,USA)测定了不同水滑石基缓蚀剂粉末的表面化学组成和元素价态。使用IC离子色谱仪(IC,DIONEEX ICS-6000),对合成的水滑石缓蚀剂的Cl-吸附和NO
使用电化学工作站(Gamry Interface 1010E)来分别对不同的样品进行电化学测试(电化学阻抗谱和极化测试)。对于水滑石粉末样品,以Q235碳钢(工作电极)、铂片(对电极)和KCl饱和甘汞(Hg/Hg2Cl2)电极(参比电极)置于含有NaCl溶液(0.05 mol/L)的电解池中,构成常规的三电极体系。然后将合成的水滑石基缓蚀剂以2 g/L的浓度,加入电解池中,进行电化学测试。样品的测试面积为1 cm2,扰动电压设置为10 mV。在105~10-2 Hz的频率范围内测量了电化学阻抗谱。此外,用ZSimpWin软件对EIS的测试结果进行拟合。在测试过程中,为防止干扰,将电解池放置在Faraday笼中,所有测试至少进行3次,以减小实验误差。对于极化测试,实验装置与EIS测试相同,测试范围为相对于开路电位-0.2~1.2 V,扫描速度0.5 mV/s。
对于涂层样品,电化学测试以涂层样品为工作电极,其他步骤与水滑石粉末样品相同。并且,使用扫描振动电极测试系统(SKP,Princeton Applied)对划伤后的涂层样品进行分析,研究涂层的自修复作用。
2 结果和讨论
2.1 水滑石的组成和结构形貌分析
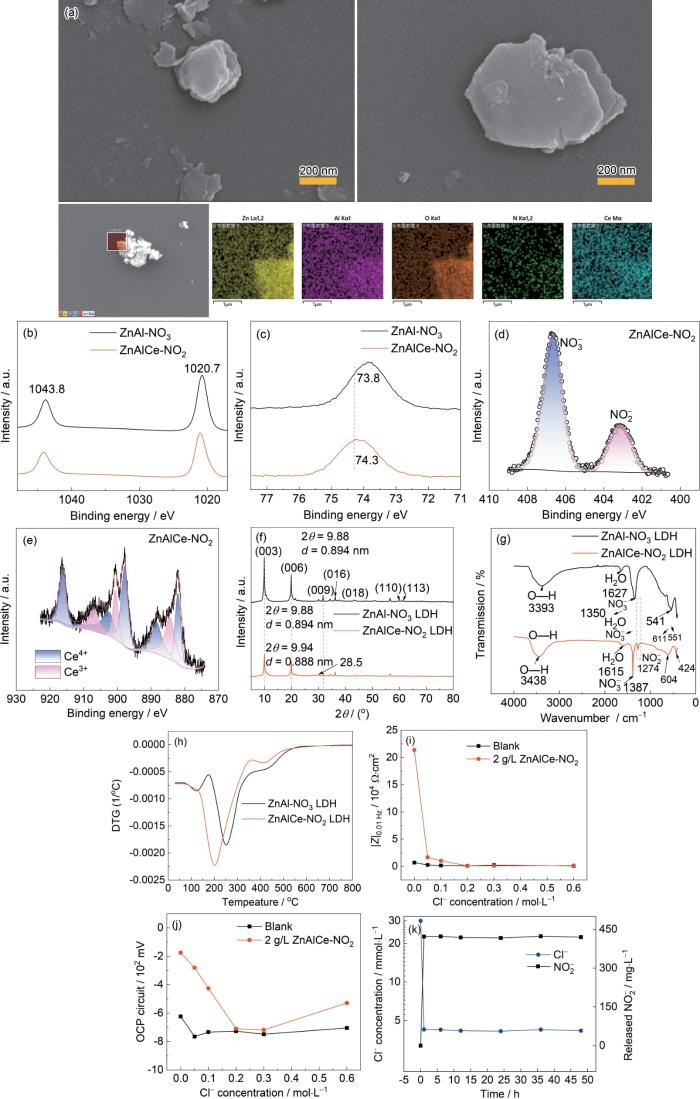
通过SEM对ZnAlCe-NO
图2
图2
ZnAl-LDH和ZnAlCe-NO2 LDH的SEM图、ZnAlCe-NO2 LDH的EDS能谱图,不同水滑石样品的XPS谱图及XRD图,FT-IR图,TG和DTG图以及不同NaCl浓度下,Q235碳钢在空白溶液和添加ZnAlCe-NO2 LDH溶液中的腐蚀电位和低频模值的变化图,ZnAlCe-NO2 LDH水滑石样品的氯离子吸附、缓蚀剂释放曲线
Fig.2
SEM images of ZnAl-LDH and ZnAlCe-NO2 LDH, EDS spectra of ZnAlCe-NO2 LDH (a), XPS spectra of different hydrotalcite samples (Zn 2p) (b), (Al 2p) (c), (N 1s) (d), (Ce 3d) (e) XRD images of different hydrotalcite samples (f), FT-IR figures (g), TG and DTG figures (h), Q235 carbon steel in blank solution and ZnAlCe-NO2 added under different sodium chloride concentrations variation of corrosion potential (i) and low-frequency modulus (j) in LDH solution, chloride ion adsorption and corrosion inhibitor release curve (k) of ZnAlCe-NO2 LDH hydrotalcite samples
2.2 水滑石的成分晶型和热稳定性分析
2.3 水滑石的化学组成分析
