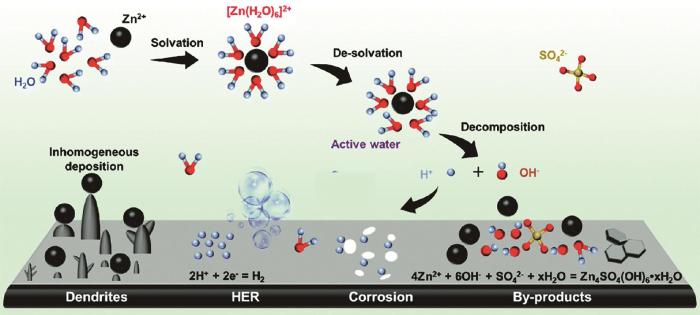
水系锌离子电池(ZIBs)因其低成本、高安全性、环境友好等特点具有强大的应用潜力[1],ZIBs研究较多的中性电解质主要有ZnCl2、ZnSO4、Zn(CF3SO3)2等锌盐,其中ZnSO4因其性能稳定、成本低而被广泛研究。然而,锌负极在中性水电解质中存在的一些核心难题阻碍了ZIBs应用。一般来说,中性电解质中锌负极的腐蚀问题包括:(1) 析氢腐蚀;(2) 钝化;(3) 枝晶生长[2]。析氢腐蚀会降低电池的Coulomb效率(CE),释放氢气,使封闭电池系统膨胀甚至爆炸;钝化会增加电极极化使电极可逆性下降;枝晶会刺穿电池的隔膜导致电池短路。迄今为止,科研工作者已经研发了许多缓解或抑制锌电极腐蚀的方法,例如在锌阳极表面引入保护层[3]、优化锌电极表面的电荷分布[4]、优化锌电极的内部结构[5]、将锌阳极与其他化学惰性金属合金化[6]、锌负极缓蚀剂[7]等。其中,添加缓蚀剂方法简单、成本较低、实用性强而备受关注[8]。
因此,本文首先介绍锌负极在中性电解质中面临的枝晶、钝化、析氢等腐蚀问题,然后针对这些问题综述缓蚀剂防护方法,从溶剂化结构调控、静电屏蔽、吸附作用、人工固态电解质界面(SEI) 4种防护机理出发介绍缓蚀剂研究进展,为抑制锌负极腐蚀提供可行思路,并在最后对缓蚀剂防护方法未来的发展方向进行了展望。
1 锌负极电极反应
锌离子电池的化学原理参考文献[9] (锌锰电池为例),锌负极发生锌的溶解/沉积反应,而正极发生锌离子的嵌入和脱出反应。放电时,锌负极失去电子生成Zn2+进入电解液中,并迁移至正极嵌入MnO2材料中;充电时,Zn2+从正极MnO2材料脱出进入电解液并迁移至锌负极,在电极表面得到电子沉积。具体反应方程如下:
负极:
正极:
总反应:
锌离子电池依靠Zn2+在正负极之间的迁移实现能量的储存与转换,锌负极是影响锌离子电池使用寿命的关键因素,锌负极上存在的析氢、钝化、枝晶问题严重限制了锌离子电池的进一步应用。
1.1 析氢副反应
图1
1.2 表面钝化
在中性电解质中,锌负极发生吸氧腐蚀并与电解质盐阴离子结合生成钝化产物,不同种类的电解质盐会生成不同的钝化物,如Zn4SO4(OH)6·xH2O[12] (ZnSO4电解质)、Zn5(OH)8Cl2·H2O[13,14] (ZnCl2电解质)、Zn x (CF3SO3)y(OH)2x-y ·xH2O[15](Zn(CF3SO3)2电解质)以及其他碱式锌盐。这些钝化物会影响锌电极表面形貌、粗糙度和电场分布,增加电极极化,导致锌电极的可逆性下降并促进枝晶的形成。Sun等[16]研究了水电解质中的溶解氧对锌电极上钝化产物的影响规律,提出了氧气参与的锌电极腐蚀钝化理论:电解质中的溶解氧是造成电池初始静置阶段钝化产物形成的主要原因,而不是质子;且锌钝化物的形成与电极电位有关。以Zn4SO4(OH)6·5H2O为例,Zhou等[17]在1 mol/L ZnSO4电解质中使用石英晶体微天平 (EQCM) 研究了Zn副产物的演化过程。研究表明,Zn的存在形式随着电极电位变化而改变 (vs. Zn2+/Zn),在开路电位至1.05 V,Zn的质荷比不变,Zn的存在形态并未发生改变;1.05 V至0.94 V,Zn的质荷比增大,此时发生Zn转化为Zn(OH)2的过程;0.94 V至0.3 V,Zn的质荷比继续增大,Zn(OH)2进一步转化为Zn4SO4(OH)6·xH2O。
1.3 枝晶生长
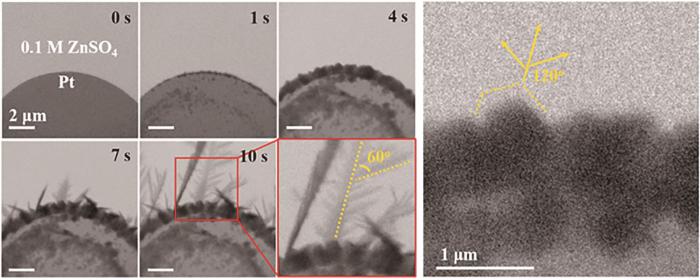
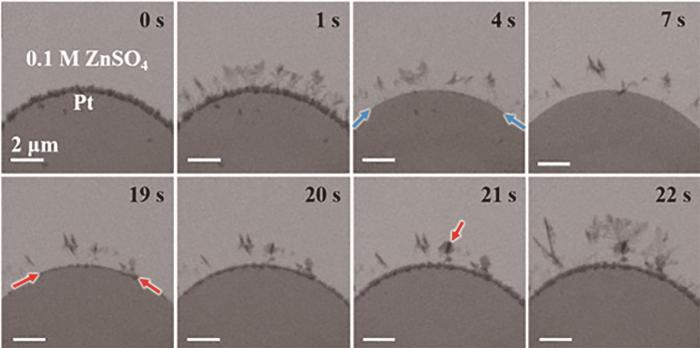
锌电极表面不均匀是形成枝晶的原因,由于实际应用中不存在完全光亮平整的锌表面,因此Zn2+在沉积时沿着电极表面扩散到达成核位点处会沉积并形成突起,突起将加剧电极表面的不均匀性,进一步影响Zn2+扩散,使Zn2+更容易在突起处沉积,如此反复最终导致枝晶生长。严重的枝晶最终会刺穿电池隔膜导致电池短路,导致使用ZnSO4电解质的锌离子电池循环寿命低于200次循环,达不到商业应用的标准。Xie等[18]从微观角度研究了锌沉积过程,结果表明商业锌箔制备加工过程中产生的晶格缺陷和残余应力是导致锌沉积过程中枝晶生长的内在因素。商业锌箔加工过程中产生的晶格缺陷将引起界面电场不均匀分布,导致界面处的不均匀沉积过程并诱导锌枝晶生长。而商业锌箔中的残余压应力高达1012 MPa,残余应力将导致金属形变和开裂,限制锌晶体外延生长并形成不规则金属基底,大部分的残余压应力可通过退火处理消除。Sasaki等[19]使用原位透射电子显微镜观察了Zn在ZnSO4电解质中的沉积溶解过程。在中性电解质中,锌枝晶为二维六边形片状物,六方紧密堆积晶体结构[20]。Zn沉积时,Zn首先在电极表面成核形成颗粒,然后颗粒逐渐长大形成六边形晶面,随后从六边形边缘生成针状沉淀并引出树状枝晶 (图2)。而在锌溶解时,Zn从靠近电极的枝晶根部开始溶解,这将使锌枝晶从电极上脱落直接导致电池容量损失 (图3)。
图2
图3
1.4 电极表面副反应间的关联性
Zn电极上的析氢、钝化、枝晶问题并不是相互独立的,而是互相影响的。电极表面发生的析氢腐蚀会使电极表面OH-浓度升高导致局部pH增加,进而促进吸氧腐蚀和枝晶的生成。而电极表面生成的钝化物会增加电极表面的不均匀度且减小电极的导电性,增加电极上的极化电压并促进枝晶生长。枝晶会增大电极表面与水的接触面积进一步促进析氢腐蚀。改善某一个问题的同时会或多或少改善其他的问题,但加重某一个问题时也会使其他问题恶化,这是腐蚀研究及缓蚀剂设计时需要考虑的问题。
2 Zn负极缓蚀剂研究
目前已经有很多关于锌负极缓蚀剂的研究,根据缓蚀剂作用机理的不同可以分为4类(见表1):(1) 溶剂化结构调控型;(2) 静电屏蔽型;(3) 吸附型;(4) 人工固体电解质界面。
表1 缓蚀剂种类及其作用机理
Table 1
| Mechanism | Substance | Way of working |
|---|---|---|
| Solvation structure | High concentration electrolytes, alcohol, carbohydrate, organic solvents, complexing agent | Change the solvation structure by replacing water in original solvation structure or form hydrogen bonds with H2O |
Self-healing electrostatic shield | Metal ion, quaternary ammonium salt | Cations adsorb at electrode surface, preventing Zn2+ from depositing at the tip to inhibiting dendrite growth |
