咪唑啉及其衍生物被认为是油气田应用广泛的有机缓蚀剂之一[7],由于其生物降解性,咪唑啉衍生物被视为“绿色”缓蚀剂[8, 9],其缓蚀效果主要归因于缓蚀剂在金属表面的吸附,从而抑制腐蚀过程中的电化学反应。咪唑啉类缓蚀剂对裸钢有优异的缓蚀效果,倪小龙等[10]合成的3种不同碳链长度的咪唑啉缓蚀剂均对裸钢有良好的缓蚀作用,缓蚀效果随碳链长度的增长而增强。然而,在实际应用中,管道表面可能覆盖有沙砾和碳酸盐等沉积物,对缓蚀剂的性能造成了重要影响。Fan等[11]研究了咪唑啉衍生物在CO2饱和盐水中对CaCO3垢下低碳钢的保护效果,其缓蚀效率最大可达到92.33%。但是,Katerina和Gubner[12]的研究表明咪唑啉缓蚀剂的缓蚀作用在很大程度上受电极表面砂粒沉积物及腐蚀产物的影响。Tan等[13]和Abd Karim等[14]研究表明,咪唑啉类缓蚀剂砂粒垢下金属缓蚀效果较差,甚至加剧了垢下金属的电偶腐蚀。咪唑啉类缓蚀剂对裸钢有良好的缓蚀作用,然而应用在垢下腐蚀中可能因垢层的存在而失效甚至加速腐蚀[15]。Pandarinathan等[16]研究了硫代苯甲酰胺(TB)在SiO2和CaCO3垢下腐蚀的缓蚀效果,结果表明TB可快速迁移穿过垢层,在3 h内可较大程度的降低腐蚀速率。Pandarinathan等[17]比较了2-巯基嘧啶(MPY)、硫代苯甲酰胺(TB)在SiO2垢下腐蚀缓蚀效果,通过吸附实验证实MPY和TB在SiO2上的吸附量很小,且缓蚀效率达到90%以上。垢层的存在导致缓蚀剂的缓蚀能力弱或完全失效,缓蚀剂只有扩散通过垢层到达金属表面,才能起到缓蚀作用。
综上所述,部分缓蚀剂在垢下腐蚀环境中会失效甚至加速腐蚀,这与缓蚀剂能否穿过惰性沉积物垢层有关。分子量较大的缓蚀剂的空间位阻大,扩散较为困难,而分子量更小的缓蚀剂迁移过程相对容易[18]。硫脲分子量小、水溶性好、扩散能力较强,而烷基咪唑啉分子量较大且拥有长烷烃链,探究二者吸附膜的形成和缓蚀协同作用对抑制垢下腐蚀有重要意义。因此,本工作深入研究了烷基咪唑啉(IM)、硫脲(TU)、烷基咪唑啉与硫脲复配(TU/IM) 3种缓蚀剂在CO2饱和模拟油田采出水中对SiO2和CaCO3惰性沉积物中的缓蚀效果及缓蚀协同作用机理。
1 实验方法
X65钢作为工作电极,其化学成分(质量分数,%)为:C 0.03、Si 0.17、Mn 1.51、P 0.02、Ni 0.17、Cu 0.04、Mo 0.16、Nb 0.06、Al 0.02、Ti 0.01,Fe余量。
工作电极试样规格为10 mm × 10 mm × 3 mm。用锡丝将试样的一端和Cu导线焊接,留下一个1 cm2的表面,试样其余部分灌入环氧树脂密封,将试样制作成电极。电极用砂纸打磨至2500目至表面光亮,用蒸馏水、无水乙醇清洗,最后用氮气干燥。
图1
图1
覆盖SiO2和CaCO3惰性沉积物的工作电极
Fig.1
Macroscopic photos of working electrodes covered with inert deposits of SiO2 (a) and CaCO3 (b)
本研究所使用的IM缓蚀剂为实验室自主合成,TU缓蚀剂购自国药化学试剂有限公司,分子结构式如图2所示。
图2