通过使用高分辨率XPS光谱技术进一步鉴定了两种水滑石样品的表面化学组成和结合状态(图2b~e)。Zn 2p3/2 (1020 eV)和Zn 2p1/2 (1043 eV)峰证实Zn2+存在(图2b);Al3+配位环境改变使Al 2p峰向低能偏移(图2c);Ce 3d (图2e)谱中882.15/900.6 eV (Ce3+)、888.65/916.45 eV (Ce4+)及卫星峰(884.5/903 eV)证实Ce3+部分取代Al3+,部分氧化为CeO2[38]。样品存在Ce3+和Ce4+的混合价态,一部分Ce3+替换了ZnAlCe-NO2水滑石主层板中的Al3+,一部分Ce3+被氧化为CeO2。综合分析表明:Ce3+成功掺入主层板,NO
2.4 水滑石的Cl- 吸附缓蚀剂释放性能
使用IC离子色谱仪,在50 mL 0.03 mol/L NaCl溶液中加入1 g的该插层化合物(ZnAlCe-NO2 LDH),考察其Cl-的吸附和缓蚀剂NO
2.5 水滑石的缓蚀性能
2.5.1 电化学分析
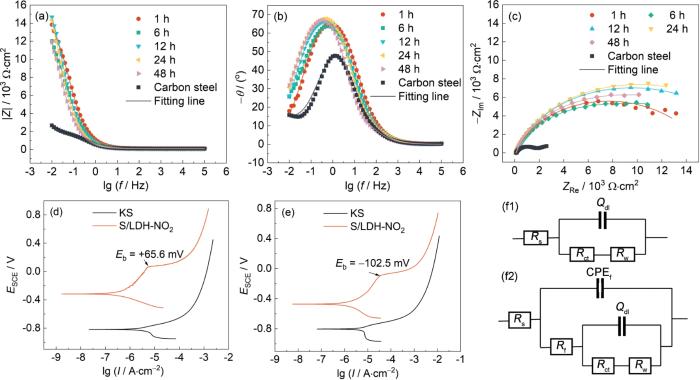
(1) 电化学阻抗谱分析 在0.05 mol/L的NaCl溶液中进行电化学测试,图3中的裸钢指Q235碳钢浸泡1h后测得的数据(在1 h时Q235碳钢已经发生腐蚀),图3a~c是添加2 g/L ZnAlCe-NO2 LDH的样品测得的电化学数据。频率为0.01 Hz时的低频模值(|Z|0.01 Hz)可以直接反映耐蚀性,一般情况下,样品的防护性能越好,|Z|0.01 Hz值越高;同时,样品Nyquist图中对应的圆弧半径越大,表明耐蚀性能越好[27,28]。由图可见,在初始浸泡时(即浸泡1 h),空白样品(裸Q235钢)的低频模值|Z|0.01 Hz为2.68 × 103 Ω·cm2,添加ZnAlCe-NO2 LDH的|Z|0.01 Hz为1.38 × 104 Ω·cm2。其初始|Z|0.01 Hz (1.38 × 104 Ω·cm2)较空白组(2.68 × 103 Ω·cm2)提升5倍,且48 h内阻抗值持续高于空白组1个数量级,24 h达峰值1.46 × 104 Ω·cm2 (图3a)。等效电路拟合(表1)证实:ZnAlCe-NO2体系Rct值较空白高1个量级(24 h达最大值),CPEf值最低(24 h:1.24 μF/cm2),表明其表面钝化膜最致密[27,28]。
图3
图3
碳钢在添加ZnAlCe-NO2 LDH溶液中的Bode和Nyquist图。碳钢在NaCl (0.05 mol/L)溶液与添加ZnAlCe-NO2 LDH的NaCl (0.05 mol/L)混合溶液中浸泡不同时间后的极化曲线图及EIS拟合的等效电路模型
Fig.3
Bode plots (a, b) and Nyquist plots (c) of carbon steel in ZnAlCe-NO2 LDH-containing solution. Polarization curves of carbon steel after immersion in 0.05 mol/L NaCl blank solution and 0.05 mol/L NaCl solution containing ZnAlCe-NO2 LDH for different durations: 24 h (d) and 48 h (e). Equivalent circuit models (a, b) for EIS fitting (f)
表1 Q235碳钢在NaCl混合溶液中浸泡不同时间的EIS拟合参数
Table 1
| Sample | Time | Rs / Ω·cm2 | CPEf / Y0 (Ω-1·cm-2·S n ) | n | Rf / Ω·cm2 | Qdl / Y0 (Ω-1·cm-2·S n ) | n | Rct / Ω·cm2 | Rw/ Ω·cm2 |
|---|---|---|---|---|---|---|---|---|---|