| Adsorption | Polymers, aldehydes, carbohydrate, surfactant | Optimize the electric field of electrode surface and adjust the electrical double layer |
| Artificial solid electrolyte interphase | Metal ion and oxide, phosphorous salts, polymer monomers | General a in situ protective layer to prevent the direct contact between electrode and electrolyte |
2.1 溶剂化结构调控型缓蚀剂
图4
“盐包水”策略具有很高的可行性,但使用这种方法会急剧增加电解质成本。因此,向电解质中加入少量缓蚀剂被认为是一种成本更低、更容易进行调控的策略。向电解质中添加亲锌络合物或者氢键受体是一种能有效破坏溶剂化结构的方法。Feng等[22]使用二甲亚砜(DMSO)缓蚀剂用于ZnSO4电解质,DMSO可以与水形成氢键网络,降低水分子的活性,同时,DMSO可直接进入Zn2+的溶剂化鞘层,取代配位的水分子并破坏溶剂化结构从而抑制析氢反应和枝晶生长。组装成的Zn||MnO2电池可在10 C的大电流下循环300次后无枝晶生长。此外,分子中含有羟基的醇类物质如甲醇[23]、乙二醇[24]、丙二醇[25, 26]、环己烷十二醇[27]、2-二(2-羟乙基)氨基-2-羟甲基-1,3-丙二醇[28](Bis-Tris)等也被研究证实具有调控Zn2+溶剂化结构的作用。
为了得到一种普适性的缓蚀剂筛选和设计原则,Li等[29]研究了3种含羰基的高极性有机溶剂N-甲基-2-吡咯烷酮(NMP)、N,N-二甲基甲酰胺(DMF)和丙酮(DMK)在ZnSO4电解质中的缓蚀作用。这些与水混溶的溶剂分子可以插入锌离子的溶剂化结构取代配位的水分子,以破坏溶剂化结构减少锌电极界面上的枝晶和析氢腐蚀,这些分子重构Zn2+溶剂化结构的作用归因于分子中的羰基。其中NMP具有最高的偶极矩(4.09)、较高的介电常数(33.0)以及最大的电负性(-5.135 eV),使其与锌离子具有最强的相互作用,去溶剂化作用最强,组装的Zn||Zn电池循环寿命增加了7倍。此外,Zhu等[30]关于甲醇和乙醇的去溶剂化研究也指出,与乙醇相比,甲醇较高的介电常数以及分子极性使甲醇分子能进入Zn2+的溶剂化鞘层取代配位的水分子,由此形成的新溶剂化鞘层具有更小的静电势,降低了锌离子去溶剂化的能垒,而介电常数和分子极性较小的乙醇无此作用。析氢极化曲线测试结果显示,添加甲醇后的析氢起始电位比添加乙醇降低了80 mV以上,甲醇具有更强的抑制析氢作用。因此,缓蚀剂分子的介电常数、极性和电负性是影响其去溶剂化作用的关键因素。
与分子外配位的缓蚀剂不同,具有大环空腔的分子如冠醚、环糊精等,其独特的分子空腔结构可以将锌离子束缚在分子空腔内部从而实现去溶剂化。Deng等[31]研究了具有大环空腔的15-冠醚-5分子对Zn2+去溶剂化的作用,15-冠醚-5分子内径(0.17~0.22 nm)接近锌离子的直径(0.15 nm)而小于Zn2+溶剂化结构的直径(0.6 nm),分子空腔内径与锌离子的尺寸匹配性较好。冠醚分子能与锌离子配位将其束缚在分子空腔中促进去溶剂化过程,并且配位后能减缓锌离子的沉积速度促进均匀沉积,组装的Zn||Zn电池能在1 mA·cm-2电流密度下稳定循环2100 h以上。此外,Zhao等[32]研究了同样具有大环空腔的α-环糊精(0.47~0.53 nm)、β-环糊精(0.60~0.65 nm)和γ-环糊精(0.75~0.83 nm)的去溶剂化作用,结果表明随着分子空腔直径的减小,环糊精的去溶剂化作用越强。但空腔直径最小的α-环糊精分子与Zn2+的配位仍然较弱,其去溶剂化作用不如15-冠醚-5分子,组装的Zn||Zn电池能在1 mA·cm-2电流密度下稳定循环1000 h,低于使用15-冠醚-5分子的2100 h,表明分子大环空腔内径与Zn2+直径相匹配才能具有较好的去溶剂化作用。
2.2 静电屏蔽型缓蚀剂
Ding等[33]在关于锂离子电池枝晶生长的研究中首次提出了自愈式静电屏蔽(SHES)机制。在电解质中添加少量还原电位比Li+低的金属离子Cs+、Rb+,金属离子在Li+沉积过程中不会被还原而是吸附在电极表面的尖端突起处,排斥锂离子在表面尖端沉积从而抑制枝晶生长。该方法被扩展到水系锌离子电池锌负极缓蚀剂的设计中并取得了一定成果。根据静电屏蔽效应,Hu等[34]首次运用稀土金属盐氯化铈(CeCl3)用于ZnSO4电解质中的腐蚀抑制,金属Ce3+在锌电极表面形成静电屏蔽层,使锌离子均匀沉积,并抑制析氢腐蚀。添加Ce3+的Zn||Zn电池的短路时间从110 h提高至2500 h以上,有效抑制了锌负极上的枝晶生长。其他金属离子如Na+[35]、Mg2+[36]、Pb2+[37]、Rb+[38]也被研究证明能对锌电极起到相同的静电屏蔽作用。进一步研究表明,这种方法不仅适用于金属离子,具有相似性质的非金属离子也具有静电屏蔽作用。研究表明性质稳定的季铵盐阳离子如四丁基硫酸铵[39](TBA2SO4)、甲基三乙基氯化铵[40](TMACl)也具有静电屏蔽作用。
2.3 吸附型缓蚀剂
缓蚀剂在电极/电解质界面吸附成膜是一种研究较多的调控策略,缓蚀剂通过吸附到电极表面形成保护膜,调控锌电极表面的电荷分布以及界面双电层结构,改善锌沉积行为,抑制电极表面各种副反应。Zhou等[41]使用大分子的木质素磺酸钠用于ZnSO4电解质,分子中含有大量磺酸基和酚羟基吸附在电极表面,抑制枝晶和副产物生成,而且木质素磺酸钠中磺酸基能加速锌离子迁移,提高电解质的电导率。添加木质素磺酸钠后Zn||Zn电池的电压极化从220 mV下降至110 mV,并在1190 h的循环后仅增加至120 mV左右,促进了锌离子沉积过程,而其他许多大分子缓蚀剂会降低电导率,这是木质素磺酸钠的优越之处。Huang等[42]将微量(2.4 mmol/L)三聚氰胺 (Mel) 添加在ZnSO4电解质中,Mel本身不具有缓蚀效果,但在弱酸性的ZnSO4溶液中会质子化形成MelH+,仅需微量的MelH+便可以在锌电极表面吸附成为单分子膜抑制副反应并调控Zn2+沉积过程,组装的Zn||Cu电池在1200次的循环中表现出99.7%的高库伦效率。Zhong等[43]将谷氨酸钠引入ZnSO4电解质,谷氨酸阴离子吸附在锌电极表面形成贫水双电层结构,促进Zn2+在电极/电解质界面的脱水过程,抑制水引起的枝晶及析氢腐蚀,并使Zn||NH4V4O10全电池的容量保持率从38.9%提高到93.6%,1000次循环后锌负极表面无Zn4SO4(OH)6·xH2O钝化物生成。部分氨基酸如甘氨酸[44]、组氨酸[45]、L-半胱氨酸[46]和丝氨酸[47]等也被证实因其分子中的氨基和羧基与金属较强的相互作用能够在锌电极表面构建稳定的单分子吸附膜。