腐蚀介质为CO2饱和的模拟油田采出水,其组成成分(g/L)为:Na2CO3 0.03,NaHCO3 3.06,Na2SO4 1.10,CaCl2 0.50,MgCl2 1.66,KCl 0.35,NaCl 16.61。含有不同缓蚀剂的腐蚀介质作为缓蚀溶液。使用CO2吹扫封闭系统中的测试溶液0.5 h,测试溶液为pH (6.10 ± 0.10)。所有实验均在25 ℃恒温水浴中进行。
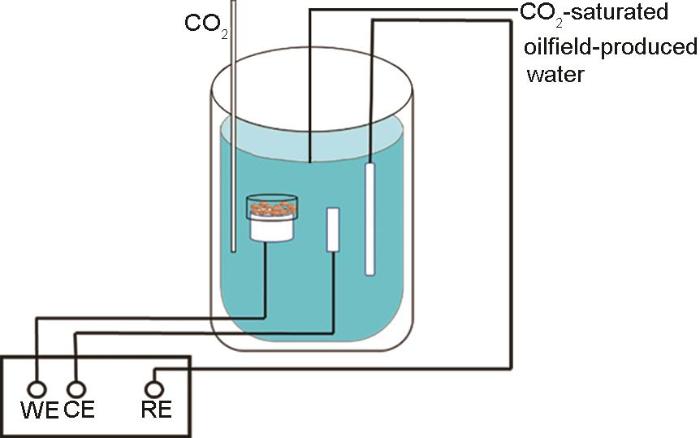
采用传统三电极体系(图3)测定动电位极化曲线和电化学阻抗谱(EIS),Pt电极为辅助电极,X65钢为工作电极,SCE为参比电极,测试仪器为Gamry Reference 600+电化学工作站。
图3
图3
以覆盖SiO2和CaCO3惰性沉积物的X65钢为工作电极的电化学测试装置示意图
Fig.3
Schematic diagram of electrochemical testing device using X65 steel covered with SiO2 or CaCO3 inert deposit as working electrode
当开路电位(OCP)波动范围小于2 mV时,即认为该测试体系达到稳定状态,随后测定动电位极化曲线和EIS谱。动电位极化曲线测试的扫描范围为-300~300 mV (vs. OCP),电位扫描速率为0.167 mV/s;电化学阻抗采用频率范围为105~10-2 Hz、幅值为10 mV的正弦波交流电压进行测试。通过Zsimpwin软件对EIS测量数据进行拟合分析,利用Cview 2软件拟合极化曲线,以得到相应的电化学参数。
工作电极在空白溶液及不同缓蚀溶液中浸泡72 h,依次用去离子水和高纯去离子水中浸泡清洗并干燥。采用扫描电子显微镜(SEM,Ultra Plus)观察腐蚀形貌,SiO2和CaCO3垢下电极在空白溶液以及不同缓蚀溶液中浸泡72 h后表面用扫描电镜配备的X射线能量色散谱仪(EDS)分析其成分。用X射线光电子能谱(XPS,ESCALAB 250 Xi)分析金属表面层元素分布信息。
2 结果与讨论
2.1 IM对SiO2 垢下腐蚀的影响
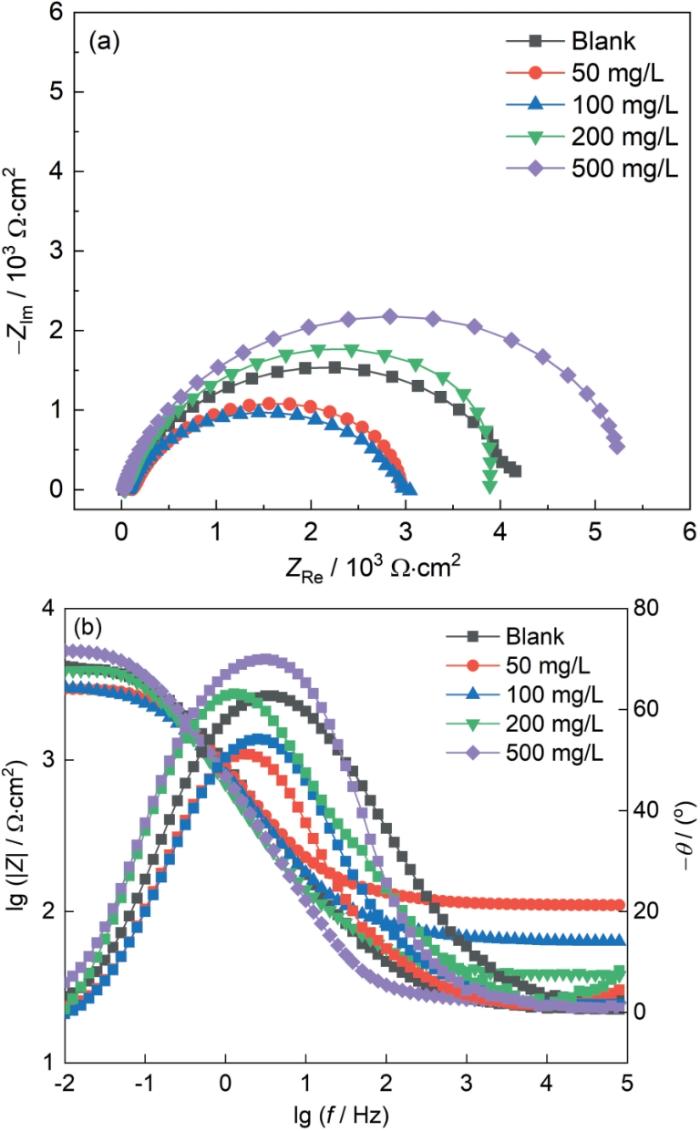
图4
图4
SiO2垢下电极在不同浓度的IM缓蚀溶液中浸泡72 h的EIS谱图
Fig.4
Nyquist (a) and Bode (b) plots of X65 electrode covered with SiO2 after immersion for 72 h in simulated solutions containing different concen-trations of IM
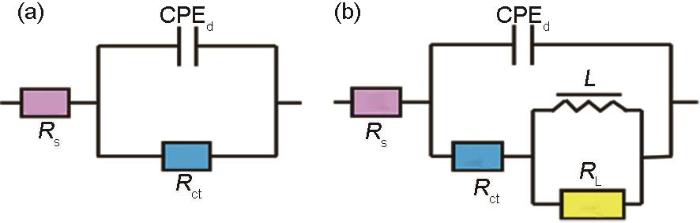
图5
图5
用于拟合EIS的等效电路图
Fig.5
Equivalent circuit models fitting EIS: (a) only three elements, (b) with inductance
式中,Y0和n为恒相角元件的组成参数,n为弥散系数,ω为角频率,j2 = 1为虚部。EIS拟合结果如表1所示,缓蚀效率(η)用下式进行定量评估:
