过去几十年,研究人员对冲刷腐蚀开展了大量研究 [7~14],主要聚焦在最为普遍的双相流腐蚀上。双相流腐蚀是由腐蚀性溶液和溶液中固体颗粒构成的双相介质共同作用所引起的材料失效,腐蚀性液体与固体颗粒特性是影响双相流腐蚀的关键因素。具体到腐蚀性液体的性质,涉及腐蚀性介质浓度、温度、pH值、流速等[10,15~17]。双相流腐蚀过程中固体颗粒的冲击是造成材料机械损伤的主要原因,影响因素包括固体颗粒形状、尺寸、进给率以及材料本身特性等。研究表明,具有尖锐棱角的颗粒对材料造成的质量损失大于圆角颗粒[18~21]。尺寸更大的颗粒可以携带更多的动能,从而深入表面导致冲刷速率增加。然而,对于圆角颗粒来说,尺寸过大反而会妨碍其钻入表面[19,22~25]。人们通常认为更高的颗粒进给率会导致更大的冲刷损害,但文献[19,20,26,27]研究表明,在高进给率下,颗粒之间存在相互干扰,导致颗粒的撞击方向和速度发生改变。撞击后反弹的颗粒会形成屏蔽效应,从而降低冲刷速率。材料自身特性在冲刷过程中也起到重要作用。Suchánek等[28]研究表明,珠光体相较于铁素体具有更好的耐冲刷性能,此外,Levy[29]研究表明,韧性在一定范围内增加有助于降低冲刷速率,超出一定范围后,冲刷速率受到强度的控制,并且球化后的球状结构具有比珠光体更好的耐冲刷性能。进一步的,腐蚀性溶液与固体颗粒之间还存在交互作用,腐蚀性溶液引起的电化学腐蚀会导致腐蚀产物层的形成,有助于降低材料的腐蚀速率。然而,固体颗粒的冲击会破坏钝化膜,使基体表面始终暴露在腐蚀性介质中,从而使材料的电荷转移过程不受钝化膜的阻碍[30~37]。此外,固体颗粒的冲击会导致材料表面出现冲击坑、剪切唇或裂纹,增加了材料在腐蚀性溶液中的暴露面积,从而促进腐蚀。同时,裂纹扩展引起的碎片剥落、剪切唇的脱落同样会导致材料质量损失的增加[38,39]。
1 实验方法
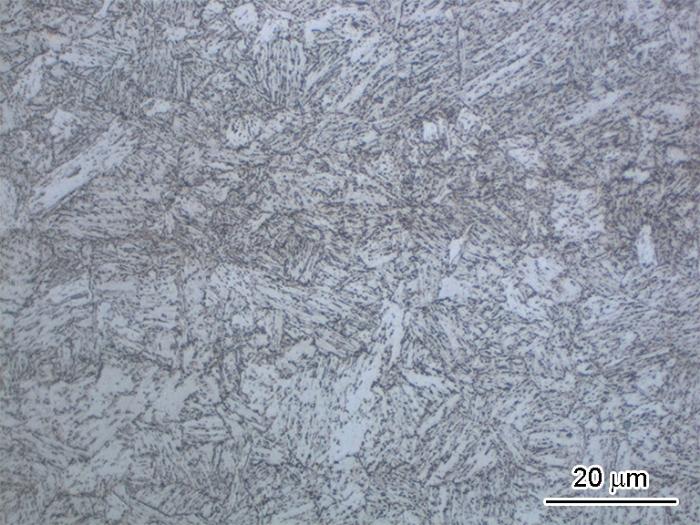
本文的研究对象为一种新研制的HSLA钢,强度不低于690 MPa,化学成分(质量分数,%)为:C ≤ 0.06,Si ≤ 0.4,Mn 0.75~1.15,P ≤ 0.02,S ≤ 0.004,Al ≥ 0.015,Cr 0.45~0.75,Ni 1.5~3.65,Ti 0.005~0.02,Mo 0.30~0.65,V ≤ 0.03,Cu 1.00~1.75,Nb 0.02~0.16,Fe余量。钢基体表面按照240#、400#、800#、1200#、2000#的顺序逐级研磨,并采用2.5 μm抛光膏抛光、4%硝酸酒精蚀刻,通过光学显微镜对蚀刻后的样品表面进行了观察,得到的显微组织结果如图1所示。结果显示HSLA钢的金相组织为板条马氏体、铁素体以及贝氏体。
图1
静态浸泡和动态冲刷选择的实验介质均为3.5% (质量分数)NaCl,实验温度为30℃。静态浸泡在恒温水浴锅中进行,动态冲刷测试在图2所示的旋转式冲刷装置中进行。该装置分为动力、样品承载及外部防护部分。动力含有电动机和传动轴,可通过调整电动机转速以改变冲刷速度。样品承载含有样品台、隔离栅。样品置于样品台中,以隔离栅互相分隔。外部防护包括外壳、盖板及特氟龙内衬等,本工作选择4.9 m/s的冲刷速度在30℃下进行测试。
图2
失重测试试样尺寸为10 mm × 10 mm × 5 mm,距边缘中心1.5 mm处有直径为1.5 mm的通孔,工作面积约为4.47 cm2。进行测试前将试样表面逐级研磨至2000#,用去离子水和无水乙醇洗涤、吹干后,使用精度为0.1 mg的分析天平称重。测试后样品用除锈剂进行超声清洗,去离子水冲洗表面并烘干,除锈剂为:3.5 g六次甲基四胺+500 mL盐酸+500 mL去离子水。对除锈后样品进行称重,计算质量损失及失重速率,每次实验取3个平行试样,以保证实验结果可靠。
电化学测试试样先用粉末涂料对四周进行涂敷以避免测试过程中产生缝隙腐蚀,对粉末涂料涂敷后的试样以环氧树脂进行封装,暴露工作面积约为1 cm2,试样工作面使用砂纸逐级研磨至2000#。使用VersaSTAT V3F电化学工作站进行电化学测试,开路电位测试600 s后进行EIS和动电位极化曲线测试。测试采用三电极体系,待测试样为工作电极,饱和甘汞电极为参比电极,铂电极为对电极。EIS测试频率范围为105~10-2 Hz,正弦波扰动振幅为10 mV,动电位极化曲线扫描范围为-0.3 V(相对开路电位)~+1.6 V(相对参比电极),扫描速度为0.333 mV/s,扫至阳极电流密度超过1 mA/cm2时停止。
表面形貌观察样品在测试前按照240#、400#、800#、1200#、2000#的顺序逐级研磨,采用2.5 μm抛光膏对表面抛光。通过体视显微镜(Zeiss SteREO)和扫描电子显微镜(Tescan Mira,SEM)对实验不同时间后的样品表面进行观察。使用Raman光谱(LabRAM HR800)对腐蚀产物的物相组成进行测试分析,使用Oxford Instruments X-Max 80能谱仪(EDS)进行EDS分析。
2 结果与讨论
2.1 失重分析
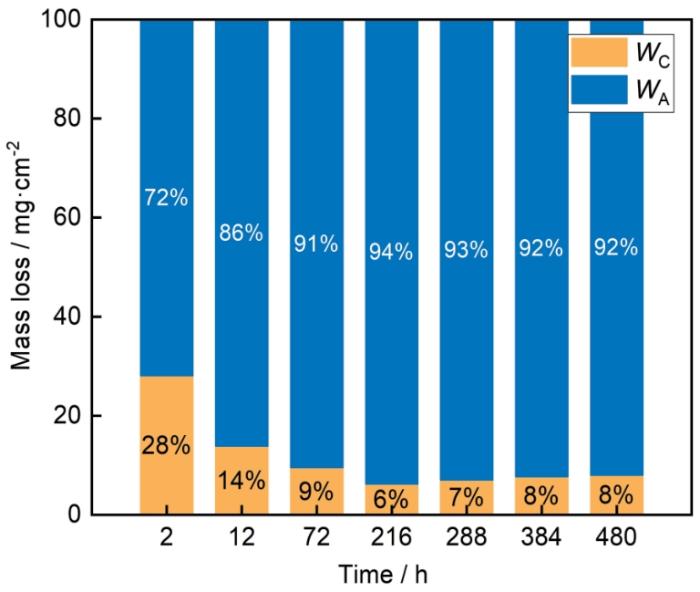