具有较多含氧基团的天然植物提取物与金属之间具有较强的相互作用,如Zhao等[48]使用香草醛分子用于ZnSO4电解质,采用密度泛函理论计算发现香草醛分子在Zn(002)晶面上的结合能(-1.1 eV)大于游离的Zn2+(-0.56 eV),并表现出良好的平行吸附构型,能够稳定吸附于锌电极表面并抑制在锌电极表面发生的副反应和枝晶生长,同时可以破坏Zn2+的溶剂化结构。Qiu等[49]使用芳香醛类物质藜芦醛用于ZnSO4电解质中,通过吸附作用调节锌离子在电极表面的扩散抑制枝晶生长。并且,锌负极的析氢起始电位降低200 mV以上(-1.1 V下降至-1.3 V, vs. Ag/AgCl),有效抑制了锌负极的析氢腐蚀,以此组装的Zn||Ti电池在200次循环中表现出超过97%的库伦效率。即使部分藜芦醛被还原为醇,其仍能在电极表面吸附抑制析氢腐蚀。
目前已有研究表明,在电解质中添加具有一定疏水性的表面活性剂可在金属表面吸附成疏水层,改善Zn2+沉积行为并抑制锌电极溶解和析氢腐蚀。表面活性剂在锌电极表面的吸附行为对调控锌沉积过程有显著影响,Guan等[50]比较了三种季铵盐表面活性剂苄基二甲基十二烷基氯化铵(DDBAC)、十二烷基三甲基氯化铵(DTAC)、苄基三甲基氯化铵 (TMBAC)在ZnSO4电解质中的缓蚀性能,其中具有单一疏水基团且空间位阻较小的TMBAC性能最好。DDBAC具有两种不同的疏水基团,在电解质中会产生多种不稳定的吸附行为,不稳定的吸附行为将导致电解质中产生大量胶束阻碍Zn2+扩散并增加锌沉积时的电压极化(200 mV)。具有单一疏水基团的DTAC能在锌电极上形成稳定的吸附层并抑制金属腐蚀,但十二烷基疏水基团的大空间位阻会增大吸附膜的厚度阻碍锌离子扩散增加电压极化 (65 mV)。而具有单一疏水基团的TMBAC能在锌电极表面呈现单一吸附行为并形成稳定的疏水层,同时其疏水基团较小的空间位阻对Zn2+扩散的影响较小,Zn||Zn对称电池的电压仅为32 mV,接近不添加TMBAC时的状态(28 mV),具有最佳的电池性能。该结果表明,疏水基团与表面活性剂在锌电极上的吸附行为有关,是影响表面活性剂类缓蚀剂性能的重要因素。
2.4 人工固态电解质界面
由于电极与电解质之间不稳定的界面状态(电极极化和尖端效应),不可避免地会产生枝晶、析氢等腐蚀问题,因此可添加缓蚀剂使电池工作状态下原位构建人工固体电解质界面以稳定电极与电解质界面的双电层状态,从而达到抑制枝晶及各种副反应的效果。Zeng等[51]在电解质中添加丙烯酰胺,电池工作过程中在锌电极表面原位电聚合形成聚丙烯酰胺聚合物膜,聚合物膜能够阻止锌电极与电解质直接接触抑制枝晶和析氢腐蚀并覆盖锌沉积活性位点改善Zn2+沉积行为,但聚合物膜会显著增大锌沉积时的电压极化,组装的Zn||Zn电池的电压极化相较于未添加状态增加了约80 mV。除了原位形成聚合物外,还可以通过添加无机盐在电极表面沉淀生成稳定致密的无机保护层[52]。Zeng等[53]在Zn(CF3SO3)2和ZnSO4电解质中加入Zn(H2PO4)2盐,磷酸盐与Zn2+结合并沉淀,在锌电极表面原位生成致密、稳定、导电的Zn3(PO4)2·4H2O保护层,确保了Zn2+的快速迁移并具备一定的自修复功能,组装的Zn||V2O5电池在长期循环中实现了近似100%的CE。Guo等[54]在ZnSO4电解质中添加LiCl,在电极表面沉淀生成Li2O/Li2CO3保护层,抑制枝晶生长及副产物的生成,并减小Zn2+沉积时的电压极化,Zn||Zn电池的电压极化从220 mV降低至38 mV。Huang等[55]在ZnSO4电解质中添加SeO2,在电极表面原位构建了ZnSe保护层。ZnSe对Zn2+具有更强的亲和力可以促进Zn2+更快地迁移和成核生长,抑制枝晶生成,并具有一定的自修复功能,组装的Zn||Zn电池能稳定循环2100 h。并且,原位形成ZnSe的锌负极在循环50次后表现出比无SEI锌负极更低的阻抗,SEI有效增加了锌负极的可逆性。
表2 不同缓蚀剂的Zn||Zn对称电池循环性能统计对比表
Table 2
| Corrosion inhibitor | Electrolytes | Current density, capacity and cycle time | Ref. |
|---|---|---|---|
| dimethyl sulfoxide | ZnSO4 | 1 mA·cm-2, 1 mAh·cm-2, 2100 h 3 mA·cm-2, 3 mAh·cm-2, 200 h | [22] |
| N-methyl-2-pyrrolidone | ZnSO4 | 1 mA·cm-2, 1 mAh·cm-2, 540 h | [29] |
| ethylene glycol | ZnSO4 | 0.5 mA·cm-2, 0.5 mAh·cm-2, 1300 h 5 mA·cm-2, 0.5 mAh·cm-2, 800 h | [24] |
| triethylmethyl ammonium | ZnCl2 | 1 mA·cm-2, 0.5 mAh·cm-2, 2145 h 5 mA·cm-2, 2.5 mAh·cm-2, 500 h | [40] |
| melamine | ZnSO4 | 2 mA·cm-2, 2 mAh·cm-2, 3000 h | [42] |
| monosodium glutamate | ZnSO4 | 2 mA·cm-2, 2 mAh·cm-2, 2200 h 5 mA·cm-2, 5 mAh·cm-2, 1700 h | [43] |
| glycine | ZnSO4 | 1 mA·cm-2, 0.5 mAh·cm-2, 2000 h 4 mA·cm-2, 2 mAh·cm-2, 600 h | [44] |
| histidine | ZnSO4 | 2 mA·cm-2, 1 mAh·cm-2, 4180 h | [45] |
| L-cysteine | ZnSO4 | 1 mA·cm-2, 0.5 mAh·cm-2, 2500 h | [46] |
| SeO2 | ZnSO4 | 2 mA·cm-2, 2 mAh·cm-2, 2100 h | [55] |
| sucrose | ZnSO4 | 1 mA·cm-2, 1 mAh·cm-2, 2000 h 2 mA·cm-2, 2 mAh·cm-2, 500 h | [57] |
| sorbitol | ZnSO4 | 1 mA·cm-2, 0.5 mAh·cm-2, 2000 h | [60] |
| alkyl polyglucoside | ZnSO4 | 1 mA·cm-2, 0.25 mAh·cm-2, 4000 h | [61] |
| silk peptide | ZnSO4 | 1 mA·cm-2, 1 mAh·cm-2, 3000 h | [64] |
| polyaspartic acid | ZnSO4 | 0.5 mA cm-2, 0.5 mAh·cm-2, 3200 h | [65] |
| ovalbumin | ZnSO4 | 1 mA·cm-2, 1 mAh·cm-2, 2200 h | [71] |