其中,R
表1 SiO2垢下电极在不同浓度的IM缓蚀剂溶液中的EIS谱拟合数据
Table 1
| Concentration | Rs | Cdl | ndl | Rct | η |
|---|---|---|---|---|---|
| / mg·L-1 | / Ω·cm2 | / μF·cm-2 | / Ω·cm2 | / % | |
| Blank | 23.69 | 238 | 0.77 | 4470 | - |
| 50 | 115.5 | 248 | 0.77 | 3019 | -32.46 |
| 100 | 67.09 | 267 | 0.77 | 2944 | -33.39 |
| 200 | 39.82 | 326 | 0.77 | 4639 | 3.643 |
| 500 | 26.05 | 256 | 0.86 | 5426 | 17.62 |
2.2 复配比对垢下腐蚀的影响
TU作为一种广泛应用在油田环境中的缓蚀剂,由于其随着浓度升高缓蚀效率反而下降的特性,通常不会单独使用,而是用作复配型缓蚀剂的主要成分之一[23]。
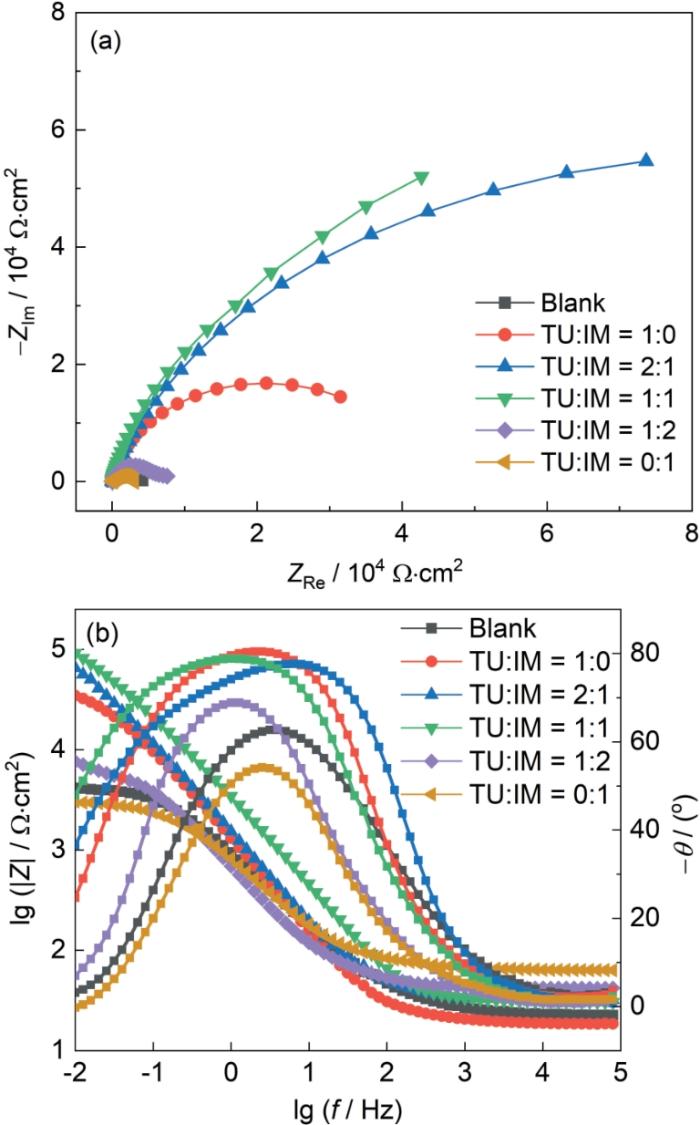
为了进一步提升缓蚀性能,将TU与IM缓蚀剂进行复配,控制TU与IM缓蚀剂的总量为100 mg/L,改变二者质量配比,其EIS谱如图6所示,复配比为TU∶IM = 1∶2和0∶1时缓蚀溶液容抗弧半径最小,与空白溶液容抗弧半径相似;并且0.01 Hz低频时阻抗模值与空白组模值相接近,表明其未产生缓蚀作用。当TU和IM复配比例为2∶1和1∶1时,Nyquist图中容抗弧半径明显的增加,Bode图中相位角峰增大,峰宽变宽。说明该缓蚀溶液下在金属表面产生了相对更加牢固的吸附层,阻止了腐蚀性离子向金属表面扩散,阻碍了腐蚀性离子与金属表面的直接接触,降低了腐蚀速率。
图6
图6
SiO2垢下电极在TU/IM不同复配比例缓蚀溶液中浸泡72 h的EIS谱图
Fig.6
Nyquist (a) and Bode (b) plots of of X65 electrode covered with SiO2 after immersion for 72 h in simulated solutions containing different ratios of TU and IM
表2 SiO2垢下电极在TU/IM不同复配比例缓蚀溶液中浸泡72 h的EIS谱拟合结果
Table 2
| Mass ratios | Rs | Cdl | ndl | Rct | ηEIS |
|---|---|---|---|---|---|
| / Ω·cm2 | / μF·cm-2 | / Ω·cm2 | / % | ||
| Blank | 92.69 | 135.0 | 0.67 | 1584 | - |
| TU:IM = 1:0 | 19.18 | 58.8 | 0.91 | 37745 | 88.15 |
| TU:IM = 2:1 | 30.49 | 61.3 | 0.87 | 92720 | 95.17 |
| TU:IM = 1:1 | 32.27 | 130.0 | 0.88 | 100720 | 95.56 |