| Carbon steel | 1 h | 110.5 | - | - | - | 4.039 × 10-4 | 0.78 | 1.876 × 103 | 5.954 × 10-3 |
| ZnAlCe-NO2 LDH | 1 h | 112.9 | - | - | - | 2.006 × 10-4 | 0.82 | 1.468 × 104 | 8.688 × 10-3 |
| 6 h | 111.2 | 1.871 × 10-4 | 0.83 | 125.3 | 8.09 × 10-5 | 0.83 | 1.162 × 104 | 1.133 × 10-3 | |
| 12 h | 101.1 | 2.234 × 10-4 | 0.81 | 134.8 | 7.022 × 10-5 | 0.90 | 1.793 × 104 | 5.202 × 10-3 | |
| 24 h | 90.6 | 2.861 × 10-4 | 0.79 | 120.1 | 6.857 × 10-5 | 0.98 | 1.915 × 104 | 5.750 × 10-3 | |
| 48 h | 111.2 | 2.718 × 10-4 | 0.80 | 78.08 | 1.470 × 10-4 | 0.94 | 1.410 × 104 | 1.600 × 10-3 |
XPS与EIS关联分析表明:LDH通过Cl-/NO
图3f中的等效电路模型分别对初始时刻和不同浸泡时间下的界面电极结构进行模拟[29]。其中,Rs是溶液电阻,Rf和CPEf分别代表膜电容和膜电阻;Rct和Qdl代表双电层电容和电荷转移电阻;W是带电离子或电子在溶液中扩散所引起的电阻[30]。在初始时刻腐蚀已经发生,但还未有大量锈迹生成,以图3f1来拟合这一阶段,在后续浸泡中,水滑石分解的Ce3+生成的氧化物和腐蚀产物(Fe的氧化物)会在基体的表面沉积,且释放的缓蚀剂离子会在表面生成钝化膜,因此以图3f2来拟合。拟合后的数据如表1所示,一般来说,CPEf的大小与基体表面保护膜阻隔性能有关CPEf值越小,膜层的阻隔性能越好,而Rct通常用来描述腐蚀速率,反映界面处电化学反应的动力学,Rct越大基体的腐蚀速率越慢,腐蚀程度越小[31]。从表中数据可知,添加ZnAlCe-NO2 LDH的样品溶液,Rct呈现先增大后减下的变化趋势,初始时刻的Rct值比空白溶液大一个数量级,并在24 h时刻达到最大值。且添加ZnAlCe-NO2 LDH的样品溶液的CPEf值在24 h时具有最小值。说明在24 h水滑石在碳钢表面作用生成的保护膜最致密,阻隔性能最好。
(2) 极化曲线测试
动电位极化曲线评价了碳钢在不同水滑石/氯化钠溶液中的缓蚀性能。NaCl(0.05 mol/L)溶液为空白组(命名为KS),添加2 g/L ZnAlCe-NO2 LDH的NaCl (0.05 mol/L)混合溶液(命名为S/LDH-NO2)。(图3d、e)表示S/LDH-NO2体系击穿电位达+65.6 mV,腐蚀电位正向偏移501 mV,腐蚀电流密度(Icorr)降至0.32 μA/cm2 (缓蚀效率97.57%),钝化区扩展显著。说明水滑石的加入可以促进钝化膜的形成,增强局部抗氯化物腐蚀的能力。根据Icorr、阴极Tafel斜率(βc)、腐蚀电位(Ecorr)等电化学动力学参数,按照
其中,Icorr, 0为碳钢在NaCl (0.05 mol/L)溶液中浸泡后的腐蚀电流密度,Icorr为碳钢在S/LDH-NO2中浸泡后的腐蚀电流密度。碳钢在不含LDH溶液中的腐蚀电流密度最大,说明钢试件处于高速腐蚀状态。添加LDH-NO2的样品,Icorr降低明显,缓蚀效率更高。