| γ-valerolactone | ZnSO4 | 1 mA·cm-2, 1 mAh·cm-2, 3500 h | [72] |
| AgNO3 | ZnSO4 | 0.5 mA·cm-2, 0.25 mAh·cm-2, 1800 h | [73] |
| KI | ZnSO4 | 1 mA·cm-2, 1 mAh·cm-2, 3000 h | [74] |
| Ni2+ | ZnSO4 | 1 mA·cm-2, 1 mAh·cm-2, 900 h | [75] |
| cholinium cations | ZnSO4 | 1 mA·cm-2, 1 mAh·cm-2, 2000 h | [76] |
| dioxane | ZnSO4 | 1 mA·cm-2, 1 mAh·cm-2, 2000 h | [77] |
| sodium tartrate | ZnSO4 | 0.5 mA·cm-2, 0.25 mAh·cm-2, 1500 h | [78] |
| tetramethylurea | Zn(OTf)2 | 1 mA·cm-2, 1 mAh·cm-2, 3000 h | [79] |
| thiourea | ZnSO4 | 1 mA·cm-2, 1 mAh·cm-2, 1200 h | [80] |
| tetrahydrofuran | ZnSO4 | 0.5 mA·cm-2, 0.5 mAh·cm-2, 1300 h 1 mA·cm-2, 1 mAh·cm-2, 500 h | [81] |
| phytic acid | ZnSO4 | 1 mA·cm-2, 1 mAh·cm-2, 1200 h | [82] |
| penta-potassium triphosphate | ZnSO4 | 1 mA·cm-2, 1 mAh·cm-2, 3800 h | [83] |
| 1-hydroxy ethylidene-1,1-diphosphonic acid | ZnSO4 | 1 mA·cm-2, 1 mAh·cm-2, 1500 h | [84] |
| taurine | ZnSO4 | 1 mA·cm-2, 1 mAh·cm-2, 3000 h | [85] |
| tetrabutylammonium 4-toluenesulfonate | Zn(PS)2 | 1 mA·cm-2, 1 mAh·cm-2, 2000 h | [86] |
| 1-phenylethylamine hydrochloride | ZnSO4 | 1 mA·cm-2, 1 mAh·cm-2, 2082 h | [87] |
| succinimide | ZnSO4 | 20 mA·cm-2, 10 mAh·cm-2, 680 h | [88] |
| hexamethylenetetramine | ZnSO4 | 5 mA·cm-2, 1 mAh·cm-2, 4000 h | [89] |
| 1-ethyl-3-methylimidazolium chloride | ZnSO4 | 1 mA·cm-2, 1 mAh·cm-2, 500 h | [90] |
| 1-butyl-3-methylimidazolium cation | ZnSO4 | 2 mA·cm-2, 1 mAh·cm-2, 3162 h 5 mA·cm-2, 5 mAh·cm-2, 1410 h 10 mA·cm-2, 10 mAh·cm-2, 1000 h | [91] |
| L-ascorbic acid sodium | ZnSO4 | 1 mA·cm-2, 1 mAh·cm-2, 1200 h | [92] |
2.5 多功能协同作用
锌电极上发生的不同副反应间相互影响,缓蚀剂的设计可考虑一剂多功能或多种缓蚀剂协同作用。已有研究工作表明,葡萄糖[56]、蔗糖[57]、木糖醇[58]、海藻糖[59]、山梨醇[60]、烷基葡萄糖苷[61]、纤维素[62]等多羟基的物质具有多种功效,缓蚀剂分子既能通过配位作用改变Zn2+的溶剂化结构,又能通过吸附作用在电极表面形成保护膜,以抑制析氢反应和枝晶生长。有些缓蚀剂会在电池工作时发生反应,反应的生成物具有与原先物质不同的防护效果。Huang等[63]将糖精引入ZnSO4电解质,糖精会吸附在锌电极表面形成贫水双电层,不仅可以抑制析氢副反应,还可以调节锌离子的扩散抑制枝晶生长,同时糖精会在锌电极表面分解原位形成ZnS保护层,组装Zn||Cu电池的库伦效率可达99.6%。
在电解质中能电离为阳离子和阴离子的物质,阳离子和阴离子具有不同的防护机理,能起到协同作用。Han等[66]将醋酸铵引入ZnSO4电解质,铵根离子具有静电屏蔽作用抑制枝晶生长,而醋酸根离子具有稳定pH的作用,将电极界面的pH稳定在约5.34抑制副反应的发生,组装的Zn||Zn电池经过3500 h循环之后仍无钝化物产生。
2.6 其他
除了上述4种主要的缓蚀剂改进策略,还有一些另辟蹊径的改进方法。Sun等[16]揭示了电解质中溶解的氧气对锌电极浸泡初期钝化产物形成的影响,提出了使用蒽醌-2-磺酸钠(AQS)作为自发脱氧缓蚀剂以抑制氧气引起的钝化反应,缓蚀剂有效抑制了静置过程中锌负极上的钝化反应,组装的Zn||Cu电池具有99.6%的高库伦效率。Gao等[67]引入H2O的同位素D2O作为电解质溶剂,D2O表现出与H2O不同的性质,D2O作为溶剂的电解质具有更宽的电化学窗口并能抑制析氢副反应,仅仅是引入同位素就使电解质表现出了不同的性质,组装的Zn||MnO2全电池循环寿命从73次提升至1000次以上。此外,通过改变水溶液的性质,在电解质溶液中加入高岭土[68]、蒙脱石[69]、膨胀粘土和膨润土[70]等以构成固液混合电解质,电解质中的固体物质可以限制水分子的移动,减小水分子的活性,抑制各种由水引起的腐蚀问题,实现与“盐包水”策略相似的“土包水”电解质。但与“盐包水”策略不同的是,引入固体物质的规整晶体结构可以提供锌离子迁移通道,实现稳定的Zn2+迁移和沉积过程。
3 总结与展望
(1) 本文总结了锌负极缓蚀剂在防治中性电解质中锌电极表面析氢、钝化、枝晶等问题研究进展。其中析氢由电解质中溶剂化的Zn2+引起,钝化物的生成与电位、溶解氧和电解质盐的种类有关,枝晶由锌电极表面不均匀引起,三者之间相互促进。
(2) 锌电极缓蚀剂主要通过溶剂化结构调控、静电屏蔽、吸附作用、原位SEI,改善锌负极电化学性能。改进策略间不互斥,研发一剂多功能,全面地提升电池的性能,且价格低廉的缓蚀剂对电池的商业化应用有重大意义。尽管缓蚀剂用于锌电极防护,取得了长足的进展,但实际使用中电池性能离商业应用的标准,仍有一定的距离。
(3) 未来负极缓蚀剂的开发策略主要包括:开发一剂多功能缓蚀剂;缓蚀剂复配协同;研发高性价比电解质;通过复配的方式综合各种缓蚀剂的优点。此外,锌离子电池最常用的为ZnSO4电解质,其优势是价格低廉,但电池性能较差,而大部分缓蚀剂基于此电解质开发。综合考虑价格和性能,开发具有更高性价比的电解质将有利于锌离子电池性能迈向更高的层次。