| TU:IM = 1:2 | 43.49 | 303.0 | 0.73 | 7208 | 37.98 |
| TU:IM = 0:1 | 27.14 | 280.0 | 0.76 | 2940 | -52.04 |
2.3 3种缓蚀剂的缓蚀性能
由前文可知当TU和IM的质量比为1∶1时,在 SiO2沉积物垢下腐蚀中缓蚀作用最佳,两者具有较好的协同作用。对IM、TU和TU/IM = 1∶1 3种缓蚀剂的缓蚀性能进行研究,进一步分析3种缓蚀剂对 SiO2和CaCO3垢下金属的缓蚀行为和吸附行为的差异。
2.3.1 动电位极化曲线
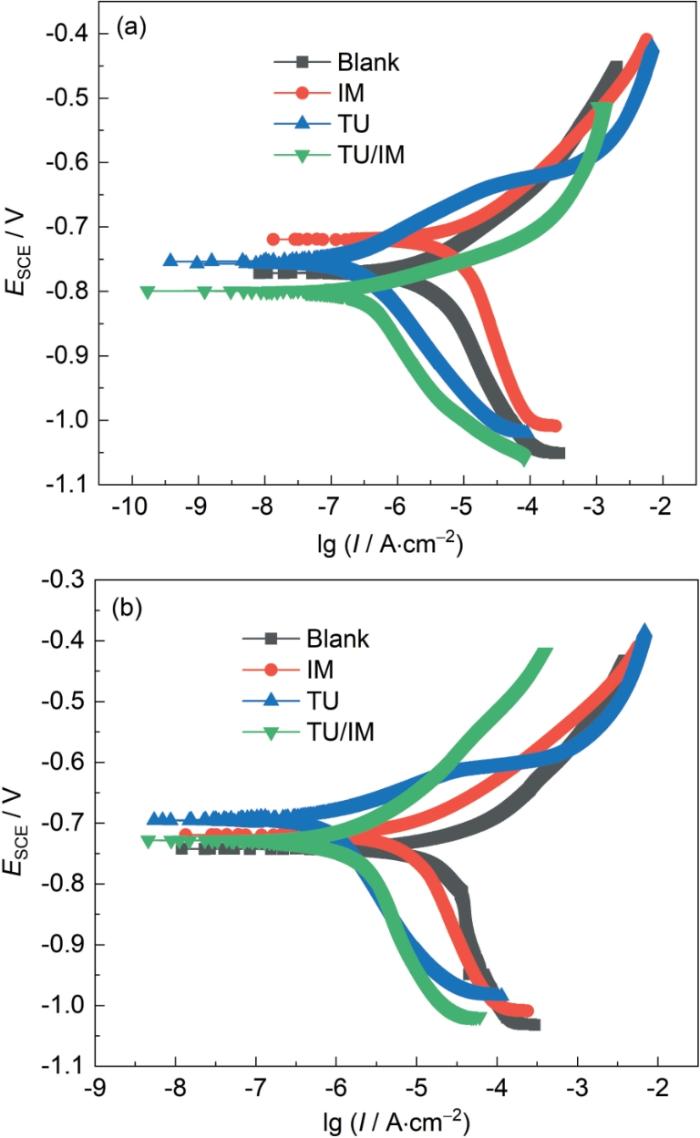
将覆盖SiO2和CaCO3沉积物的电极分别置于空白溶液和不同缓蚀溶液中进行动电位极化测试,如图7所示。在SiO2惰性沉积物下,IM缓蚀剂的加入使得阴阳极曲线均向电流增大的方向移动,表明IM并未抑制SiO2沉积物电极的腐蚀过程,反而加速了腐蚀过程;然而加入TU和TU/IM后,无论是阳极还是阴极的曲线都向电流密度小的方向移动,这说明X65钢的阳极Fe的溶解反应和阴极析氢反应都受到了明显的抑制作用[24],且在TU/IM缓蚀体系中,与阳极曲线相比,其阴极曲线的移动程度更大。在CaCO3惰性沉积物下,与空白溶液相比,加入IM后其阴阳极曲线向电流减小的方向的移动程度较小,而TU和TU/IM缓蚀体系中的阴阳极曲线有明显左移,二者既抑制了Fe的阳极溶解也抑制了阴极析氢反应,其中加入TU/IM后极化曲线移动幅度最大,复配缓蚀剂的抑制效果最好。
图7
图7
25 ℃下垢下电极在CO2饱和的空白溶液以及含100 mg/L缓蚀溶液中浸泡72 h后的动电位极化曲线
Fig.7
Potentiodynamic polarization curves of X65 electrodes covered with SiO2 (a) and CaCO3 (b) after 72 h immersion at 25 oC in CO2-saturated blank solution and simulated solutions contain-ing 100 mg/L corrosion inhibitors of TU, IM and TU/IM, respectively
动电位极化曲线的拟合结果如表3所示,其中ηPOT的计算过程如下式:
表3 图7动电位极化曲线拟合结果
Table 3
| Deposits | Inhibitor | βa / mV·dec-1 | -βc / mV·dec-1 | Icorr / μA·cm-2 | Ecorrvs. SCE / V | ηPOT / % |
|---|---|---|---|---|---|---|
| SiO2 | Blank | 75.95 | 194.8 | 3.01 | -0.760 | - |
| IM | 68.03 | 192.7 | 4.53 | -0.744 | -50.49 | |
| TU | 128.40 | 108.6 | 0.82 | -0.756 | 72.65 | |
| TU/IM | 168.40 | 79.7 | 0.30 | -0.800 | 90.10 | |
