目前关于夹杂物诱发腐蚀的机理主要有两种:一种是由于夹杂物周围的缺陷,包括缝隙、局部应力等诱发并促进局部腐蚀的发生[7~10];另一种是夹杂物与周围基体存在电势差,形成微电偶,从而产生电偶腐蚀,进而导致腐蚀破坏[11~14]。夹杂物自身的物理和化学性质也会影响其耐蚀性,有研究[15~17]表明,夹杂物尺寸越小,则抗点蚀性能越好。Yang等[18]运用不同的冷却工艺得到了具有不同尺寸MnS夹杂物样品的低碳钢。宏观电化学腐蚀实验和夹杂物浸泡腐蚀实验表明MnS夹杂物存在一个诱发点蚀的临界尺寸,但由于受到MnS团簇影响的限制,文中未具体提出临界尺寸的大小。Shi等[19]通过原位浸泡方法研究了弹簧钢中不同尺寸MnS夹杂物的腐蚀行为,结果表明链条状MnS夹杂物在长度为5 μm时诱发的局部腐蚀最严重。对于螺纹钢而言,当前的研究主要集中在环境(Cl-、电流和温度等)对于螺纹钢腐蚀行为的影响[20~22]。于彦冲等[2]利用浸泡实验方法研究表明螺纹钢HRB500E发生局部腐蚀是由MnS夹杂物的溶解造成的。Liu等[23]也利用浸泡实验研究了含稀土螺纹钢HRB400E的初期腐蚀行为,认为点蚀是由(RE)2O2S夹杂物的溶解进而导致腐蚀坑底部局部酸化引起的。而当前对于螺纹钢中不同尺寸夹杂物诱发腐蚀的研究却较少。
因此,本文采用全自动夹杂物分析仪分析了HRB400E钢样品的夹杂物尺寸分布,同时使用原位浸泡实验和电子背散射衍射仪研究了该螺纹钢中MnS夹杂物尺寸对局部腐蚀的影响,拟为工业生产中通过调控MnS尺寸提高螺纹钢耐蚀性能提供新思路。
1 实验方法
实验材料为HRB400E螺纹钢,成品钢材直径为12 mm,化学成分(质量分数,%)为:C 0.24,Si 0.46,Mn 1.42,P 0.044,S 0.027,Al 0.002,V 0.020,Fe余量。将螺纹钢沿钢筋轴线进行线切割,所用的样品尺寸为10 mm × 10 mm × 4 mm,并且观察面为样品轧制方向所在面。参照YB/T 4367-2014,浸泡腐蚀溶液为2%(质量分数)NaCl溶液(pH为~7.0)。
利用Leica 光学显微镜 (OM)和Exploer 4 Analyzer 全自动夹杂物分析仪(Aspex)对样品中的夹杂物种类和尺寸面积进行统计分析,其中,Aspex扫描面积为5 mm × 5 mm。本文中所提及夹杂物尺寸均由Aspex分析获得[24]。利用Apreo 2场发射扫描电子显微镜(SEM)和Quantax 200 X Flash 60能谱仪(EDS)原位观察0、15、30 和60 min时夹杂物及周围基体腐蚀形貌的变化。利用Senox 3D光学轮廓仪测量样品浸泡60 min后的点蚀坑深度。对振动抛光后的样品利用Bruker QUANTAX EBSD 400i e-FlashFS电子背散射衍射仪(EBSD)获取目标夹杂物周围的晶体学信息。
2 结果与讨论
2.1 典型夹杂物分析
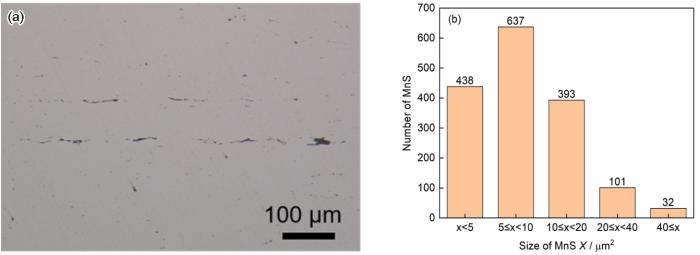