HSLA钢静态浸泡和动态冲刷480 h后的失重结果如图3a所示。结果表明,动态冲刷条件下的失重显著高于静态浸泡,并呈线性上升的变化趋势,在480 h后增加至68.3 mg·cm-2,是静态浸泡480 h后5.5 mg·cm-2的13倍。图3b显示了HSLA钢在两种条件下的腐蚀速率。明显可见,在每个时间节点,HSLA钢动态冲刷后的腐蚀速率均显著大于静态浸泡后的腐蚀速率,动态冲刷12 h后的腐蚀速率为0.172 mg·cm-2·h-1,远高于静态浸泡12 h后的0.024 mg·cm-2·h-1。动态冲刷216 h后的腐蚀速率为0.170 mg·cm-2·h-1,是静态浸泡相同时间后0.011 mg·cm-2·h-1的16倍。实验480 h后,动态冲刷条件下HSLA钢的腐蚀速率为0.142 mg·cm-2·h-1,是静态浸泡条件下腐蚀速率的13倍。对于静态浸泡环境,HSLA钢的腐蚀速率在初期的2~12 h达到最大,随后显著下降并逐渐趋于稳定。对于动态冲刷条件,HSLA钢的腐蚀速率相比于静态浸泡始终维持在较高的水平,动态冲刷2~216 h后,腐蚀速率在0.134~0.172 mg·cm-2·h-1的范围内波动,实验至216~480 h后,腐蚀速率有所下降并逐渐平缓。可以将HSLA钢在动态冲刷后的失重分为两个部分,一方面是静态浸泡下单纯由腐蚀性离子作用所造成的质量损失,简称为纯腐蚀;另一方面是腐蚀性介质流动冲刷引起的质量损失,简称为腐蚀性流体的加速腐蚀作用。图4显示了纯腐蚀和腐蚀性流体的加速作用对质量损失的贡献比例。通过将动态冲刷后的失重减去静态浸泡后的失重,计算得到由腐蚀性流体的加速腐蚀作用所导致的失重,如
图3
图3
HSLA钢在静态浸泡和动态冲刷过程中的失重及腐蚀速率
Fig.3
Mass losses (a) and corrosion rates (b) of HSLA steel during static immersion and dynamic erosion
式中,WA为腐蚀性流体的加速作用所导致的失重;WEC和WC分别为动态冲刷后和静态浸泡后的失重结果。结果表明,相比于WC,WA始终占据相当高的比例,480 h内平均占比88.6%,是纯腐蚀作用11.4%的7.8倍,特别是在72~480 h,其平均占比高达92%。这表明流动性腐蚀介质极大促进了HSLA钢的腐蚀,是导致动态冲刷后失重及腐蚀速率激增的重要原因,这与Hu等[46]的研究结果相一致。
图4
图4
纯腐蚀与流动性介质的加速作用所导致的质量损失占比
Fig.4
Proportions of mass losses caused by accelerated corrosion of corrosive fluid and static immersion
2.2 电化学测试分析
2.2.1 低频阻抗模值结果
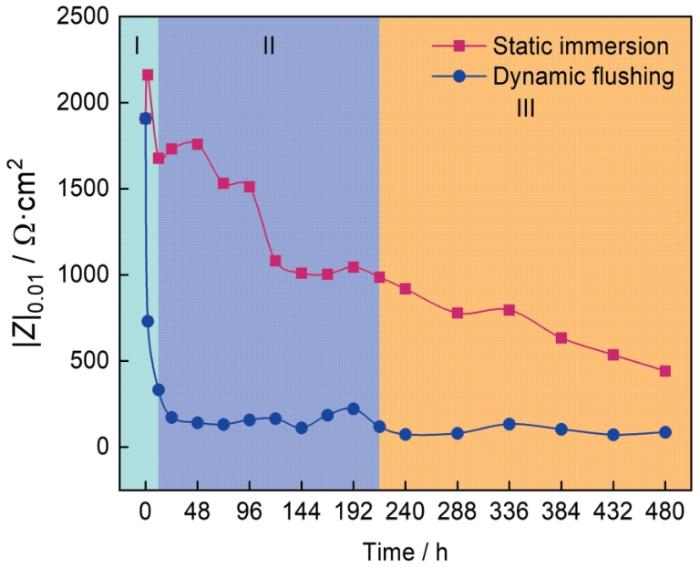
HSLA钢静态浸泡和动态冲刷480 h内电化学阻抗谱低频阻抗模值(0.01 Hz)随时间的变化如图5所示。从图中可以观察到,480 h的实验周期内,HSLA钢在动态冲刷条件下的低频阻抗模值明显低于静态浸泡条件。HSLA钢初始的低频阻抗模值为1908 Ω·cm2,静态浸泡12 h后降为1676 Ω·cm2,而动态冲刷12 h后则迅速降至333 Ω·cm2,不足原始值的1/5。实验480 h后,动态冲刷条件下的低频阻抗模值进一步降低为86 Ω·cm2,远低于静态浸泡后的442 Ω·cm2。虽然HSLA钢的低频阻抗模值在静态浸泡与动态冲刷条件下均呈下降的趋势,但两者之间存在明显差异,静态浸泡后的低频阻抗模值随着时间增加而逐渐下降,动态冲刷后的低频阻抗模值先急剧降低而后趋于平稳。根据HSLA钢低频阻抗模值在两种条件下的变化趋势,可将HSLA钢的腐蚀失效过程分为三个阶段:第Ⅰ阶段(0~12 h),第Ⅱ阶段(12~216 h),第Ⅲ阶段(216~480 h)。
图5
图5
HSLA钢在静态浸泡及动态冲刷后的低频阻抗模值变化趋势
Fig.5
Changes of low frequency impedance modulus of HSLA steel during static immersion and dynamic erosion
2.2.2 EIS结果
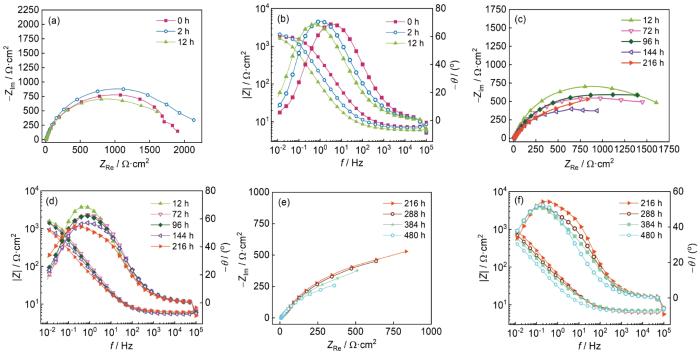
按照图5的阶段划分,静态浸泡条件下3个阶段的EIS结果如图6所示。可以看出,HSLA钢在静态浸泡的整个阶段均呈容抗弧特征,容抗弧的半径随着时间的增加而逐渐减小。在第Ⅰ阶段,容抗弧半径和低频阻抗模值呈现先增加后降低的趋势,高频相位角逐渐降低,低频相位角逐渐升高,低频阻抗模值由0 h的1908 Ω·cm2降低至12 h的1676 Ω·cm2,降低了12%。第Ⅱ阶段,容抗弧半径与低频阻抗模值持续降低,相位角峰值也逐渐降低,低频阻抗模值在216 h时降低至987 Ω·cm2,降低了48%。第Ⅲ阶段,容抗弧半径、低频阻抗模值继续降低,低频阻抗模值在480 h后降至442 Ω·cm2,较原始值降低了77%。从降低幅度上判断,HSLA钢低频阻抗模值3个阶段的降低幅度分别为12%、36%和29%,耐蚀性能的降低主要集中在第Ⅱ和第Ⅲ阶段。
图6
图6
HSLA钢在静态浸泡过程中的EIS结果
Fig.6
EIS results of HSLA steel after static immersion: (a, b) stage Ⅰ, (c, d) stage Ⅱ, (e, f) stage Ⅲ