| CaCO3 | Blank | 70.48 | 147.1 | 13.11 | -0.750 | - |
| IM | 56.19 | 165.1 | 5.61 | -0.720 | 57.20 | |
| TU | 55.21 | 151.4 | 0.99 | -0.690 | 92.45 | |
| TU/IM | 164.20 | 101.3 | 0.28 | -0.740 | 97.81 |
式中,I
2.3.2 EIS谱
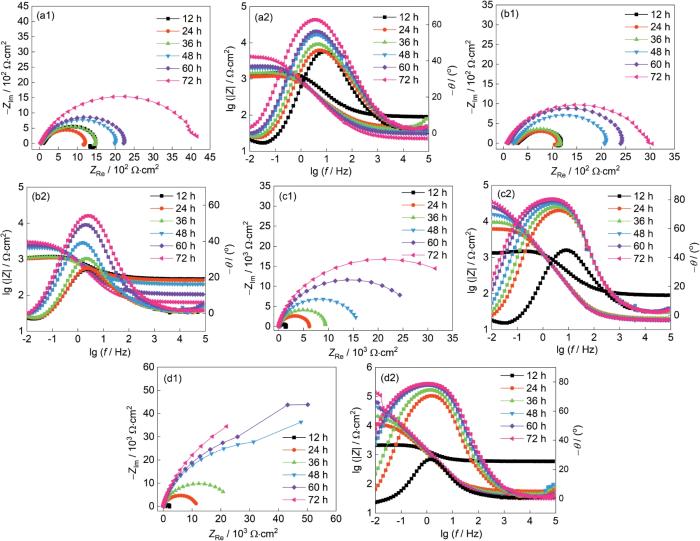
垢下电极在空白溶液及3种缓蚀溶液中浸泡0~72 h的EIS谱如图8所示。空白溶液中,由SiO2垢下电极的Nyquist图中可以看出,容抗弧较小且随时间变化不大。加入IM缓蚀剂后,12~72 h阻抗半径均较小,甚至72 h时的容抗弧小于空白溶液的容抗弧,表明该缓蚀剂未对SiO2垢下腐蚀产生缓蚀效果。加入TU缓蚀剂后,电极的容抗弧随着时间而明显增大,在24 h时低频的感抗弧即消失,表明其透过SiO2垢层扩散到金属表面的浓度逐渐增加,自24 h后逐渐形成较为致密的吸附膜,对垢下金属具有一定的保护性。当加入TU/IM复配缓蚀剂后,Nyquist图容抗弧半径大幅度增大,垢下金属表面腐蚀过程所受到的阻力明显增大。缓蚀效率与容抗弧的半径大小呈正比[25],因此可以看出3种缓蚀剂的缓蚀效率顺序为:η(TU/IM) > η(TU) > η(IM)。在Bode图中的低频区,IM缓蚀剂溶液中模值没有明显变化,而TU和TU/IM溶液中0.01 Hz对应的模值逐渐增大,表明缓蚀剂在SiO2垢下金属表面均形成了吸附膜,有效屏蔽了腐蚀粒子,减缓腐蚀的发生[26]。表4 是SiO2垢下电极在空白和3种缓蚀溶液中的EIS谱拟合结果。在IM缓蚀溶液中72 h时Rct低于空白组,IM缓蚀剂的加入反而加速了垢下电极的腐蚀。在TU缓蚀溶液中的拟合数据可以看出,双电层电容Cdl不断减小,表明电极表面介电常数减小或双电层厚度增加;弥散系数ndl呈现逐渐增加的趋势,表明缓蚀剂吸附膜越接近电容特性;电荷转移电阻Rct逐渐增大,腐蚀速率降低。在TU/IM复配缓蚀溶液中,双电层电容Cdl逐渐降低,电荷转移电阻Rct逐渐增大,缓蚀剂不断的透过沉积物垢层吸附在金属表面。其缓蚀效率顺序为:η(TU/IM) > η(TU) > η(IM),这也与极化曲线中的结果相一致。
图8
图8
SiO2垢下电极在空白溶液及不同缓蚀溶液中浸泡不同时间后的EIS谱图
Fig.8
EIS of X65 electrode covered with SiO2 after immersion for different time in blank solution (a) and corrosion inhibition solutions containing IM (b), TU (c) and TU/IM (d)
表4 SiO2沉积物覆盖下的电极在空白溶液及缓蚀溶液中浸泡不同时间的EIS谱拟合结果
Table 4
| Inhibitor | t / h | Rs / Ω·cm2 | Cdl / μF·cm-2 | ndl | Rct / Ω·cm2 | L / H·cm-2 |