(4) 正极缓蚀剂研发。Zn||Zn对称电池的循环性能是说明锌负极缓蚀剂性能的主要指标,但其只揭示了缓蚀剂对锌负极的作用,而忽略了缓蚀剂对正极材料的影响。电池是一个整体,对电池负极和正极进行整体评价才能更好地反映缓蚀剂的性能。
参考文献
Recent advances in the electrolytes of rechargeable zinc-ion batteries
[J].
可充电锌离子电池电解质的研究进展
[J].锌离子电池以其低价、安全、高可用性、生态友好、易于制备等特点而受到了广泛的研究。作为连通锌离子电池其他部分的纽带,电解质与电化学性能的发挥有着重要关系。为了进一步具备应用的可能性,增强电解质对电池电化学性能的释放具有深刻的意义。尽管在电解质方面已经进行了诸多探索并取得了很多优秀的成果,然而对于锌离子电池电解质的一些问题仍然需要引起足够的关注。本文首先阐述了锌离子电池的一般原理。然后分别从水溶液电解质、有机溶液电解质、凝胶电解质、全固态电解质等四个方面回顾了锌离子电池各种电解质的研究进展。其中,水溶液电解质部分重点介绍了对应电解质存在的溶剂化问题、相关的改性策略及添加剂对电极稳定性的提升机理。有机溶液电解质部分阐述了电池电化学性能的提升。凝胶电解质部分说明了相关电解质的形成原理和其在柔性储能领域的应用。全固态电解质部分则对相应电解质的无液特性和本质导电优势进行了简要说明。最后,对各种电解质的发展近况进行了总结,并对未来可能进行的研究方向进行了展望。
Progress of electrolyte additives for aqueous zinc-ion battery
[J].
水系锌离子电池电解液添加剂研究进展
[J].
Eliminating dendrites and side reactions via a multifunctional ZnSe protective layer toward advanced aqueous Zn metal batteries
[J].
Revealing the role of crystal orientation of protective layers for stable zinc anode
[J].Rechargeable aqueous zinc-ion batteries are a promising candidate for next-generation energy storage devices. However, their practical application is limited by the severe safety issue caused by uncontrollable dendrite growth on zinc anodes. Here we develop faceted titanium dioxide with relatively low zinc affinity, which can restrict dendrite formation and homogenize zinc deposition when served as the protective layer on zinc anodes. The as-prepared zinc anodes can be stripped and plated steadily for more than 460 h with low voltage hysteresis and flat voltage plateau in symmetric cells. This work reveals the key role of crystal orientation in zinc affinity and its internal mechanism is suitable for various crystal materials applied in the surface modification of other metal anodes such as lithium and sodium.
Wiring zinc in three dimensions re-writes battery performance—dendrite-free cycling
[J].
Effects of alloying elements on electrochemical performance of zinc air battery anode
[J].
合金元素对锌空气电池阳极电化学性能的影响
[J].
Study of the mechanism for electrodeposition of dendrite-free zinc in an alkaline electrolyte modified with 1-ethyl-3-methylimidazolium dicyanamide
[J].
Research progress and prospect on electrolyte additives for stabilizing the zinc anode interface in aqueous batteries
[J].
电解液添加剂稳定水系电池锌负极界面的研究进展
[J].水系锌金属电池(AZMBs)由于价格低廉、安全性高,在大规模储能领域极具潜力。然而,锌金属在常规水系电解液中并不稳定,界面处容易产生锌枝晶、析氢和腐蚀等副反应,导致AZMBs循环寿命较短。其中,电解液添加剂可以有效调控锌负极界面的化学特性和反应过程,显著提升其界面稳定性,大幅延长AZMBs的循环寿命。因此,对电解液添加剂稳定锌负极的相关研究进行总结,并对目前存在的关键问题提出新的解决思路非常必要。本文通过对近期相关文献进行探讨,简要介绍了锌负极目前面临的主要挑战及其相关机理,重点阐述了电解液添加剂对锌负极界面的主要调控机制,包括设计静电屏蔽层、贫水双电层(EDL)、原位固体电解质界面(SEI)层以及调控锌离子溶剂化鞘层。此外,还对不同添加剂类型进行了分类讨论,包括阳离子型、阴离子型、有机小分子型、有机聚合物型和其他类型,并分析了其各自的调控机理和对电化学性能的影响。最后,本文还对电解液添加剂策略稳定锌负极的未来发展方向提出了展望。
Energetic zinc ion chemistry: the rechargeable zinc ion battery
[J].
Strategies of regulating Zn2+ solvation structures for dendrite-free and side reaction-suppressed zinc-ion batteries
[J].
Toward practical high-areal-capacity aqueous zinc-metal batteries: quantifying hydrogen evolution and a solid-ion conductor for stable zinc anodes
[J].