HSLA钢在动态冲刷后3个阶段的EIS结果如图7所示。可以看出,HSLA钢在动态冲刷的整个阶段同样呈容抗弧特征,容抗弧的半径随着动态冲刷时间的增加而减小。在第Ⅰ阶段,容抗弧半径、低频阻抗模值及相位角峰值均出现了明显降低,低频阻抗模值由0 h的1908 Ω·cm2急剧降低至12 h的333 Ω·cm2,降低了83%。在第Ⅱ阶段,容抗弧半径与低频阻抗模值继续降低,相位角峰值同样逐渐减小,低频阻抗模值降低至119 Ω·cm2,降低了94%。在第Ⅲ阶段,容抗弧半径、低频阻抗模值和低频相位角仍然保持降低的趋势,低频阻抗模值在480 h后降低至86 Ω·cm2,较原始值降低了95%。从降幅上分析,HSLA钢低频阻抗模值3个阶段的降幅分别为83%、11%和1%,耐蚀性能的降低主要集中在第Ⅰ阶段。
图7
图7
HSLA钢在动态冲刷过程中的EIS结果
Fig.7
EIS results of HSLA steel after dynamic erosion: (a, b) stage Ⅰ, (c, d) stage Ⅱ, (e, f) stage Ⅲ
图8
图8
电化学阻抗谱等效电路
Fig.8
Equivalent circuits of EIS of HSLA steel after static immersion (a) and dynamic erosion (b)
图9显示了HSLA钢静态浸泡和动态冲刷过程中Rf和Rct的变化趋势。Rct的变化趋势与图5低频阻抗模值的变化趋势相近,这里不再作赘述。总的来说,HSLA钢在动态冲刷条件下的Rf值要明显高于静态浸泡下的Rf值,尤其是第Ⅰ阶段动态冲刷12 h后,HSLA钢的Rf值迅速上升至506.1 Ω·cm2,是静态浸泡相同时间Rf值的36倍。进一步具体分析两种条件下HSLA钢Rf值的变化趋势,对于动态冲刷:第Ⅰ阶段,Rf值激增至初始状态的35倍,预示着腐蚀产物的快速形成与堆积;第Ⅱ阶段,Rf值总体表现为逐渐降低的趋势,在216 h时降低为最大值的21%,表明腐蚀产物的保护性快速下降;第Ⅲ阶段,Rf值在50~217 Ω·cm2之间波动,代表着腐蚀产物剥落及重新生成的动态过程。对于静态浸泡,第Ⅰ阶段的Rf值无明显变化,在第Ⅱ阶段末期上升至263 Ω·cm2,预示着腐蚀产物层的形成,在第Ⅲ阶段,静态浸泡后Rf值再次降低,随后趋于稳定。
图9
图9
HSLA钢腐蚀产物膜电阻和电荷转移电阻随时间的变化
Fig.9
Variations of corrosion product layer resistance (a) and charge transfer resistance (b) of HSLA steel with time
2.2.3 动电位极化曲线
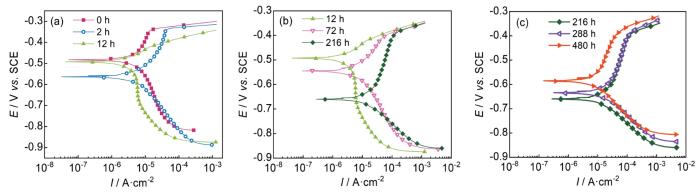
为了表征HSLA钢在静态浸泡和动态冲刷过程中的阳极和阴极变化,对实验不同时间后的HSLA钢进行动电位极化曲线测试,如图10和11所示,并对相应的电化学参数进行了拟合,结果如表1所示。可以看出,静态浸泡条件下HSLA钢具有更低的自腐蚀电流密度与更高的自腐蚀电位,动态冲刷480 h后的自腐蚀电流密度为8.06 × 10-5 A·cm-2,比静态浸泡480 h后的4.55 × 10-6 A·cm-2高出一个数量级,这预示着腐蚀性流体的动态冲刷极大的促进了材料的腐蚀。具体分析HSLA钢在两种条件下的电化学行为。对于图10所示的静态浸泡条件,HSLA钢的阳极在480 h的整个浸泡周期内,阳极大部分时间均表现出微弱的钝化(12 h除外),整个浸泡过程中击破电位基本保持不变。第Ⅰ阶段,2 h后阳极受到明显的促进,导致自腐蚀电位下降,12 h后阳极由钝化转变为活性溶解的特征,说明HSLA钢的初始氧化层被逐步破坏,而完整的腐蚀产物层尚未形成。第Ⅱ阶段,随着浸泡时间的增加,HSLA钢的阳极和阴极均受到促进,但阳极的促进作用占据主导,导致HSLA钢的自腐蚀电位继续负移。第Ⅲ阶段,HSLA钢的阳极受到抑制,阴极受到促进,导致材料的自腐蚀电位升高。图11展示了动态冲刷过程中的动电位极化曲线。与静态浸泡明显不同的是,HSLA钢在480 h的整个试验周期内,阳极大部分时间均表现为活性溶解(除前12 h的第Ⅰ阶段外)。具体的,在第Ⅰ阶段,阳极的促进作用占据主导,导致HSLA钢的自腐蚀电位大幅度负移。第Ⅱ阶段,HSLA钢阴极受到抑制的程度逐渐与阳极受到促进的程度相当,导致自腐蚀电位基本不变。第Ⅲ阶段,HSLA钢的阳极受抑制程度大于阴极受促进程度,导致材料自腐蚀电位的升高。
图10
图10
HSLA钢静态浸泡不同时间后的动电位极化曲线
Fig.10
Polarization curves of HSLA steel after static immersion for different time: (a) stage Ⅰ, (b) stage Ⅱ, (c) stage Ⅲ
图11
图11
HSLA钢动态冲刷不同时间后的动电位极化曲线
Fig.11
Polarization curves of HSLA steel after dynamic erosion for different time: (a) stage Ⅰ, (b) stage Ⅱ, (c) stage Ⅲ
表1 静态浸泡和动态冲刷后的动电位极化曲线拟合参数
Table 1
| State | Time / h | Ecorr / V | Icorr / A·cm-2 | Eb / V | Ip / A·cm-2 |
|---|---|---|---|---|---|