|---|---|---|---|---|---|---|
| Blank | 12 | 92.69 | 135 | 0.67 | 1584 | 706 |
| 24 | 47.21 | 251 | 0.66 | 1339 | 600 | |
| 36 | 41.22 | 250 | 0.71 | 1661 | - | |
| 48 | 34.68 | 245 | 0.73 | 2227 | - | |
| 60 | 32.04 | 237 | 0.74 | 2415 | - | |
| 72 | 23.69 | 238 | 0.77 | 4420 | - | |
| IM | 12 | 18.34 | 284 | 0.68 | 1055 | 716 |
| 24 | 18.47 | 276 | 0.69 | 1092 | 551 | |
| 36 | 11.68 | 289 | 0.71 | 1678 | - | |
| 48 | 21.32 | 305 | 0.69 | 2544 | - | |
| 60 | 11.15 | 279 | 0.73 | 2920 | - | |
| 72 | 27.14 | 280 | 0.76 | 2940 | - | |
| TU | 12 | 27.83 | 256 | 0.76 | 8554 | 501 |
| 24 | 21.57 | 166 | 0.85 | 6972 | - | |
| 36 | 21.60 | 161 | 0.87 | 10830 | - | |
| 48 | 20.48 | 157 | 0.87 | 17212 | - | |
| 60 | 19.46 | 63 | 0.88 | 21170 | - | |
| 72 | 19.18 | 59 | 0.89 | 37745 | - | |
| TU/IM | 12 | 62.23 | 173 | 0.83 | 1629 | - |
| 24 | 57.86 | 182 | 0.85 | 11888 | - | |
| 36 | 47.48 | 167 | 0.86 | 24370 | - | |
| 48 | 39.72 | 146 | 0.87 | 65057 | - | |
| 60 | 36.81 | 143 | 0.87 | 88136 | - | |
| 72 | 32.27 | 130 | 0.88 | 100720 | - |
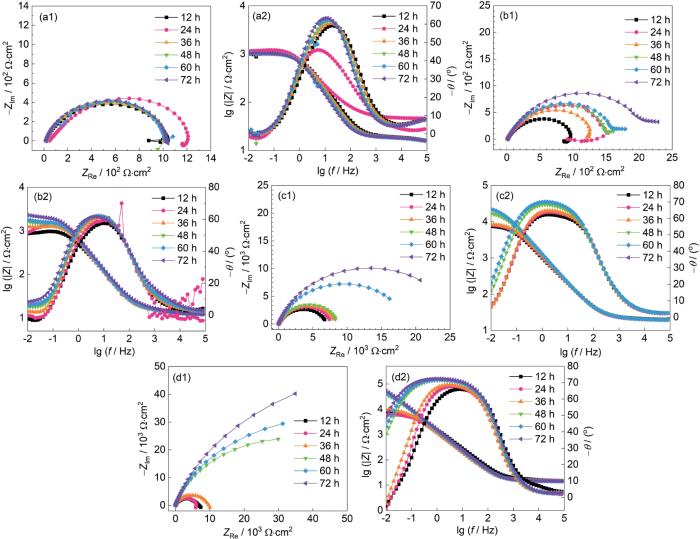
图9
图9
CaCO3垢下电极在空白溶液及不同缓蚀溶液中浸泡不同时间后的EIS谱图
Fig.9
EIS of X65 electrode covered with CaCO3 after immersion for different time in blank solution (a) and corrosion inhibition solutions containing IM (b), TU (c) and TU/IM (d)
表5 CaCO3 沉积物覆盖下的电极在空白溶液及缓蚀溶液中浸泡不同时间的EIS谱拟合结果
Table 5