2.5.2 碳钢浸泡24 h后的表面性质分析
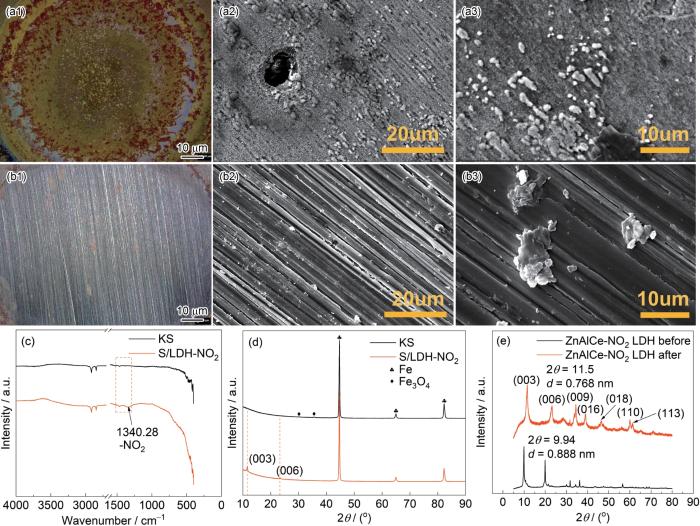
浸泡实验是为了评定金属腐蚀行为、金属防蚀措施的有效性或环境的腐蚀性所进行的实验[34]。以KS表示空白0.05 mol/L NaCl溶液,取ZnAlCe-NO2 LDH样品与0.05 mol/L的NaCl溶液配置2 g/L LDH/NaCl混合溶液(S/LDH-NO2),将Q235碳钢分别放入空白0.05 mol/L NaCl溶液、S/LDH-NO2中24 h,并记录碳钢表面的腐蚀状况。表面形貌与成分分析(图4a、b)显示:S/LDH-NO2体系碳钢表面无明显腐蚀(仅残留LDH片层)。XRD (图4d)证实含LDH体系在2θ =11.74° (d(003) = 0.75 nm)出现Cl-插层特征峰,层间距收缩(Δd≈0.12 nm)印证Cl-/NO
图4
图4
碳钢分别在0.05 mol/L NaCl溶液、S/LDH-NO2溶液中浸泡24 h后的显微形貌;碳钢分别在0.05 mol/L NaCl空白溶液和S/LDH-NO2溶液中浸泡24 h后的FT-IR图和XRD图谱及ZnAlCe-NO2 LDH样品在NaCl溶液中离子交换前后的XRD图谱
Fig.4
High-resolution micrographs of carbon steel after 24 h immersion in (a) 0.05 mol/L NaCl solution and (b) S/LDH-NO2 solution. FT-IR spectrum (c) and XRD pattern (d) of carbon steel immersed in blank 0.05 mol/L NaCl solution and S/LDH-NO2 solution for 24 h. XRD patterns of ZnAlCe-NO2-LDH before and after ion exchange in NaCl solution (e)
2.6 水滑石掺杂溶胶凝胶涂层的分析
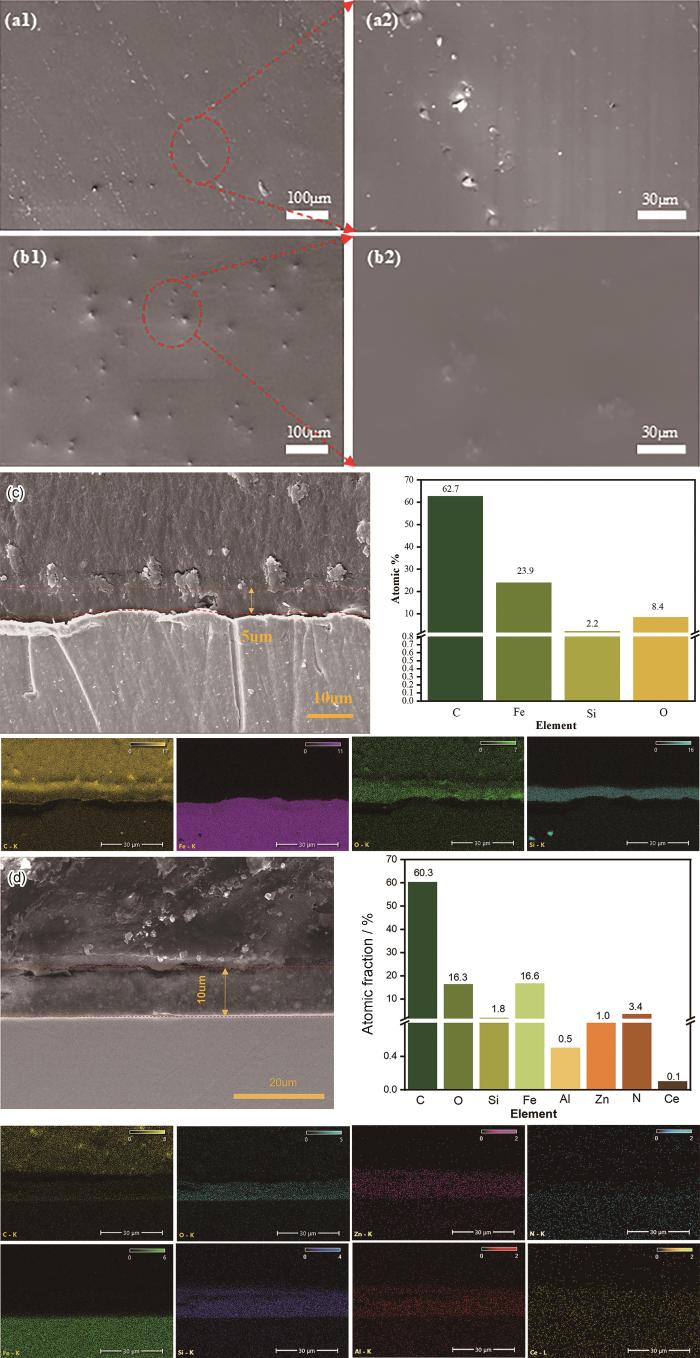
2.6.1 溶胶凝胶膜层截面组成分析
图5
图5
空白溶胶凝胶涂层、ZnAlCe-NO2 LDH掺杂的溶胶凝胶涂层的表面SEM图谱、空白溶胶凝胶涂层截面及能谱图及ZnAlCe-NO2 LDH掺杂的溶胶凝胶涂层截面及能谱图
Fig.5
SEM patterns (a), (b) of blank sol gel coating, ZnAlCe-NO2 LDH-doped sol gel coating, cross-section and energy spectrum (c) of blank sol gel coating, ZnAlCe-NO2 LDH doped sol gel coating cross-section and energy spectrum (d)
2.6.2 溶胶凝胶涂层的耐蚀性分析
采用电化学方法研究了不含LDH和含LDH硅烷涂层在0.05 mol/L NaCl溶液中的防腐性能。图6a1和b2是涂覆空白溶胶凝胶(SC)、图6b~d涂覆了掺杂1 mg/mL (图6b1和b2) (SC/NO2-LDH1),2.5 mg/mL ((图6c1和c2) SC/NO2-LDH2.5),10 mg/mL (图6d1和d2) (SC/NO2-LDH10))的ZnAlCe-NO2 LDH的3种硅烷涂层的低碳钢试样在0.05 mol/L NaCl中浸泡6 h内的Bode和Nyquist图。