由图1a可见,样品中夹杂物数量较多,并且有较多的长条状夹杂物。Aspex结果表明,夹杂物的种类主要是MnS夹杂物,占夹杂物总量的64.6%。由于样品中的部分MnS夹杂物在形状上呈现端部较窄且弯曲,中间略宽的形状,不易准确定义其粒径。因而,本文统一使用Aspex统计夹杂物的面积来表征其尺寸[24]。其尺寸分布如图1b所示,结果表明典型MnS夹杂物的尺寸大小主要分布在0~20 μm2之间,数量约占MnS夹杂物总量的91.7%。并且所有MnS夹杂物数量最多的是5~10 μm2之间,其次是在0~5 μm2之间和10~20 μm2之间。另外,有少量的20~40 μm2和大于40 μm2的MnS夹杂物。样品中MnS夹杂物平均尺寸大小约为11 μm2。
图1
图1
样品中夹杂物的OM图像和MnS夹杂物的尺寸分布
Fig.1
OM image of inclusions (a) and the size distribution of MnS inclusions in the sample (b)
2.2 浸泡实验
图2
图2
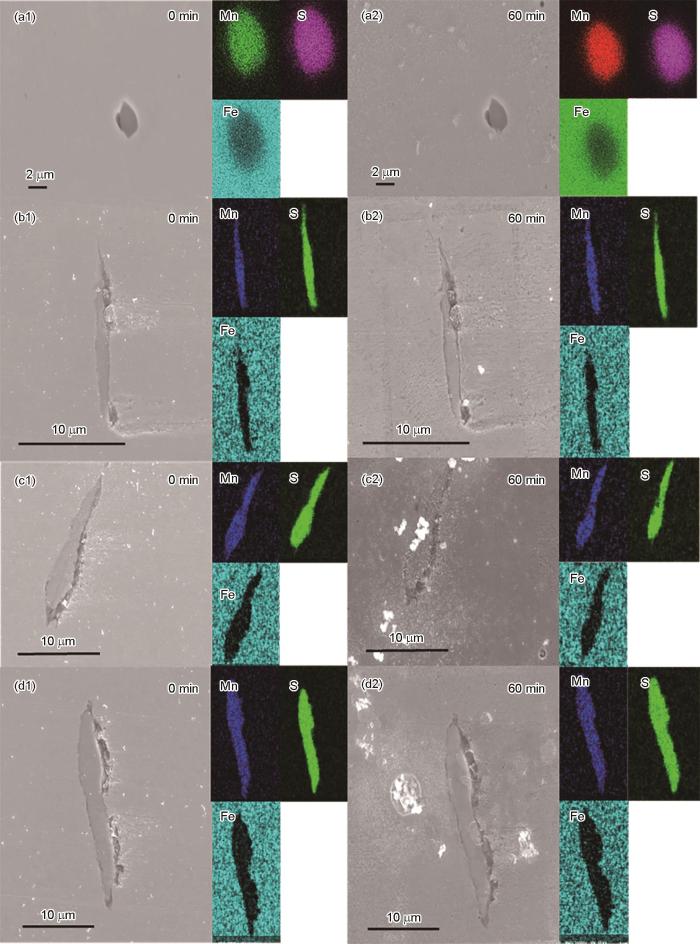
尺寸分布在0~5、5~10、10~20和20~40 μm2的MnS夹杂物浸泡60 min前后的SEM-EDS图
Fig.2
SEM-EDS images of the MnS with (0-5 μm2 (a), 5-10 μm2 (b), 10-20 μm2 (c) and 20-40 μm2 (d)) inclusions in HRB400E steel unimmered (a1-d1) and immersed (a2-d2) for 60 min
图3
图3
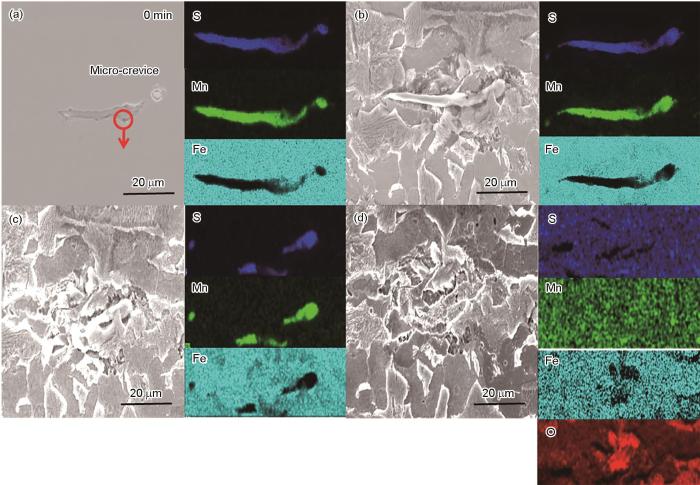
尺寸约为60.5 μm2的MnS夹杂物浸泡60 min时SEM-EDS图
Fig.3
SEM-EDS images of the MnS (~60.5 μm2) inclusions in HRB400E steel unimmersed (a) and immersed for 15 min (b), 30 min (c) and 60 min (d)
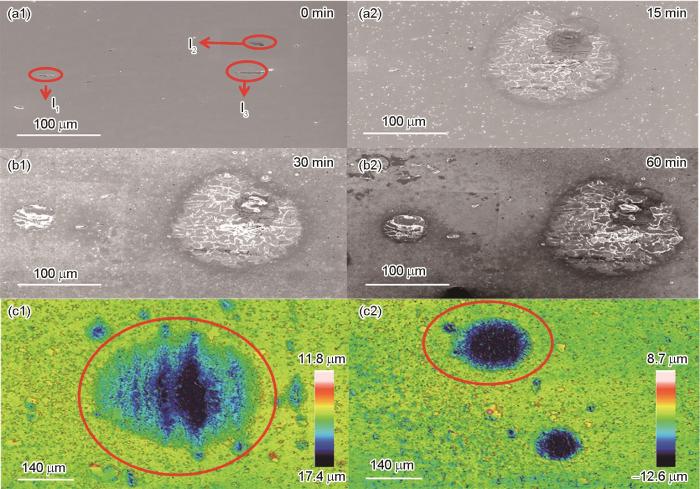
图4为团簇夹杂物与单一夹杂物浸泡60 min的SEM图和3D轮廓图,在浸泡60 min后,图4a1中标号为I1(47.9 μm2)、I2(50.7 μm2)和I3(60.5 μm2)处的MnS夹杂物发生了溶解,周围基体也发生腐蚀。标号为I2和I3的夹杂物在腐蚀过程中共同形成了一个蚀坑,导致基体腐蚀更为严重。图4b2中对比标号为I2、I3和I1形成的蚀坑,可见I2、I3形成的团簇夹杂物引发的蚀坑面积(19136.9 μm2)远大于单一夹杂物形成的蚀坑面积(1902.5 μm2)。图4中红色位置内的蚀坑分别是由团簇夹杂物和单一夹杂物形成的,蚀坑最大深度分别是3.2和1.9 μm,最大点蚀深度也说明夹杂物聚集诱发腐蚀比单一夹杂物诱发腐蚀严重。Liu等[25]也通过原位浸泡实验表明团簇夹杂物会在腐蚀过程中发生耦合作用。