| Inhibitor | t / h | Rs / Ω·cm2 | Cdl / μF·cm-2 | ndl | Rct / Ω·cm2 | L / H·cm-2 |
|---|---|---|---|---|---|---|
| Blank | 12 | 17.96 | 166 | 0.66 | 1053 | 290 |
| 24 | 46.31 | 294 | 0.66 | 1603 | 600 | |
| 36 | 17.72 | 173 | 0.72 | 1021 | - | |
| 48 | 17.90 | 183 | 0.73 | 1021 | - | |
| 60 | 17.58 | 204 | 0.73 | 1018 | - | |
| 72 | 17.82 | 204 | 0.75 | 1031 | - | |
| IM | 12 | 13.91 | 378 | 0.76 | 1107 | 915 |
| 24 | 10.67 | 370 | 0.77 | 1472 | 784 | |
| 36 | 13.31 | 361 | 0.77 | 1600 | 523 | |
| 48 | 13.26 | 322 | 0.77 | 1641 | - | |
| 60 | 13.10 | 329 | 0.78 | 1784 | - | |
| 72 | 13.15 | 323 | 0.78 | 2385 | - | |
| TU | 12 | 20.22 | 250 | 0.74 | 7765 | - |
| 24 | 20.69 | 226 | 0.76 | 8618 | - | |
| 36 | 19.77 | 210 | 0.77 | 9139 | - | |
| 48 | 20.67 | 205 | 0.78 | 9607 | - | |
| 60 | 21.14 | 184 | 0.80 | 20391 | - | |
| 72 | 20.70 | 173 | 0.81 | 28174 | - | |
| TU/IM | 12 | 15.66 | 129 | 0.79 | 6682 | - |
| 24 | 15.46 | 172 | 0.82 | 6665 | - | |
| 36 | 15.02 | 178 | 0.81 | 9924 | - | |
| 48 | 14.66 | 160 | 0.81 | 62240 | - | |
| 60 | 14.32 | 155 | 0.81 | 78113 | - | |
| 72 | 14.45 | 140 | 0.82 | 117130 | - |
基于以上分析可知,各种缓蚀剂进入到SiO2和CaCO3垢层下且在垢下金属表面形成吸附膜均需要一定的时间,其中TU/IM缓蚀剂吸附膜形成的最快、膜的致密性最好。直至72 h时,TU和TU/IM缓蚀体系下仍保持较高的腐蚀阻力,吸附膜具有良好的稳定性、持效性。
2.3.3 腐蚀形貌分析
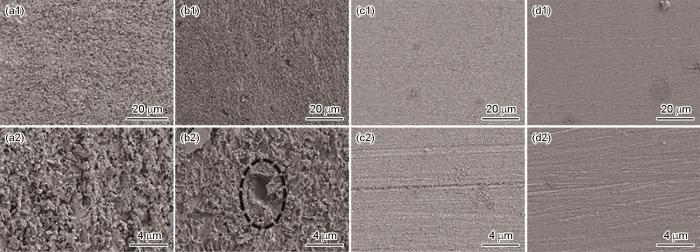