由Bode图可见,随着浸泡时间的增加,除了SC/NO2-LDH2.5 (图6c1和c2)硅烷涂层,所有样品的低频模值(|Z|0.01 Hz)和Nyquist曲线的圆弧半径都呈逐渐减小的趋势,低频模值(|Z|0.01 Hz)可以直接反映涂层耐腐蚀性能的好坏[45,46],说明随着浸泡时间的增加,侵蚀性离子逐渐进入涂层内部,破环了涂层的腐蚀屏障作用[39,40]。SC/NO2-LDH2.5初始低频阻抗模量达9.72 × 105 Ω·cm2 (较空白组高29.6倍),6 h后仍保持9.23 × 104 Ω·cm2 (衰减率仅5%),显著优于其他掺杂体系,所有LDH溶胶-凝胶涂层都比空白溶胶凝胶涂层高大约4倍,这说明LDH颗粒在胶凝胶涂层中的掺杂,提高了涂层的完整性和阻隔性能[40]。
图6
图6
在0.05 mol/L NaCl溶液中浸泡不同时间后的不同的涂层的Bode图和Nyquist图模型a及等效电路(R(Q(R(QR))))
Fig.6
Bode plots (a1-d1) and Nyquist plots (a2-d2) of different coatings after immersion in 0.05 mol/L NaCl solution for varying durations: SC (a1, a2), SC/NO2-LDH1 (b1, b2), SC/NO2-LDH2.5 (c1, c2), SC/NO2-LDH10 (d1, d2). Equivalent circuit Model a: R(Q(R(QR))) (e)
图7
图7
EIS拟合得到的Rc、Qc、Rct随浸泡时间的变化曲线和低频模值的变化曲线,开路变化曲线及不同涂层样品在NaCl溶液中浸泡6 h后的极化曲线图
Fig.7
Variation curves of EIS-fitted parameters with immersion time: (a) coating resistance (RcRc), (b) coating capacitance (QcQc), (c) charge transfer resistance (RctRct). Low-frequency modulus variation curve (d) and open-circuit potential variation curve (e). Polarization curves of different coating samples after 6 h immersion in NaCl solution (f)
一般来说,Rc越大,涂层的抗渗性越好[30]。阻渗电阻Rc(6 h:2.85 × 104 Ω·cm2)较空白高1个量级,过量掺杂(10 mg/mL)则使Rc降低76% (图7a),电容值Qc稳定在3.2 × 10-9 F/cm2,而高掺杂体系Qc增幅达320% (图7b),佐证其孔隙率控制优势[44]。一般来说,电荷转移电阻(Rct)是由于金属基体表面电子的转移而形成的电阻,与腐蚀过程有关[31]。低频模值(|Z|0.01 Hz)可以直接反映涂层耐腐蚀性能的好坏。随着浸泡时间增加,Rct和|Z|0.01 Hz都呈逐渐减小的趋势(图7c、7d),这说明随着电解液(NaCl)的渗透入侵,腐蚀正逐渐加速,涂层逐渐恶化失去保护作用,其中空白溶胶凝胶涂层防护作用最差。对于SC/NO2-LDH2.5涂层,Rct值随浸泡时间先减小,后增大,在4 h时达到最小值1.113 × 105 Ω·cm2,整体具有最高的电荷转移电阻。(SC/NO2-LDH2.5)硅烷涂层的低频模值(|Z|0.01 Hz)随浸泡时间整体变化不大,屏障性能几乎没有破环。