图4
图4
团簇与单一夹杂物浸泡60 min后的SEM图和3D轮廓图
Fig.4
SEM images (a, b) and 3D profilometer images (c) of the clustered (a, b, c1) and single (a, b, c2) inclusions not immersed (a1) and immersed for 15 min (a2), 30 min (b1) and 60 min (b2)
2.3 EBSD表征
图5a和b为MnS夹杂物的EBSD结果图。其尺寸分别为9.8和43.5 μm2,可以反映本文定义的大小尺寸夹杂物。另外,图5c是为保证大尺寸夹杂物分析时与小尺寸夹杂物观察一致的局部放大图。图5a1~c1是其对应的平均取向差分布图,平均取向差越大,则缺陷密度越大,越容易发生腐蚀[7]。可以看出,相对于小尺寸MnS而言,大尺寸MnS夹杂物周围的平均取向差绿色聚集程度更大,因此更容易发生腐蚀。图5a2~c2中红色代表的是晶界角度在3°~15°之间,即为小角度晶界,而黑色代表的是晶界角度大于15°,即为大角度晶界。一般而言,小角度晶界越多,则越容易发生腐蚀[26,27]。可以看出,大尺寸MnS夹杂物周围的小角度晶界更多,因此更容易发生腐蚀,这与平均取向差图得出的结果一致。夹杂物的存在破坏了钢材基体的均匀性和连续性。夹杂物会与基体之间形成不连续的界面,夹杂物使晶界形核点增加,改变不同晶界类型的分布。图5表明大尺寸夹杂物周围的小角度晶界数量增多,且大尺寸夹杂物周围的局部应力集中增强,位错密度较高。相关研究也表明,小角度晶界与位错密度之间的关系为正相关[28, 29]。
图5
图5
大小尺寸MnS夹杂物的平均取向差图和大小角度晶界图
Fig.5
Kernel average misorientation (a1-c1) and grain boundaries (a2-c2) of different size of MnS inclusions
2.4 MnS夹杂物在Cl- 环境下的腐蚀机理分析
图6
图6
不同尺寸MnS夹杂物在Cl-环境下的腐蚀机理图
Fig.6
Schematic diagrams of corrosion mechanism of MnS inclusions with a size of less than (a, b) and larger than (c-f) 40 μm2 in chloride environment
随着Fe2+的产生,与Cl-结合逐渐生成腐蚀产物,化学反应式如下[30]:
因此如图6b所示,小尺寸MnS夹杂物在浸泡60 min后周围基体出现点蚀坑,但是数量较少且难以扩展到夹杂物本身及其周围的缝隙,因此小尺寸MnS夹杂物未受到周围蚀坑的影响而发生溶解。