| Static immersion | 0 | -0.483 | 2.88 × 10-6 | -0.341 | 1.51 × 10-5 |
| 2 | -0.567 | 3.28 × 10-6 | -0.338 | 4.50 × 10-5 | |
| 12 | -0.493 | 2.02 × 10-6 | |||
| 72 | -0.539 | 4.68 × 10-6 | -0.398 | 6.50 × 10-5 | |
| 216 | -0.661 | 8.34 × 10-6 | -0.394 | 1.31 × 10-4 | |
| 288 | -0.634 | 9.42 × 10-6 | -0.383 | 1.47 × 10-4 | |
| 480 | -0.586 | 4.55 × 10-6 | -0.377 | 6.50 × 10-5 | |
| Dynamic flushing | 0 | -0.479 | 3.05 × 10-6 | -0.341 | 1.51 × 10-5 |
| 2 | -0.599 | 4.02 × 10-6 | -0.402 | 4.90 × 10-5 | |
| 12 | -0.616 | 5.68 × 10-6 | -0.351 | 1.16 × 10-4 | |
| 72 | -0.674 | 1.84 × 10-5 | |||
| 216 | -0.602 | 3.42 × 10-5 | |||
| 288 | -0.560 | 3.90 × 10-5 | |||
| 480 | -0.519 | 8.06 × 10-5 |
2.3 腐蚀形貌分析
2.3.1 宏观形貌
HSLA钢静态浸泡不同时间后的宏观形貌如图12所示。浸泡2 h后表面出现明显局部腐蚀(图12a),并且周围沉积有腐蚀产物。随着时间增加至12 h,局部腐蚀逐渐向四周扩展,腐蚀产物覆盖面积逐渐增加(图12b)。72 h后表面完全被黑色腐蚀产物所覆盖,仍可见局部腐蚀聚集的痕迹(图12c)。至216 h后,表面被黑色腐蚀产物覆盖的同时可见明显的絮状红褐色腐蚀产物(图12d)。浸泡288 h后,表面絮状红褐色腐蚀产物有所增加(图12e),384 h时再次出现类似72 h时的局部腐蚀区域(图12f),480 h后HSLA钢表面完全被黑色腐蚀产物覆盖,并零星分布有红褐色产物(图12g)。根据HSLA钢在3.5%NaCl溶液中静态浸泡不同时间后的宏观形貌演变,可以将其与图5的3个阶段对应起来并赋予不同的意义:第Ⅰ阶段(0~12 h)为局部腐蚀的萌生与扩展阶段,第Ⅱ阶段(12~216 h)为腐蚀产物的成膜阶段,第Ⅲ阶段(216~480 h)为稳定腐蚀阶段。
图12
图12
HSLA钢在静态浸泡不同时间后的宏观形貌
Fig.12
Macroscopic morphologies of HSLA steel after static immersion for 2 h (a), 12 h (b), 72 h (c), 216 h (d), 288 h (e),384 h (f) and 480 h (g)
HSLA钢动态冲刷不同时间后的宏观形貌见图13。动态冲刷2 h后材料表面立即被腐蚀产物覆盖,并带有明显红褐色锈迹(图13a)。12 h后整个表面被带有裂纹的红褐色腐蚀产物所覆盖,部分脱落暴露出基体(图13b)。72~216 h(图13c和d),红褐色腐蚀产物完全覆盖表面。288 h与384 h时表面仍然被完整的红褐色腐蚀产物覆盖(图13e和f),但480 h后,表面腐蚀产物呈黑色,并存在破碎的红褐色腐蚀产物残留(图13g)。根据HSLA钢动态冲刷后的形貌演变,同样可以将其分为3个阶段:第Ⅰ阶段(0~12 h)为快速成膜阶段,第Ⅱ阶段(12~216 h)为膜层开裂和堆积阶段,第Ⅲ阶段(216~480 h)为动态往复阶段。
图13
图13
HSLA钢在动态冲刷不同时间后的宏观形貌
Fig.13
Macroscopic morphologies of HSLA steel after dynamic erosion for 2 h (a), 12 h (b), 72 h (c), 216 h (d), 288 h (e),384 h (f) and 480 h (g)
综上所述,HSLA钢在动态冲刷后的腐蚀较静态浸泡后更为严重。最为明显的是,动态冲刷2 h,钢的表面完全被腐蚀产物覆盖。而静态浸泡72 h后HSLA钢表面才被完全覆盖。显然,流动性腐蚀介质显著促进了腐蚀的萌生与扩展。
2.3.2 微观形貌
对HSLA钢的显微组织形貌进行表征,结果如图14所示,表明在HSLA钢的表面存在夹杂物,对夹杂物进行EDS分析,结果表明夹杂物的主要成分(质量分数,%)为:C 9.23,O 16.61,Mg 1.81,Al 10.01,S 1.11,Ti 5.09,Mn 1.04,Fe 50.20,Ni 1.29,Cu 3.62。表明其为氧化物、硫化物及碳化物的复合夹杂。
图14
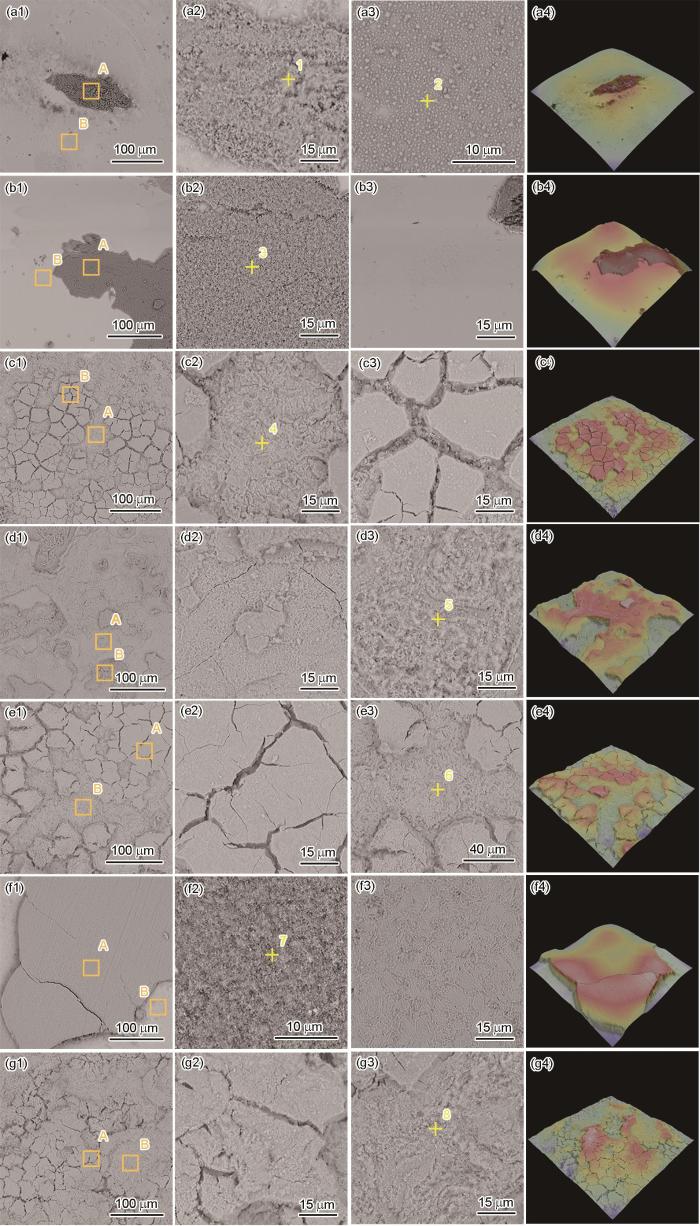