说明2.5 mg/mL水滑石掺杂的溶胶凝胶涂层(SC/NO2-LDH2.5)具有最好的腐蚀防护作用,而少量水滑石掺入,不能完全弥补硅烷涂层形成过程中产生的缺陷,少量的ZnAlCe-NO2 LDH的氯离子的吸附和缓蚀剂的释放量都有限,不能起到很好的防腐效果。过量水滑石物质的掺杂,则会影响溶胶凝胶涂层的网状结构,导致产生更大的缺陷如涂层内部的孔隙甚至裂纹,最终影响涂层的耐腐蚀性能开路电位(图7e)监测显示LDH涂层初始电位(-0.36~-0.40 V)显著高于空白组(-0.46 V),且SC/NO2-LDH2.5在6 h浸泡后电位降幅最小(维持-0.38 V),印证其阻隔性能优势[38]。极化曲线(图7f)验进一步研究了添加水滑石的溶胶凝胶涂层样品在0.05 mol/L NaCl溶液中浸泡6 h时的腐蚀防护情况,SC/NO2-LDH2.5使腐蚀电位正移109 mV,阳极电流密度降低2个量级。其性能提升源于LDH层间NO
2.6.3 掺杂溶胶凝胶涂层的自修复性能分析
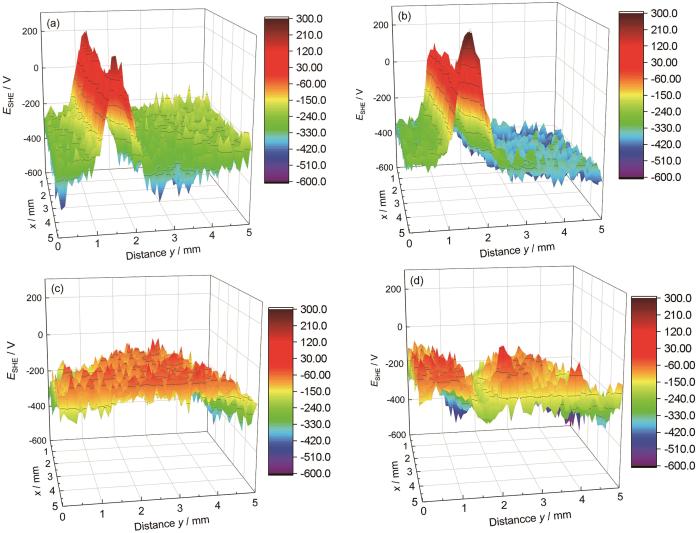
扫描Kelvin探针技术(SKP)研究溶胶凝胶涂层的腐蚀状况以及在涂层和金属衬底之间的界面反应情况[47,48]。SKP微区电位分析(图8)揭示划伤后的SC及SC/NO2-LDH2.5涂层在0.05 mol/L NaCl溶液中浸泡30/90 min后,电位分布差异显著。SC体系30 min时电位差达765 mV (299~-466 mV) (图8a),90 min扩大至879 mV (图8b);而SC/NO2-LDH2.5体系电位差始终低于500 mV (0~-450 mV) (图8c、d),证实了掺杂LDH所负载缓蚀剂对腐蚀介质的响应释放能够有效抑制微区局部腐蚀。结合EIS和极化数据,该现象源于LDH通过Cl-/NO
图8
图8
空白硅烷涂层(SC)和溶胶凝胶涂层(SC/NO2-LDH2.5)在NaCl溶液中浸泡30 min和90 min后测得的SKP图
Fig.8
SKP (Scanning Kelvin Probe) maps of (a, b) blank silane coating (SC) and (c, d) sol-gel coating (SC/NO2-LDH2.5) measured after immersion in 0.05 mol/L NaCl solution for (a, c) 30 min and (b, d) 90 min
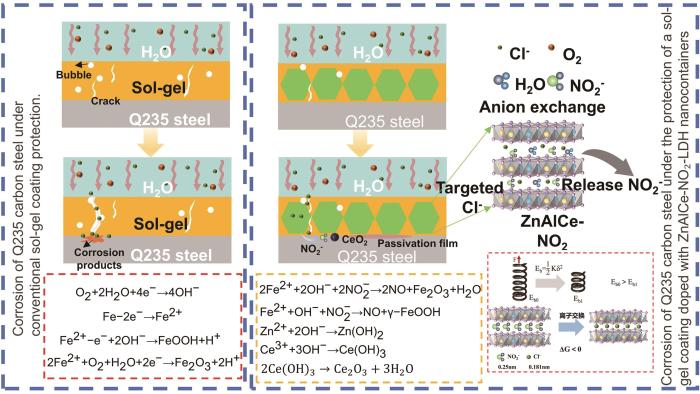
2.7 掺杂溶胶凝胶涂层的腐蚀防护机理
图9
图9
掺杂溶胶凝胶涂层的腐蚀防护机理
Fig.9
Corrosion protection mechanism of doped Sol-Gel coatings
从热力学视角看,LDH层间NO
3 结论
(1) 通过离子色谱法验证了ZnAlCe-NO2 LDH能够固定自发智能捕捉Cl-的同时释放NO
(2) 在0.05 mol/L的NaCl溶液中,ZnAlCe-NO2 LDH对碳钢表现出优异的缓蚀性能,缓蚀效率可达97.57%。
(3) 相较于空白溶胶凝胶涂层,2.5 mg/mL添加量的ZnAlCe-NO2 LDH纳米容器能显著提升溶胶凝胶涂层耐腐蚀能力,原因是纳米容器的加入不仅降低了溶胶-凝胶涂层的孔隙率,而且能够对侵入涂层的Cl-智能响应,在捕捉侵入Cl-的同时,释放层间NO
参考文献
Investigation on the crystallography of reverted structure and its effect on the properties of low carbon steel
[J].