3 结论
(1) 螺纹钢HRB400E的夹杂物中64.6%为MnS,其中MnS夹杂物尺寸主要分布在0~20 μm2之间,数量约占MnS夹杂物总量的91.7%。
(2) 质量分数2%NaCl浸泡实验表明,尺寸小于40 μm2的MnS夹杂物耐蚀性优于尺寸大于40 μm2的MnS夹杂物。尺寸小于40 μm2的MnS夹杂物没有溶解, 基体有轻微腐蚀;大于40 μm2的MnS夹杂物浸泡60 min后溶解消失且周围基体腐蚀严重,在蚀坑表面存在氧化物。
(3) 浸泡腐蚀实验表明,团簇大尺寸夹杂物诱发局部腐蚀形成的蚀坑面积远远大于单独的夹杂物诱发的局部腐蚀。
(4) 相比大尺寸MnS夹杂物,小尺寸MnS夹杂物周围存在较少的小角度晶界和缺陷,因此不容易发生腐蚀。
参考文献
Development of an embedded UHF-RFID corrosion sensor for monitoring corrosion of steel in concrete
[J].
Pitting corrosion behavior and corrosion resistance of high strength seismic reinforcement rebar with trace rare earth
[J].
稀土微合金化高强抗震钢筋点蚀行为及耐蚀性能研究
[J].
Influence of cementite coarsening on the corrosion resistance of high strength low alloy steel
[J].
Why stainless steel corrodes
[J].
Review on effect of non-metallic inclusions on pitting corrosion resistance of stainless steel
[J].
非金属夹杂物对不锈钢耐点蚀性能影响的综述
[J].首先,总结了3种常用的非金属夹杂物对不锈钢耐点蚀性能影响的研究方法,即原位腐蚀观察、微区电化学法、原子力显微镜。其次,总结了硫化物、氧化物、稀土夹杂物3种不同类型夹杂物对不锈钢耐腐蚀性能影响。随着硫化物含量的增多,不锈钢的耐点蚀性能会下降;对于氧化物的影响,目前的研究集中在氧化物的成分对不锈钢耐点蚀性能的影响。不同成分的夹杂物对不锈钢耐点蚀性能的影响机制还不是很清楚;稀土夹杂物对不锈钢点蚀的影响主要与稀土对不锈钢中夹杂物改性有关。而后,汇总了目前提出的夹杂物对不锈钢耐点蚀性能影响的机制,即贫Cr区机制、微缝隙机制、活性机制。贫Cr区机制主要用于解释硫化物引起的点蚀,后2种主要用于解释氧化物引起的点蚀。最后,提出了夹杂物控制提升不锈钢耐点蚀性能的展望。
Research progress on initiation mechanism of local corrosion induced by inclusions in low alloy steel
[J].