结合图12的宏观形貌观察,对HSLA钢静态浸泡不同时间后的微观形貌进行了表征,并对矩形方框标记处进行了局部放大。第Ⅰ阶段,局部腐蚀的萌生与扩展阶段,2 h后出现了圆形局部腐蚀区(图15a1),合金中的夹杂物可能是腐蚀的萌生处(图15a2和a3)。12 h后,夹杂物脱落,由夹杂物引起的局部腐蚀相互连接,导致腐蚀面积进一步扩大,并形成带有裂纹的腐蚀产物层(图15b1~b3)。第Ⅱ阶段,腐蚀产物成膜阶段,72 h后夹杂物脱落形成的位置被腐蚀产物覆盖,HSLA钢表面腐蚀产物膜形成(图15c1~c3),216 h后腐蚀产物膜在生长应力的作用下发生龟裂(图15d1~d3)。第Ⅲ阶段,稳定腐蚀阶段,在该阶段所有时间点,均观察到钢基体表面被龟裂的腐蚀产物所覆盖,局部区域发生了腐蚀产物的脱落(图15e1~e3,f1~f3和g1~g3)。
图15
图15
HSLA钢在静态浸泡不同时间后的微观形貌
Fig.15
Microscopic morphologies of HSLA steel after static immersion for 2 h (a), 12 h (b), 72 h (c), 216 h (d), 288 h (e),384 h (f) and 480 h (g)
HSLA钢在3.5%NaCl溶液中动态冲刷不同时间后的微观形貌如图16所示。第Ⅰ阶段,快速成膜阶段,流动性腐蚀介质加快了O2、Cl-等的传质,导致材料表面2 h后即发生了全面腐蚀(图16a1),绝大部分的表面被絮状腐蚀产物覆盖(图16a3),局部区域出现腐蚀产物堆积(图16a2和a4)。随着时间的增加,12 h后表面被腐蚀产物覆盖(图13b),但腐蚀产物较薄且附着性较差(图16b1和b2),容易露出钢基体表面(图16b3和b4)。第Ⅱ阶段,膜层开裂和堆积阶段,72 h后,腐蚀产物明显增厚且发生了龟裂(图16c1和c4),表现为块状腐蚀产物(图16c3)的下方并非钢基体,而是另外一种层状结构的腐蚀产物(图16c2),216 h后,腐蚀产物进一步堆积增厚(图16d1和d4),裂纹明显减少(图16d2),产物脱落下方仍可见层状腐蚀产物(图16d3)。第Ⅲ阶段,动态往复阶段,288 h后,表面腐蚀产物的裂纹再次增多(图16e1和e4),龟裂的腐蚀产物(图16e2)及其下方的层状腐蚀产物两种形态共存(图16e3),384和480 h后,再次出现了块状腐蚀产物堆积、表面裂纹减少(图16f1~f4)以及腐蚀产物减薄、表面裂纹增多的现象(16g1~g4)。可见,动态冲刷条件下,腐蚀产物会在HSLA钢表面迅速生成,而后堆积、增厚,经历“开裂-剥落-重新生成”的动态过程。
图16
图16
HSLA钢在动态冲刷不同时间后的微观形貌
Fig.16
Microscopic morphologies of HSLA steel after dynamic erosion for 2 h (a), 12 h (b), 72 h (c), 216 h (d), 288 h (e), 384 h (f) and 480 h (g)
2.4 腐蚀产物分析
为了确定HSLA钢在3.5%NaCl溶液中静态浸泡和动态冲刷后的腐蚀产物,对图15和16中十字形标记的位置进行Raman光谱检测,结果见图17。表2展示了文献中Raman光谱的峰值位置[47~50]。在静态浸泡后的第Ⅰ阶段,225、292~293、410和1320 cm-1处出现的峰表明其存在α-Fe2O3,同时在497和655 cm-1同样出现峰,对应物相为γ-FeOOH。而Fe3O4或γ-Fe2O3受到Raman测量激光照射,很容易使其局部加热氧化为α-Fe2O3[51],这表明第Ⅰ阶段腐蚀产物可能为γ-FeOOH、Fe3O4或γ-Fe2O3。在静态浸泡后的72、216、288和384 h,腐蚀产物均与第Ⅰ阶段相同,为γ-FeOOH、Fe3O4或γ-Fe2O3。在浸泡480 h后,220和288 cm-1处出现的峰是由于Fe3O4或γ-Fe2O3受热氧化为α-Fe2O3,同时396、483、684和1304 cm-1处出现峰,表明存在致密稳定的α-FeOOH。
图17
图17
HSLA钢在静态浸泡和动态冲刷不同时间后腐蚀产物Raman光谱分析
Fig.17
Raman spectra of corrosion products formed on HSLA steel after static immersion (a) and dynamic erosion (b) for different time
| Phase | Raman shift / cm-1 |
|---|---|
| Lepidocrocite (γ-FeOOH) | 166, 217, (248-257), 310, 350, ( |
| Goethite (α-FeOOH) | 203, (241-250), ( |
| Akaganeite (β-FeOOH) | 139, (308-314), 331, (385-390), (415-420), (497-499), (526-541), (720-745) |
| Magnetite (Fe3O4) | (298-306), (535-550), 616, (663-670) |
| Maghemite (γ-Fe2O3) | (339-386), (461-512), (500-506), (671-717), (700-720), (1400-1440) |
| Hematite (α-Fe2O3) | (220-228), (238-250), (288-299), (400-415), (497-502), (609-625), 670, (1320-1330) |
Bold: The strongest peak.
Underlined: The next strongest peak.