The effect of temperature and concentration on the inhibition of acid corrosion of carbon steel by newly synthesized Schiff base
[J].
Biofriendly vegetable oil healing agents used for developing self-healing coatings: a review
[J].
Enhancement of active anticorrosion via Ce-doped Zn-Al layered double hydroxides embedded in sol-gel coatings on aluminum alloy
[J].
Hybrid sol-gel/thiourea binary coating for the mitigation of copper corrosion in neutral medium
[J].
Sol-gel-based coatings for oxidation protection of TiAl alloys
[J].
Sol-gel coatings with hydrothermal hydroxylation as pre-treatment for 2198-T851 corrosion protection performance
[J].
A comparative study of different sol-gel coatings for sealing the plasma electrolytic oxidation (PEO) layer on AA2024 alloy
[J].
Hybrid sol-gel coatings for corrosion mitigation: A critical review
[J].The corrosion process is a major source of metallic material degradation, particularly in aggressive environments, such as marine ones. Corrosion progression affects the service life of a given metallic structure, which may end in structural failure, leakage, product loss and environmental pollution linked to large financial costs. According to NACE, the annual cost of corrosion worldwide was estimated, in 2016, to be around 3%–4% of the world’s gross domestic product. Therefore, the use of methodologies for corrosion mitigation are extremely important. The approaches used can be passive or active. A passive approach is preventive and may be achieved by emplacing a barrier layer, such as a coating that hinders the contact of the metallic substrate with the aggressive environment. An active approach is generally employed when the corrosion is set in. That seeks to reduce the corrosion rate when the protective barrier is already damaged and the aggressive species (i.e., corrosive agents) are in contact with the metallic substrate. In this case, this is more a remediation methodology than a preventive action, such as the use of coatings. The sol-gel synthesis process, over the past few decades, gained remarkable importance in diverse areas of application. Sol–gel allows the combination of inorganic and organic materials in a single-phase and has led to the development of organic–inorganic hybrid (OIH) coatings for several applications, including for corrosion mitigation. This manuscript succinctly reviews the fundamentals of sol–gel concepts and the parameters that influence the processing techniques. The state-of-the-art of the OIH sol–gel coatings reported in the last few years for corrosion protection, are also assessed. Lastly, a brief perspective on the limitations, standing challenges and future perspectives of the field are critically discussed.
Potentiodynamic evaluation of sol-gel coatings with inorganic inhibitors
[J].
Nanostructured sol-gel coatings doped with cerium nitrate as pre-treatments for AA2024-T3: Corrosion protection performance
[J].
Electrodeposited silica films post-treated with organosilane coupling agent as the pretreatment layers of organic coating system
[J].
Supermolecular layered double hydroxides
[J].
Layered double hydroxide/eggshell membrane: an inorganic biocomposite membrane as an efficient adsorbent for Cr(VI) removal
[J].
Numerical and experimental analysis of self-protection in reinforced concrete due to application of Mg-Al-NO2 layered double hydroxides
[J].
Active protection coatings with layered double hydroxide nanocontainers of corrosion inhibitor
[J].
Controlled release of nitrate and molybdate intercalated in Zn-Al-layered double hydroxide nanocontainers towards marine anticorrosion applications
[J].
Fabrication and characterization of layered double hydroxide/silane nanocomposite coatings for protection of mild steel
[J].
The urea method for the direct synthesis of ZnAl layered double hydroxides with nitrate as the interlayer anion
[J].
Surface-modified LDH nanosheets with high dispersibility in oil for friction and wear reduction
[J].
Antifouling composites with self-adaptive controlled release based on an active compound intercalated into layered double hydroxides
[J].
Thermochemical radii of complex ions
[J].
Enhanced inhibition performance of NO
Delamination and self-assembly of layered double hydroxides for enhanced loading capacity and corrosion protection performance
[J].
Preparation of MgAl layered double hydroxides intercalated with nitrite ions and corrosion protection of steel bars in simulated carbonated concrete pore solution
[J].
Polyelectrolyte-modified layered double hydroxide nanocontainers as vehicles for combined inhibitors
[J].
Three birds with one stone: contemporaneously boosting passive, active and self-healing properties for long-term anticorrosion coatings
[J].
A stable anticorrosion coating with multifunctional linkage against seawater corrosion
[J].