低合金钢中夹杂物诱发局部腐蚀萌生机制的研究进展
[J].
Initiation mechanism of localized corrosion induced by Al2O3-MnS composite inclusion in low-alloy structural steel
[J].
Pitting corrosion of steel induced by Al2O3 inclusions
[J].
Effects of niobium and rare earth elements on microstructure and initial marine corrosion behavior of low-alloy steels
[J].In the present paper, the effects of Nb and rare earth elements on initial marine corrosion behavior of low-alloy steels were investigated by first principle modeling as well as multifarious analytical techniques, such as scanning electron microscopy with X-ray microanalysis (SEM/EDS), transmission electron microscopy (TEM), scanning vibrating electrode technique (SVET), and electrochemical workstation. It was found that inclusions played key roles during the corrosion process. Spherified fine (Al, RE)-oxy-sulfide inclusions were formed in RE-bearing steel, which was dissolved preferentially in 0.5 wt% NaCl solution, and inhibited the propagation of initial corrosion. In contrast, coarse Al(2)O(3 )inclusions were formed in Nb-bearing steel, thus resulting in the selective dissolution of Fe matrix and further development of initial corrosion. RE-bearing steel demonstrated higher corrosion resistance as compared to Nb-bearing steel. Moreover, the work functions of low-indexed crystallographic planes of the inclusions and the substrate were calculated by first principle modeling, and the findings indicated consistent corrosion tendencies with experimental results, (RE)(2)O2S > REAlO3 > Fe > Al2O3. Finally, a schematic model was proposed to observe the influences of Nb and RE elements on corrosion initiation and propagation behavior of low-alloy steels.
Effect of cerium on the cleanliness of spring steel used in fastener of high-speed railway
[J].
Correlation between hyperfine structure of inclusion and localized corrosion mechanism of DSS2101 with Ce microalloying in simulated marine environment
[J].
Effect of rare earth addition on corrosion sensitivity of GCr15 bearing steel in marine environment
[J].
Dissolution kinetics of the sulfide-oxide complex inclusion and resulting localized corrosion mechanism of X70 steel in deaerated acidic environment
[J].
Effect of TiN inclusion on pitting of an ultra-pure ferritic stainless steel
[J].
TiN夹杂物对超纯铁素体不锈钢点蚀的影响
[J].
The pitting to uniform corrosion evolution process promoted by large inclusions in mooring chain steels
[J].
Effect of composition and size of oxide inclusions on pitting initiation of 2205 duplex stainless steel
[J].
2205双相不锈钢中氧化物夹杂的成分和尺寸对点蚀萌生的影响
[J].利用恒电位脉冲方法、毛细管微电极技术结合扫描电镜等研究了夹杂物对2205双相不锈钢点蚀萌生的影响。结果表明:2205双相不锈钢中夹杂物均为氧化物,且为2205双相不锈钢点蚀萌生的主要因素;氧化物夹杂通过影响其周围钢基体的点蚀电位从而影响点蚀的萌生,且点蚀是沿夹杂物而不是金属基体扩展。不仅氧化物夹杂的化学成分,其尺寸也会影响该不锈钢点蚀的萌生,其中Al<sub>2</sub>O<sub>3</sub>-CaO-SiO<sub>2</sub>和Al<sub>2</sub>O<sub>3</sub>-MgO-CaO-SiO<sub>2</sub>型夹杂物易引起点蚀,且尺寸越大越易萌生点蚀,而Al<sub>2</sub>O<sub>3</sub>-MgO夹杂物不易引起点蚀。
Pitting corrosion behavior in advanced high strength steels
[J].
Induced-pitting behaviors of MnS inclusions in steel
[J].
Understanding the corrosion mechanism of spring steel induced by MnS inclusions with different sizes
[J].
Corrosion behavior of Cr micro-alloyed corrosion-resistant rebar in neutral Cl- -containing environment
[J].