图17b显示了动态冲刷不同时间后HSLA钢表面的Raman光谱结果。其中,Point1和Point2为动态冲刷后2 h的光谱结果。对于Point1,在225、290、406和615 cm-1处出现峰,证明其含有α-Fe2O3,而α-Fe2O3同样是由Fe3O4或γ-Fe2O3受热氧化形成,同时在1310 cm-1处的峰对应γ-FeOOH的存在。这证明在2 h局部堆积的腐蚀产物由γ-FeOOH、Fe3O4或γ-Fe2O3组成(图16a2)。Point2存在248、380和650 cm-1处的峰,表明2 h时大面积覆盖的絮状腐蚀产物为γ-FeOOH(图16a3)。12 h后,650 cm-1处的峰对应物相为γ-FeOOH。在其余时间点,所有腐蚀产物均为γ-FeOOH、Fe3O4或γ-Fe2O3。
结果显示,无论是动态冲刷还是静态浸泡,腐蚀产物在大部分时间均由γ-FeOOH、Fe3O4或γ-Fe2O3组成。然而,静态浸泡480 h后出现了更为稳定的α-FeOOH。而在动态冲刷后的所有时间点,均未发现α-FeOOH的存在。
2.5 腐蚀性流体的加速腐蚀机制
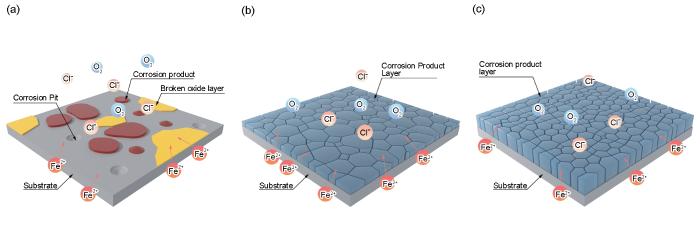
为了分析腐蚀性流体的加速腐蚀机制,需要对动态冲刷和静态浸泡后的实验结果进行综合对比,分析腐蚀性流体的加速作用机制。HSLA钢静态浸泡时,第Ⅰ阶段局部腐蚀的萌生与扩展阶段,初始氧化膜逐渐被Cl-迅速破坏,HSLA钢的阳极受到明显的促进,表面由钝态转变为活性溶解(图10a)。此时腐蚀产物层尚未形成,电荷转移直接在基体与溶液间发生,HSLA钢表现出最高的腐蚀速率(图3b)。具体的,钝化膜首先在夹杂物处破裂,导致局部腐蚀的发生(图15a2)。随着时间的增加,局部腐蚀的范围逐渐增大,多个在夹杂物处发生的局部腐蚀互相连接,形成更大的腐蚀区域(图15b1~b3),示意图见图18a。第Ⅱ阶段,腐蚀产物成膜阶段,腐蚀持续进行,生成的腐蚀产物逐步堆积成膜(图15d1~d3),覆盖的腐蚀产物将腐蚀介质与基体相互隔离,大大减缓了电荷转移速度,从而导致了较低的腐蚀速率(图3b)。然而,腐蚀产物层存在的裂纹为腐蚀介质的传输提供了通道(图15d2),导致HSLA钢的阳极和阴极均受到促进作用(图10b),示意图见图18b。第Ⅲ阶段,稳定腐蚀阶段,腐蚀产物逐渐堆积增厚,并形成稳定的腐蚀产物α-FeOOH(图17a),进一步阻碍了腐蚀性离子与基体的接触,虽然表面裂纹仍然存在,但HSLA钢阳极受到抑制的程度大于阴极受到促进的程度(图10c),导致腐蚀速率的进一步降低,示意图见图18c。
图18
图18
HSLA钢在静态浸泡过程中腐蚀机制
Fig.18
Corrosion mechanism of HSLA steel during static immersion: (a) stage Ⅰ, (b) stage Ⅱ, (c) stage Ⅲ
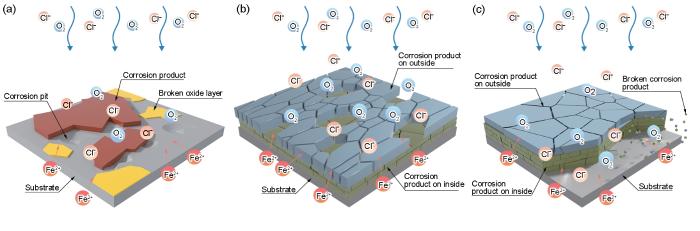
综合以上实验结果,具体分析HSLA钢在3.5%NaCl溶液中动态冲刷过程中的3个阶段。第Ⅰ阶段为快速成膜阶段,首先,流体的机械冲击造成夹杂物处的初始氧化膜迅速被破坏,并引发夹杂物的脱落,使HSLA钢的阳极受到明显的促进(图11a)。同时,腐蚀性流体以远高于静态浸泡条件下依靠浓度梯度差进行扩散的速度迅速将溶解氧及Cl-传输至材料表面,导致HSLA钢的腐蚀速率明显高于静态浸泡(图3b),絮状腐蚀产物迅速在表面生成并伴随有局部腐蚀产物堆积(图16a2~16a3)。随着实验时间的延长,流体的机械冲击将表面附着性较差且不稳定的絮状γ-FeOOH带离材料表面(图16b3),同时,附着性较好的腐蚀产物逐渐堆积扩展,在局部表面上形成腐蚀产物层(图16b2和16b4),导致HSLA钢的膜电阻迅速上升(图9a),示意图见图19a。第Ⅱ阶段为膜层开裂和堆积阶段,由于流体的冲击首先作用于腐蚀产物层外侧,并且Cl-的存在会对腐蚀产物造成破坏,使腐蚀产物在逐渐堆积增厚的同时,产生表面裂纹(图16c3),这些表面存在的裂纹为溶解氧及Cl-提供快速传输的通道,并且在流体作用下,溶解氧及Cl-以远高于静态条件下的扩散速度通过裂纹向材料表面传输,从而导致相比于静态浸泡更低的电荷转移电阻Rct (图9b),使HSLA钢阳极和阴极均受到明显的促进(图11b),并使HSLA钢的腐蚀速率依然居高不下(图3b),但另一方面,腐蚀过程伴随着腐蚀产物的生成,积累的腐蚀产物在内部堆积,使其具有比静态浸泡条件下更高的膜电阻Rf (图9a),此外,外层的腐蚀产物在流体的冲击下优先脱落,暴露出内侧的腐蚀产物(图16c2和d2),示意图见图19b。第Ⅲ阶段为动态往复阶段,在流动性腐蚀介质的持续冲击下,腐蚀产物的裂纹不断产生和扩展,最终破坏腐蚀产物完整性,导致腐蚀产物脱落,重新暴露出基体(图16f3)。同时,充足的溶解氧与Cl-立即与基体发生反应,腐蚀产物再次堆积,产生了腐蚀产物“开裂-剥落-重新生成”的动态往复(图16f2)。在这个动态往复的过程中,部分区域的腐蚀产物发生破裂脱落,但仍存在部分附着性好的腐蚀产物覆盖表面,使腐蚀产物逐渐在内层堆积并增厚,使得HSLA钢的阳极反应受到一定程度的抑制(图11c),腐蚀速率出现一定程度的降低(图3b),示意图见图19c。
图19
图19
HSLA钢在动态冲刷过程中的腐蚀机制
Fig.19
Corrosion mechanism of HSLA steel during dynamic erosion: (a) stage Ⅰ, (b) stage Ⅱ, (c) stage Ⅲ
总结而言,腐蚀性流体的加速作用主要体现在两个方面:第一是对电化学反应的影响,流速的增加极大促进了溶解氧及Cl-的传质过程,使材料始终被充足的去极化剂和腐蚀性离子所环绕,从而加剧电化学过程的进行。第二是对腐蚀产物的机械破坏作用,腐蚀性流体通过破坏腐蚀产物的完整性,促进腐蚀产物内部裂纹的扩展,当裂纹逐渐衍生并相互交错时,腐蚀产物发生破碎剥落。在实际测试中,腐蚀性流体对电化学反应及腐蚀产物完整性的影响同时发生。材料首先在充满腐蚀性离子的环境中发生腐蚀,即使存在钝化膜覆盖也会在腐蚀性流体的加速腐蚀作用下开裂,并形成大量毛细管孔隙,使材料仍然存在大量与腐蚀性介质直接接触的微小区域,从而导致极高的腐蚀速率。值得注意的是,腐蚀性流体的加速腐蚀作用会阻止稳定腐蚀产物的形成,在本研究中,HSLA钢在动态冲刷后的480 h内均未发现α-FeOOH。这是由于α-FeOOH需要由FeO x (OH)3x 与OH-还原转变而来,而FeO x (OH)3x 的形成需要γ-FeOOH的溶解与沉淀[52,53]。然而,与静态浸泡不同,腐蚀性流体始终处于运动状态,即使γ-FeOOH已经沉淀也会因流体作用而被带离表面,从而阻止了α-FeOOH生成。
3 结论
通过失重测试、EIS、动电位极化曲线、Raman光谱及腐蚀形貌表征等手段,综合研究了HSLA钢在3.5%NaCl溶液中的冲刷腐蚀行为,并对腐蚀性流体的加速作用进行了量化,结果表明:
(1) 动态冲刷造成的腐蚀失重是静态浸泡的10倍以上,流动性介质的加速腐蚀作用是导致HSLA钢在动态冲刷条件下腐蚀速率激增的主要原因,其对质量损失的贡献平均可达88.6%,是纯腐蚀作用的7.8倍。
(2) HSLA钢在3.5%NaCl溶液中静态浸泡和动态冲刷过程中的失效均可分为3个阶段,静态浸泡的3个阶段为局部腐蚀的萌生与扩展、腐蚀产物的成膜以及稳定腐蚀阶段,动态冲刷的3个阶段为快速成膜、膜层开裂和堆积以及腐蚀产物“开裂-剥落-重新生成”的动态往复阶段。两种条件下HSLA钢的腐蚀产物主要是γ-FeOOH、Fe3O4或γ-Fe2O3,所不同的是静态浸泡480 h后,HSLA钢表面形成了稳定的α-FeOOH,动态冲刷条件下介质的流动性是阻碍稳定腐蚀产物生成的主要原因。
(3) 在静态浸泡和动态冲刷的3个阶段,HSLA钢阳极和阴极表现出相似的规律特征,在第一和第二阶段,主要表现为HSLA钢阳极的促进,而第三阶段阳极受到抑制作用占据主导,导致腐蚀速率的降低。所不同的是,静态浸泡条件下,HSLA钢的性能恶化主要体现在第二和第三阶段,而动态冲刷条件下,HSLA钢耐蚀性能的降低主要集中在第一阶段。
(4) 流动性介质的加速腐蚀作用机制主要体现在两个方面:第一,加快传质过程,溶解氧及Cl-等会随着流体的运动被源源不断的运送至材料表面,使材料始终处在被充足去极化剂和侵蚀性离子包围的环境中;第二,破坏钝化膜以及腐蚀产物膜的完整性,流体冲击的动能会直接造成钝化膜的破坏以及腐蚀产物膜的开裂,从而为溶解氧及Cl-等的传输提供通道。
参考文献
High-strength, low-alloy steels