Ni-Co hydrotalcite modified diatom to achieve corrosion inhibition and Cl- adsorption for long-term corrosion protection of steel
[J].
Mxene structure: a key parameter in corrosion barrier performance of organic coatings
[J].
Nanocontainer-based anticorrosive coatings: Effect of the container size on the self-healing performance
[J].
Electrochemical and AFM studies of mussel adhesive protein (Mefp-1) as corrosion inhibitor for carbon steel
[J].
Effect of cations on the activity coefficient of NO
A smart anti-corrosion coating based on triple functional fillers
[J].
Bioinspired layered hybrid coatings with greatly enhanced barrier effect and active corrosion protection performance
[J].
Zn-Al layered double hydroxides as chloride nanotraps in active protective coatings
[J].
Corrosion mechanism of carbon steel in chloride solution
[J].
碳钢在含氯离子环境中腐蚀机理的研究
[J].
Superhydrophobic composite coating with active corrosion resistance for AZ31B magnesium alloy protection
[J].
Organic/inorganic hybrid waterborne polyurethane coatings with self-healing properties for anticorrosion application
[J].
Anti-corrosion and self-healing behaviors of waterborne polyurethane composite coatings enhanced via chitosan-modified graphene oxide and phosphate intercalated hydrotalcite
[J].
Characterization of hybrid sol-gel coatings doped with hydrotalcite-like compounds to improve corrosion resistance of AA2024-T3 alloys
[J].
Synergistic effect of graphene oxide@phosphate-intercalated hydrotalcite for improved anti-corrosion and self-healable protection of waterborne epoxy coating in salt environments
[J].
A novel fly ash bifunctional filler for epoxy coating with long-term anti-corrosion performance under harsh conditions
[J].
A novel bilayer system comprising LDH conversion layer and sol-gel coating for active corrosion protection of AA2024
[J].
In-situ observation of the growth behavior of ZnAl layered double hydroxide film using EQCM
[J].
Effects of Al2O3 Nano-additive on performance of micro-arc oxidation coatings formed on AZ91D Mg alloy
[J].Ceramic coatings were prepared on AZ91D Mg alloy by micro-arc oxidation (MAO) in aluminate electrolytes, with Al<sub>2</sub>O<sub>3</sub> nano-additive suspending at different concentrations. Effects of nano-additive concentration on the structure, phase composition, hardness and anti-corrosion property of the MAO coatings were analyzed by scanning electron microscopy, X-ray diffraction, micro-hardness test and electrochemical method, respectively. The results revealed that Al<sub>2</sub>O<sub>3</sub> nano-particles were mostly incorporated into ceramic coating chemically, transferred into MgAl<sub>2</sub>O<sub>4</sub>, rather than being trapped mechanically during MAO process. With the increase of Al<sub>2</sub>O<sub>3</sub> concentration, the voltage-time response, content of MgAl<sub>2</sub>O<sub>4</sub>, hardness and anti-corrosion property increased. However, when the concentration varied from 10 g/L to 15 g/L, these behaviors and properties changed only a little. This result indicated that, after the concentration of Al<sub>2</sub>O<sub>3</sub> nano-additive reaching 10 g/L, the incorporation of Al<sub>2</sub>O<sub>3</sub> nano-particles turned into a saturation state, due to the complex process during MAO treatment. Therefore, 10 g/L might be a proper concentration for MAO coating to incorporate Al<sub>2</sub>O<sub>3</sub> nano-particles.
AN SKP and EIS study of microporous nickel-chromium coatings in copper containing electrolytes
[J].The corrosion behaviour of microporous nickel-chromium systems was studied by means of conventional electrochemical techniques and localized ones using two chloride based electrolytes with and without cupric ions in their composition. The conscious combination of different methodologies using these techniques has provided valuable information about the corrosion process. Open Circuit Potential (OCP) and Electrochemical Impedance Spectroscopy (EIS) measurements were performed in bulk solution, whilst Scanning Kelvin Probe (SKP) measurement were carried out using two methodologies: i) electrolyte droplets monitoring (measuring simultaneously potential and droplet height with time), and ii) potential maps of dried surfaces that previously were exposed to droplets. Further characterization was done based on the morphology of the attack and composition on the surface by Optical Microscopy (OM), Field Emission-Scanning Electron Microscope (FE-SEM) and X-ray Photoelectron Spectroscopy (XPS) together with the analysis of electrolyte composition with time by Inductively Coupled Plasma-Optical Emission Spectrometry (ICP-OES). Results have shown the harmful effect of Cu2+ cations in the corrosion resistance and a different morphological impact on the surface. Such negative effect has revealed a new time constant at high frequency in the impedance diagrams as well as an abrupt potential decrease (due to a change in the cathodic reaction involved: Cu2+ + e(-) Cu+ takes place) using SKP droplet test. Apparently, Cu+ species were stabilized in bulk solution by the formation of chloride complexes, as was confirmed by the precipitation of a white CuCl compound during droplet evaporation, pointing out the key role of Cu thorn in the corrosion process. (C) 2019 Elsevier Ltd.