Designing dendrite-free zinc anodes for advanced aqueous zinc batteries
[J].
ZnCl2 “water-in-salt” electrolyte transforms the performance of vanadium oxide as a Zn battery cathode
[J].
Corrosion behavior of galvanized steel in a simulated marine atmospheric environment
[J].
模拟海洋大气环境中镀锌钢的腐蚀行为和机理
[J].
Bio-inspired design of an in situ multifunctional polymeric solid-electrolyte interphase for Zn metal anode cycling at 30 mA cm-2 and 30 mA h cm-2
[J].
A self-deoxidizing electrolyte additive enables highly stable aqueous zinc batteries
[J].
Exploring the interfacial chemistry between zinc anodes and aqueous electrolytes via an in situ visualized characterization system
[J].
Discovering the intrinsic causes of dendrite formation in zinc metal anodes: Lattice defects and residual stress
[J].
In situ electron microscopy analysis of electrochemical Zn deposition onto an electrode
[J].
Dendrites in Zn-based batteries
[J].
Highly reversible zinc metal anode for aqueous batteries
[J].Metallic zinc (Zn) has been regarded as an ideal anode material for aqueous batteries because of its high theoretical capacity (820 mA h g(-1)), low potential (-0.762 V versus the standard hydrogen electrode), high abundance, low toxicity and intrinsic safety. However, aqueous Zn chemistry persistently suffers from irreversibility issues, as exemplified by its low coulombic efficiency (CE) and dendrite growth during plating/stripping, and sustained water consumption. In this work, we demonstrate that an aqueous electrolyte based on Zn and lithium salts at high concentrations is a very effective way to address these issues. This unique electrolyte not only enables dendrite-free Zn plating/stripping at nearly 100% CE, but also retains water in the open atmosphere, which makes hermetic cell configurations optional. These merits bring unprecedented flexibility and reversibility to Zn batteries using either LiMn2O4 or O-2 cathodes-the former deliver 180 W h kg(-1) while retaining 80% capacity for > 4,000 cycles, and the latter deliver 300 W h kg(-1) (1,000 W h kg(-1) based on the cathode) for > 200 cycles.
Immunizing aqueous Zn batteries against dendrite formation and side reactions at various temperatures via electrolyte additives
[J].
Boosting zinc electrode reversibility in aqueous electrolytes by using low-cost antisolvents
[J].Antisolvent addition has been widely studied in crystallization in the pharmaceutical industries by breaking the solvation balance of the original solution. Here we report a similar antisolvent strategy to boost Zn reversibility via regulation of the electrolyte on a molecular level. By adding for example methanol into ZnSO electrolyte, the free water and coordinated water in Zn solvation sheath gradually interact with the antisolvent, which minimizes water activity and weakens Zn solvation. Concomitantly, dendrite-free Zn deposition occurs via change in the deposition orientation, as evidenced by in situ optical microscopy. Zn reversibility is significantly boosted in antisolvent electrolyte of 50 % methanol by volume (Anti-M-50 %) even under harsh environments of -20 °C and 60 °C. Additionally, the suppressed side reactions and dendrite-free Zn plating/stripping in Anti-M-50 % electrolyte significantly enhance performance of Zn/polyaniline coin and pouch cells. We demonstrate this low-cost strategy can be readily generalized to other solvents, indicating its practical universality. Results will be of immediate interest and benefit to a range of researchers in electrochemistry and energy storage.© 2021 Wiley-VCH GmbH.
Ethylene glycol as an antifreeze additive and corrosion inhibitor for aqueous zinc-ion batteries
[J].
Self-smoothing deposition behavior enabled by beneficial potential compensating for highly reversible Zn-metal anodes
[J].
Modulated bonding interaction in propanediol electrolytes toward stable aqueous zinc-ion batteries
[J].
丙二醇电解液键结构调控及其水系锌离子电池稳定性研究
[J].
Cyclohexanedodecol-assisted interfacial engineering for robust and high-performance zinc metal anode
[J].Aqueous zinc-ion batteries (AZIBs) can be one of the most promising electrochemical energy storage devices for being non-flammable, low-cost, and sustainable. However, the challenges of AZIBs, including dendrite growth, hydrogen evolution, corrosion, and passivation of zinc anode during charging and discharging processes, must be overcome to achieve high cycling performance and stability in practical applications. In this work, we utilize a dual-functional organic additive cyclohexanedodecol (CHD) to firstly establish [Zn(HO)(CHD)] complex ion in an aqueous Zn electrolyte and secondly build a robust protection layer on the Zn surface to overcome these dilemmas. Systematic experiments and theoretical calculations are carried out to interpret the working mechanism of CHD. At a very low concentration of 0.1 mg mL CHD, long-term reversible Zn plating/stripping could be achieved up to 2200 h at 2 mA cm, 1000 h at 5 mA cm, and 650 h at 10 mA cm at the fixed capacity of 1 mAh cm. When matched with VO cathode, the resultant AZIBs full cell with the CHD-modified electrolyte presents a high capacity of 175 mAh g with the capacity retention of 92% after 2000 cycles under 2 A g. Such a performance could enable the commercialization of AZIBs for applications in grid energy storage and industrial energy storage.© 2022. Crown.
Dendrite-free zinc anode enabled by zinc-chelating chemistry
[J].
A universal additive strategy to reshape electrolyte solvation structure toward reversible Zn storage
[J].
A new insight of anti-solvent electrolytes for aqueous zinc-ion batteries by molecular modeling
[J].
Subnanocyclic molecule of 15-Crown-5 Inhibiting interfacial water decomposition and stabilizing zinc anodes via regulation of Zn2+ solvation shell
[J].
Boosting the kinetics and stability of Zn anodes in aqueous electrolytes with supramolecular cyclodextrin additives
[J].The hydrophobic internal cavity and hydrophilic external surface of cyclodextrins (CDs) render promising electrochemical applications. Here, we report a comparative and mechanistic study on the use of CD molecules (α-, β-, and γ-CD) as electrolyte additives for rechargeable Zn batteries. The addition of α-CD in aqueous ZnSO solution reduces nucleation overpotential and activation energy of Zn plating and suppresses H generation. Computational, spectroscopic, and electrochemical studies reveal that α-CD preferentially adsorbs in parallel on the Zn surface via secondary hydroxyl groups, suppressing water-induced side reactions of hydrogen evolution and hydroxide sulfate formation. Additionally, the hydrophilic exterior surface of α-CD with intense electron density simultaneously facilitates Zn deposition and alleviates Zn dendrite formation. A formulated 3 M ZnSO + 10 mM α-CD electrolyte enables homogenous Zn plating/stripping (average Coulombic efficiency ∼ 99.90%) at 1 mA cm in Zn|Cu cells and a considerable capacity retention of 84.20% after 800 cycles in Zn|VO full batteries. This study provides insight into the use of supramolecular macrocycles to modulate and enhance the interface stability and kinetics of metallic anodes for aqueous battery chemistry.