[J].High-strength, low-alloy (HSLA) steels have nearly the same composition as plain carbon steels. However, they are up to twice as strong and their greater load-bearing capacity allows engineering use in lighter sections. Their high strength is derived from a combination of grain refinement; precipitation strengthening due to minor additions of vanadium, niobium, or titanium; and modifications of manufacturing processes, such as controlled rolling and controlled cooling of otherwise essentially plain carbon steel. HSLA steels are less formable than lower strength steels, but dualphase steels, which evolved from HSLA steels, have ferrite-martensite microstructures and better formability than HSLA steels of similar strength. This improved formability has substantially increased the utilization potential of high-strength steels in the manufacture of complex components. This article reviews the development of HSLA and dual-phase steels and discusses the effects of variations in microstructure and chemistry on their mechanical properties.
High-strength low-alloy (HSLA) steels: visokotrdna malolegirana (HSLA) konstrukcijska jekla
[J].
Development and certification of HSLA-100 steel for naval ship construction
[J].
Fundamental investigation of stress corrosion cracking of E690 steel in simulated marine thin electrolyte layer
[J].The mechanism of stress corrosion cracking (SCC) of E690 high-strength steel in a marine thin electrolyte layer (TEL) was investigated by performing in-situ mechanical-electrochemical tests, slow strain rate tensile (SSRT) tests, and characterization of corrosion morphology. It was concluded that E690 steel was highly sensitive to SCC, which was jointly determined by local anodic dissolution (AD) and hydrogen embrittlement (HE) both caused by dissolved O-2. In addition to these functions, hydrogen oxidation catalyzed by ferric ion was found. There was a critical oxygen concentration, approximately 21% by volume, between these two different roles. Below this value, the increase in the oxygen concentration promoted the synergistic effect of AD and HE, resulting in the increase in SCC susceptibility. However, above this value, worse general corrosion offset crack initiation as well as the oxidation of hydrogen catalyzed by ferric ions reduced the SCC susceptibility.
The effect of corrosion evolution on the stress corrosion cracking behavior of mooring chain steel
[J].
Environmentally assisted stress corrosion cracking behaviour of low alloy steel in SO2-containing nacl solution
[J].
Investigation of erosion-corrosion behavior of Q235B steel in liquid-solid flows
[J].
Effects of flow velocity and impact angle on erosion-corrosion of an API-5 L X65 steel coated by plasma nitriding of hard chromium underlayer
[J].
Evaluation of a semi-empirical model in predicting erosion–corrosion
[J].
Review of mechanisms of erosion-corrosion of alloys at elevated temperatures
[J].
The effect of low pH in erosion-corrosion resistance of high chromium cast irons and stainless steels
[J].
Corrosion behavior of B10 Cu-Ni alloy pipe in static and dynamic seawater
[J].
静态和动态海水中B10铜镍合金管的腐蚀行为研究
[J].
Progress in research methods for erosion-corrosion
[J].
冲刷腐蚀研究方法进展
[J].
Progress of study on erosion-corrosion
[J].
冲刷腐蚀的研究现状
[J].
Effect of temperature on the marine immersion corrosion of carbon steels
[J].
Effect of temperature, pH and sulphide on the cavitation erosion behaviour of super duplex stainless steel
[J].
Intergranular erosion corrosion of pure copper tube in flowing NaCl solution
[J].
The effects of erodent composition and shape on the erosion of steel
[J].
The effect of erodent particle characteristics on the erosion of metals
[J].
Effect of impact angle and velocity on erosion of API X42 pipeline steel under high abrasive feed rate
[J].
Experimental study of particle size, shape and particle flow rate on erosion of stainless steel
[J].