Distribution of stray current induced corrosion of reinforced bars within concrete based on electric field analysis and experiment with transparent imitated concrete
[J].
基于电场分析和仿混凝土实验的杂散电流腐蚀分布规律研究
[J].为了研究杂散电流作用下混凝土等导电基体中钢筋腐蚀的宏观分布规律,开展了三维电场的有限元模拟和基于仿混凝土材料的钢筋腐蚀实验。建立三根钢筋嵌入矩形基体的有限元模型来开展数值模拟研究,讨论钢筋的锈蚀状态和基体的杂散电流电场,并进行参数分析。然后使用与数值模型相同尺寸的试件来开展腐蚀实验,将得到的钢筋腐蚀速率与模拟结果进行了对比。结果表明,应用界面极化反应与外加Maxwell宏观电场相结合的方法,可以实现杂散电流作用下钢筋腐蚀速率与基体电场的有限元模拟。此外,通过对透明基体试件的观察和三维激光扫描,可以定性和定量地评估腐蚀量的分布。
The coupled effect of temperature and carbonation on the corrosion of Rebars in the simulated concrete pore solutions
[J].
Attempt to optimize the corrosion resistance of HRB400 steel rebar with Cr and RE
[J].
Effects of rare earth modifying inclusions on the pitting corrosion of 13Cr4Ni martensitic stainless steel
[J].In this study, the pitting corrosion behavior of 13Cr4Ni martensitic stainless steel (BASE) and that modified with rare earth (REM) in 0.1 mol/L NaCl solution were characterized. Techniques such as automatic secondary electron microscope (ASPEX PSEM detector), scanning electron microscope (SEM), transmission electron microscope (TEM), scanning Kelvin probe force microscope (SKP), potentiodynamic and potentiostatic polarizations were employed. The results obtained indicate that BASE steel contains Al2O3/MnS, Al2O3 and MnS inclusions, while REM steels contain (La, Ce, Cr, Fe)-O and (La, Ce, Cr, Fe)-O-S inclusions. Compared with BASE steel, REM steel is more susceptible to induce the metastable pitting nucleation and repassivation, whereas it restrains the transition from metastable pitting to stable pitting. Adding 0.021% rare earth element to BASE steel can reduce the number and area of inclusions, while that of 0.058% can increase the number and enlarged the size of inclusions, which is also the reason that pitting corrosion resistance of 58REM steel is slightly lower than that of 21REM steel. In the process of pitting corrosion induced by Al2O3/MnS inclusions, MnS is preferentially anodic dissolved, and also the matrix contacted with Al2O3 is subsequently anodic dissolved. For REM steels, anodic dissolution preferentially occurs at the boundary between inclusions and matrix, while (La, Ce, Cr, Fe)-O inclusions chemically dissolve in local acidic environment or are separated from steel matrix. The chemically dissolved substance (La3+ and Ce3+) of (La, Ce, Cr, Fe)-O inclusions are concentrated in pitting pits, which inhibits its continuous growth.
Role of Al2O3 inclusions on the localized corrosion of Q460NH weathering steel in marine environment
[J].
Effect of creep ageing on the corrosion behaviour of an Al-Cu-Li alloy
[J].
Insight into microstructure, microhardness and corrosion performance of 2205 duplex stainless steel: Effect of plastic pre-strain
[J].
Free dislocations and boundary dislocations in tempered martensite ferritic steels
[J].
Continuum model and numerical method for dislocation structure and energy of grain boundaries
[J].
Corrosion evolution and stress corrosion cracking behavior of a low carbon bainite steel in the marine environments: Effect of the marine zones
[J].
Mechanism of MnS-mediated pit initiation and propagation in carbon steel in an anaerobic sulfidogenic media
[J].
Occluded solution chemistry control and the role of alloy sulfur on the initiation of crevice corrosion in type 304ss
[J].
Initiation and propagation of localized corrosion induced by (Zr, Ti, Al)-O x inclusions in low-alloy steels in marine environment
[J].
Effect of manganese sulfide inclusion morphology on the corrosion resistance and pitting corrosion behavior of free-cutting austenitic stainless steel
[J].