Dendrite-free lithium deposition via self-healing electrostatic shield mechanism
[J].Rechargeable lithium metal batteries are considered the "Holy Grail" of energy storage systems. Unfortunately, uncontrollable dendritic lithium growth inherent in these batteries (upon repeated charge/discharge cycling) has prevented their practical application over the past 40 years. We show a novel mechanism that can fundamentally alter dendrite formation. At low concentrations, selected cations (such as cesium or rubidium ions) exhibit an effective reduction potential below the standard reduction potential of lithium ions. During lithium deposition, these additive cations form a positively charged electrostatic shield around the initial growth tip of the protuberances without reduction and deposition of the additives. This forces further deposition of lithium to adjacent regions of the anode and eliminates dendrite formation in lithium metal batteries. This strategy may also prevent dendrite growth in lithium-ion batteries as well as other metal batteries and transform the surface uniformity of coatings deposited in many general electrodeposition processes.
A self-regulated electrostatic shielding layer toward dendrite-free Zn batteries
[J].
Structural absorption by barbule microstructures of super black bird of paradise feathers
[J].Many studies have shown how pigments and internal nanostructures generate color in nature. External surface structures can also influence appearance, such as by causing multiple scattering of light (structural absorption) to produce a velvety, super black appearance. Here we show that feathers from five species of birds of paradise (Aves: Paradisaeidae) structurally absorb incident light to produce extremely low-reflectance, super black plumages. Directional reflectance of these feathers (0.05-0.31%) approaches that of man-made ultra-absorbent materials. SEM, nano-CT, and ray-tracing simulations show that super black feathers have titled arrays of highly modified barbules, which cause more multiple scattering, resulting in more structural absorption, than normal black feathers. Super black feathers have an extreme directional reflectance bias and appear darkest when viewed from the distal direction. We hypothesize that structurally absorbing, super black plumage evolved through sensory bias to enhance the perceived brilliance of adjacent color patches during courtship display.
Mechanistic insights of Mg2+-electrolyte additive for high-energy and long-life zinc-ion hybrid capacitors
[J].
Refining the grain size of zinc electrodeposit by Pb2+ ion grinding for compact and stable zinc anode
[J].
Efficient suppression of dendrites and side reactions by strong electrostatic shielding effect via the additive of Rb2SO4 for anodes in aqueous zinc-ion batteries
[J].
Cationic surfactant-type electrolyte additive enables three-dimensional dendrite-free zinc anode for stable zinc-ion batteries
[J].
A versatile cation additive enabled highly reversible zinc metal anode
[J].
Stabilizing zinc deposition with sodium lignosulfonate as an electrolyte additive to improve the life span of aqueous zinc-ion batteries
[J].Thanks to high safety and low cost, rechargeable zinc-ion batteries (RZIBs) have become a promising candidate for grid-scale energy storage systems. However, zinc anodes suffer from severe dendrite growth and irreversible side reactions, leading to poor cyclability of RZIBs. In this work, low-cost sodium lignosulfonate (SL) is utilized as the electrolyte additive to solve this problem. The added amount of SL is optimized to be 0.02%, which enables the Zn//α-MnO battery to deliver a large capacity of 146 mAh g after 1000 cycles at 1 A g, corresponding to a high capacity retention of 83.5%. The Zn//Zn symmetric cell with the modified electrolyte also shows excellent cyclability even under a commercial level of areal specific capacity (4 mAh cm). Overall, the results of this study confirm that the SL additive can improve the ionic conductivity of electrolyte, restrict the two-dimensional planar diffusion of Zn ions at the electrode/electrolyte interface, lower the nucleation overpotential of Zn ions, prevent side reactions, and inhibit the corrosion of Zn metal. Therefore, the dendrite growth and byproduct formation can be effectively suppressed. This study provides new insights into protecting metal electrodes of electrochemical energy storage devices.Copyright © 2021 Elsevier Inc. All rights reserved.
Highly reversible zinc metal anodes enabled by protonated melamine
[J].
Monosodium glutamate, an effective electrolyte additive to enhance cycling performance of Zn anode in aqueous battery
[J].
Electrolyte regulation of bio-inspired zincophilic additive toward high-performance dendrite-free aqueous zinc-ion batteries
[J].
Inhibition of dendrite growth and side reactions using histidine as electrolyte additive for aqueous Zn-ion batteries
[J].
Self-assembled multilayers direct a buffer interphase for long-life aqueous zinc-ion batteries
[J].
Facet-termination promoted uniform Zn (100) deposition for high-stable zinc-ion batteries
[J].
Stabilizing zinc electrodes with a vanillin additive in mild aqueous electrolytes
[J].
Manipulating interfacial stability via absorption-competition mechanism for long-lifespan Zn anode
[J].The stability of Zn anode in various Zn-based energy storage devices is the key problem to be solved. Herein, aromatic aldehyde additives are selected to modulate the interface reactions between the Zn anode and electrolyte. Through comprehensively considering electrochemical measurements, DFT calculations and FEA simulations, novel mechanisms of one kind of aromatic aldehyde, veratraldehyde in inhibiting Zn dendrite/by-products can be obtained. This additive prefers to absorb on the Zn surface than HO molecules and Zn, while competes with hydrogen evolution reaction and Zn plating/stripping process via redox reactions, thus preventing the decomposition of active HO near the interface and uncontrollable Zn dendrite growth via a synactic absorption-competition mechanism. As a result, Zn-Zn symmetric cells with the veratraldehyde additive realize an excellent cycling life of 3200 h under 1 mA cm/1 mAh cm and over 800 h even under 5 mA cm/5 mAh cm. Moreover, Zn-Ti and Zn-MnO cells with the veratraldehyde additive both obtain elevated performance than that with pure ZnSO electrolyte. Finally, two more aromatic aldehyde additives are chosen to prove their universality in stabilizing Zn anodes.© 2021. The Author(s).
Anti-corrosion for reversible zinc anode via a hydrophobic interface in aqueous zinc batteries
[J].
In-situ constructing polyacrylamide interphase enables dendrite-free zinc anode in aqueous batteries
[J].
Degradation behavior of pure zinc and Zn-xLi alloy in artificial urine
[J].
Zn及锌锂合金在人工尿液中的腐蚀行为
[J].通过体外浸泡实验及电化学测试的方法,研究了Zn-xLi (x=0,0.5%,0.8%) 在人工尿液 (AU) 中长达28 d的腐蚀行为。结果表明,尽管浸泡过程中,样品依然存在结壳现象,但对比前人研究过的几种金属,Zn/Zn-xLi结壳现象有所缓解,这在输尿管植入应用中是十分可喜的现象。样品在人工尿液中的腐蚀产物为CaZn<sub>2</sub>(PO<sub>4</sub>)<sub>2</sub>·2H<sub>2</sub>O,电化学测定浸泡28 d后样品的腐蚀速率为0.21~0.34 mm·a<sup>-1</sup>。
Electrolyte design for in situ construction of highly Zn2+-conductive solid electrolyte interphase to enable high-performance aqueous Zn-ion batteries under practical conditions
[J].
Alleviation of dendrite formation on zinc anodes via electrolyte additives
[J].
Self-healing SeO2 additives enable zinc metal reversibility in aqueous ZnSO4 electrolytes
[J].
Simultaneous regulation on solvation shell and electrode interface for dendrite-free Zn ion batteries achieved by a low-cost glucose additive
[J].
Unraveling the regulation of a polyhydroxy electrolyte additive for a reversible, dendrite-free zinc anode
[J].