Testing and modeling of particle size effect on erosion of steel and cobalt-based alloys
[J].
The role of abrasive particle size on erosion characteristics of stainless steel
[J].Understanding the erosion characteristics in oil and gas equipment and abrasive transportation is of vitally important to implement the engineering design and subsequently prevent failures. In this study, an in-house erosion test facility was designed and built up and its performance was tested through studying the size effect of abrasive sand particle on the erosion behaviour of a commonly used stainless steel material in the oil exploitation field. The experimental results reveal that the erosion rate (kg/impact), the depth of erosion as well as the surface roughness increased while the width of wear scar decreased with the increasing of particle size. In addition, its interesting to observe the shape of erosion scar changing from "W" to "U" shape when the particle size became bigger. Further, four main types of erosion mechanisms involved in the erosion process, namely, plastic deformation induced indentation, ploughing, cutting and sliding, and correlation between the erosion characteristics and microstructure is discussed.
Effect of particle size on erosion characteristics
[J].
Effect of erosion on corrosion of API X120 steel in relation to erodent particle size
[J].The nature of the solid erodent particles present in corrosive petroleum fluid can cause transporting pipeline to experience severe erosion and corrosion damages. The effect of erosion on corrosion behavior of API X120 steel was investigated using aluminum oxide and silicon carbide particles with different sizes as erodent and 3.5 wt.% NaCl aqueous solution saturated with carbon dioxide as a corrosive medium. The effect of the erodent particle size on the corrosion behavior of the steel material at different particle speeds and impact angles was investigated using weight loss, potentiodynamic polarization and surface analysis techniques. The erosion results confirmed that the material damage increased with increasing particle speed. It was observed that in carbon dioxide-saturated saline solution, deposition of protective iron carbonate film occurred on the steel surface. It was found that the corrosion film can provide better protection at lower particle speed than at higher speed. The ratio of total erosion-corrosion (S)/effect of erosion on corrosion (T) analysis confirmed that at higher S/T ratio, the particle speed and material removal rate are low and vice versa at lower S/T ratio. Lower S/T values for the combined erosion and corrosion tests performed with erodent silicon carbide particle compared to erodent aluminum oxide particle showed that erosion enhancement of corrosion is more evident in the test performed using aluminum oxide particle than using silicon carbide particle. The result also suggests that when subjected to larger size erodent particle, the damage to pipeline due to effect of erosion on corrosion process can be more significant compared to smaller size erodent particle.
Flux effects in solid particle erosion
[J].
Effect of sand size and temperature on synergistic effect of erosion-corrosion for 20 steel in simulated oilfield produced fluid with sand
[J].
粒径和温度对20号钢冲刷腐蚀协同作用的影响
[J].
Influence of microstructure on erosion resistance of steels
[J].
The solid particle erosion behavior of steel as a function of microstructure
[J].
Prediction of erosion-corrosion penetration rate in a carbon dioxide environment with sand
[J].
Flow accelerated corrosion and erosion-corrosion behavior of marine carbon steel in natural seawater
[J].In this work, flow accelerated corrosion (FAC) and erosion−corrosion of marine carbon steel in natural seawater were electrochemically studied using a submerged impingement jet system. Results show that the formation of a relatively compact rust layer in flowing natural seawater would lead to the FAC pattern change from ‘flow marks’ to pits. The increase of the flow velocity was found to have a negligible influence on the FAC rate at velocities of 5−8 m s−1. The synergy of mechanical erosion and electrochemical corrosion is the main contributor to the total steel loss under erosion−corrosion. The increase of the sand impact energy could induce the pitting damage and accelerate the steel degradation. The accumulation of the rust inside the pits could facilitate the longitudinal growth of the pits, however, the accumulated rusts retard the erosion of the pit bottom. The erosion and corrosion could work together to cause the steel peeling at the pit boundary. The steel degradation would gradually change from corrosion-dominated to erosion-dominated along with the impact energy increasing.
A study of the erosion-corrosion behaviour of engineering steels for marine pumping applications
[J].
Examining corrosion effects and corrosion/erosion interactions on metallic materials in aqueous slurries
[J].
Characterization of synergistic effects between erosion and corrosion in an aqueous environment using electrochemical techniques
[J].
Erosion-corrosion and synergistic effects in disturbed liquid-particle flow
[J].
Erosion–corrosion resistance of engineering materials in various test conditions
[J].
Flow-dependent corrosion. I. Current understanding of the mechanisms involved
[J].
The platelet mechanism of erosion of ductile metals
[J].
Effect of the sea mud on erosion-corrosion behaviors of carbon steel and low alloy steel in 2.4% nacl solution
[J].
Effects of shear stress on the erosion-corrosion behaviour of X-65 carbon steel: a combined mass-loss and profilometry study
[J].
Erosion-corrosion behaviour of high manganese steel used in slurry pipelines
[J].
Erosion-corrosion behaviour of steels used in slurry pipelines
[J].
The mechanism of erosion-corrosion of API X65 steel under turbulent slurry flow: effect of nominal flow velocity and oxygen content
[J].
Analysis on erosion of pipe bends induced by liquid-solid two-phase flow
[J].
固体颗粒对液/固两相流弯管冲蚀作用分析
[J].采用计算流体动力学 (CFD) 方法分析了不同颗粒参数包括固体颗粒流速、流量及粒径大小对于弯管不同截面冲蚀速率的影响,求解得到固体颗粒运动轨迹,结合颗粒碰撞模型得到颗粒运动对管壁的冲蚀作用。结果表明:冲蚀严重区域主要存在于下游直管段与弯头连接处的侧壁以及下游直管段与弯头连接处的外侧;Stokes数的变化会导致冲蚀严重区域的移动,下游直管段与弯头连接处侧壁区域并不是都会发生严重冲蚀。
Erosion-corrosion behavior of 90° horizontal elbow in single phase flow
[J].
单相流条件下90°水平弯管冲刷腐蚀行为研究
[J].通过自行设计的管流式实验装置,采用失重测量、表面分析等方法,研究了单相流条件下90°水平弯管不同部位的冲刷腐蚀行为。结果表明:单相流条件下,90°水平弯管不同部位的冲刷腐蚀速率主要集中在2.11~3.29 mm/a,弯管的内侧及出口处的外侧冲刷腐蚀比较严重。流动条件下的冲刷腐蚀速率远远大于静止条件下的纯腐蚀速率,机械冲刷对腐蚀过程起到促进作用,介质流动是引起冲刷腐蚀速率大大增加的主要原因。试样表面存在面积较大的冲刷腐蚀坑点和沟槽,沟槽具有明显的方向性,沟槽的方向与局部流体流动的方向一致。
SEM/Micro-Raman characterization of the morphologies of marine atmospheric corrosion products formed on mild steel
[J].
Spectroscopic and thermoanalytical characterization of standard substances for the identification of reaction products on iron electrodes
[J].
Raman spectroscopic studies of the corrosion of model iron electrodes in sodium chloride solution
[J].
The evolution of the corrosion of iron in hydraulic binders analysed from 46- and 260-year-old buildings
[J].
Raman mapping of corrosion products formed onto spring steels during salt spray experiments. A correlation between the scale composition and the corrosion resistance
[J].
The mechanism of atmospheric rusting and the protective amorphous rust on low alloy steel
[J].