Modulating cation migration and deposition with xylitol additive and oriented reconstruction of hydrogen bonds for stable zinc anodes
[J].
Polyhydroxylated organic molecular additives for durable aqueous zinc battery
[J].
Electrolyte additive of sorbitol rendering aqueous zinc-ion batteries with dendrite-free behavior and good anti-freezing ability
[J].
Surface control behavior toward crystal regulation and anticorrosion capacity for zinc metal anodes
[J].
Cellulose-complexing strategy induced surface regulation towards ultrahigh utilization rate of Zn
[J].
Stabilizing zinc anodes by regulating the electrical double layer with saccharin anions
[J].
Synergistic solvation and interface regulations of eco-friendly silk peptide additive enabling stable aqueous zinc-ion batteries
[J].
Toward stable zinc aqueous rechargeable batteries by anode morphology modulation via polyaspartic acid additive
[J].
A self-regulated interface toward highly reversible aqueous zinc batteries
[J].
When it's heavier: interfacial and solvation chemistry of isotopes in aqueous electrolytes for Zn-ion batteries
[J].
Quasi-decoupled solid–liquid hybrid electrolyte for highly reversible interfacial reaction in aqueous zinc-manganese battery
[J].
Constructing ionic self-concentrated electrolyte via introducing montmorillonite toward high-performance aqueous Zn-MnO2 batteries
[J].
A low-cost quasi-solid-state “water-in-swelling-clay” electrolyte enabling ultrastable aqueous zinc-ion batteries
[J].
In situ buildup of zinc anode protection films with natural protein additives for high-performance zinc battery cycling
[J].
Manipulating the solvation structure and interface via a bio-based green additive for highly stable Zn metal anode
[J].
Chemical and electrochemical synergistic weaving stable interface enabling longevous zinc plating/stripping process
[J].
Low-concentration redox-electrolytes for high-rate and long-life zinc metal batteries
[J].
Reversible Zn metal anodes enabled by trace amounts of underpotential deposition initiators
[J].
Cholinium cations enable highly compact and dendrite-free Zn metal anodes in aqueous electrolytes
[J].
Addition of dioxane in electrolyte promotes (002)-textured zinc growth and suppressed side reactions in zinc-ion batteries
[J].The reversibility and cyclability of aqueous zinc-ion batteries (ZIBs) are largely determined by the stabilization of the Zn anode. Therefore, a stable anode/electrolyte interface capable of inhibiting dendrites and side reactions is crucial for high-performing ZIBs. In this study, we investigated the adsorption of 1,4-dioxane (DX) to promote the exposure of Zn (002) facets and prevent dendrite growth. DX appears to reside at the interface and suppress the detrimental side reactions. ZIBs with the addition of DX demonstrated a long-term cycling stability of 1000 h in harsh conditions of 10 mA cm with an ultrahigh cumulative plated capacity of 5 Ah cm and shows a good reversibility with an average Coulombic efficiency of 99.7%. The Zn//NHVO full battery with DX achieves a high specific capacity (202 mAh g at 5 A g) and capacity retention (90.6% after 5000 cycles), much better than that of ZIBs with the pristine ZnSO electrolyte. By selectively adjusting the Zn deposition rate on the crystal facets with adsorbed molecules, this work provides a promising modulation strategy at the molecular level for high-performing Zn anodes and can potentially be applied to other metal anodes suffering from instability and irreversibility.
A double-functional additive containing nucleophilic groups for high-performance Zn-ion batteries
[J].
A co-solvent in aqueous electrolyte towards ultralong-life rechargeable zinc-ion batteries
[J].
Building metal-molecule interface towards stable and reversible Zn metal anodes for aqueous rechargeable zinc batteries
[J].
Decreasing water activity using the tetrahydrofuran electrolyte additive for highly reversible aqueous zinc metal batteries
[J].
Dual-function electrolyte additive enabling simultaneous electrode interface and coordination environment regulation for zinc-ion batteries
[J].
Tuning the electrode/electrolyte interface enabled by a trifunctional inorganic oligomer electrolyte additive for highly stable and high-rate Zn anodes
[J].
Surface protection and interface regulation for Zn anode via 1-hydroxy ethylidene-1,1-diphosphonic acid electrolyte additive toward high-performance aqueous batteries
[J].
Simultaneous regulation on coordination environment and interfacial chemistry via taurine for stabilized Zn metal anode
[J].Aqueous Zn-ion batteries (AZIBs) are the potential options for the next-generation energy storage scenarios due to the cost effectiveness and intrinsic safety. Nevertheless, the industrial application of AZIBs is still impeded by a series of parasitic reactions and dendrites at zinc anodes. In this study, taurine (TAU) is used in electrolyte to simultaneously optimize the coordination condition of the ZnSO<sub>4</sub> electrolyte and interfacial chemistry at the anode. TAU can preferentially adsorb with the zinc metal and induce an in situ stable and protective interface on the anode, which would avoid the connection between H<sub>2</sub>O and the zinc metal and promote the even deposition of Zn<sup>2+</sup>. The resulting Zn//Zn batteries achieve more than 3000 hours long cyclic lifespan under 1 mA cm<sup>-2</sup> and an impressive cumulative capacity at 5 mA cm<sup>-2</sup>. Moreover, Zn//Cu batteries can realize a reversible plating/stripping process over 2,400 cycles, with a desirable coulombic efficiency of 99.75% (1 mA cm<sup>-2</sup>). Additionally, the additive endows Zn//NH<sub>4</sub>V<sub>4</sub>O<sub>10</sub> batteries with more stable cyclic performance and ultrafast rate capability. These capabilities can promote the industrial application of AZIBs.
Coordination modulation of hydrated zinc ions to enhance redox reversibility of zinc batteries
[J].The dendrite growth of zinc and the side reactions including hydrogen evolution often degrade performances of zinc-based batteries. These issues are closely related to the desolvation process of hydrated zinc ions. Here we show that the efficient regulation on the solvation structure and chemical properties of hydrated zinc ions can be achieved by adjusting the coordination micro-environment with zinc phenolsulfonate and tetrabutylammonium 4-toluenesulfonate as a family of electrolytes. The theoretical understanding and in-situ spectroscopy analysis revealed that the favorable coordination of conjugated anions involved in hydrogn bond network minimizes the activate water molecules of hydrated zinc ion, thus improving the zinc/electrolyte interface stability to suppress the dendrite growth and side reactions. With the reversibly cycling of zinc electrode over 2000 h with a low overpotential of 17.7 mV, the full battery with polyaniline cathode demonstrated the impressive cycling stability for 10000 cycles. This work provides inspiring fundamental principles to design advanced electrolytes under the dual contributions of solvation modulation and interface regulation for high-performing zinc-based batteries and others.© 2023. The Author(s).
A multifunctional organic electrolyte additive for aqueous zinc ion batteries based on polyaniline cathode
[J].
Stable Zn metal anodes enabled by restricted self-diffusion via succinimide surfactant
[J].
In situ construction of anode-molecule interface via lone-pair electrons in trace organic molecules additives to achieve stable zinc metal anodes
[J].
Designing anion-type water-free Zn2+ solvation structure for robust Zn metal anode
[J].
Inducing the preferential growth of Zn (002) plane for long cycle aqueous Zn-ion batteries
[J].
Reconstructing the anode interface and solvation shell for reversible zinc anodes
[J].