图10为SiO2垢下电极在不同溶液中浸泡72 h后的腐蚀形貌。在空白溶液中,电极表面较为粗糙,腐蚀产物呈疏松多孔结构,并且有球状晶体腐蚀产物析出。在IM缓蚀溶液下,金属表面较为粗糙,腐蚀产物不均匀,伴有大小不一的孔洞,这些孔洞的产生更易于腐蚀性离子的侵蚀,可能会导致金属基体发生严重点蚀[27,28]。在TU和TU/IM缓蚀溶液中的试样表面均匀平整,仍可见打磨后的划痕,只有极少量的腐蚀产物附着。表6为SiO2垢下电极在空白溶液以及不同缓蚀溶液中浸泡72 h后表面扫描EDS能谱结果。可以看出在加入3种缓蚀剂后,Fe含量明显增大,这可能表明金属表面Fe氧化物含量降低;并且在TU和TU/IM缓蚀溶液中出现了S元素,说明TU和TU/IM缓蚀剂稳定吸附在金属表面。
图10
图10
SiO2垢下电极在空白溶液及不同缓蚀溶液中浸泡72 h后腐蚀形貌
Fig.10
Corrosion morphologies of X65 electrode covered with SiO2 after 72 h immersion in blank solution (a) and corrosion inhibition solutions containing IM (b), TU (c) and TU/IM (d)
表6 SiO2垢下电极在空白溶液及不同缓蚀溶液中浸泡72 h后表面EDS结果
Table 6
| Inhibitor | Fe | S |
|---|---|---|
| Blank | 58.16 | - |
| IM | 69.37 | - |
| TU | 72.13 | 0.36 |
| TU/IM | 71.51 | 0.46 |
图11
图11
CaCO3垢下电极在空白溶液及不同缓蚀溶液中浸泡72 h后腐蚀形貌
Fig.11
Corrosion morphologies of X65 electrode covered with CaCO3 after 72 h immersion in blank solution (a) and corrosion inhibition solutions containing IM (b), TU (c) and TU/IM (d)
表7 CaCO3垢下电极在空白溶液及不同缓蚀溶液中浸泡72 h后表面EDS结果 (atomic fraction / %)
Table 7
| Inhibitor | Fe | S |
|---|---|---|
| Blank | 46.73 | - |
| IM | 27.35 | - |
| TU | 62.55 | 0.34 |
| TU/IM | 72.06 | 0.49 |
腐蚀形貌图表明,IM没有起到很好的缓蚀作用,而TU和TU/IM的加入抑制了腐蚀,这与电化学测量结果相对应。
2.3.4 表面成分分析
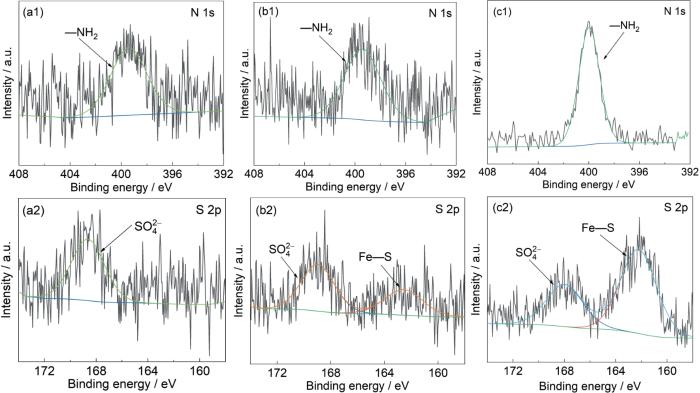
图12为SiO2垢下金属在IM、TU和TU/IM 3种缓蚀溶液浸泡后表面各元素的高分辨率能谱,3种缓蚀溶液的金属表面均出现N 1s和S 2p峰,其中S 2p来源于油田采出水中的硫酸盐和缓蚀剂中的硫脲基团[29],而N 1s仅来源于缓蚀剂。如图12a所示,在IM缓蚀溶液中浸泡的金属表面的精细谱图,其表面未检测到Fe—N,这可能是由IM缓蚀剂在垢下金属表面的吸附量较低造成的;但是检测到了400 eV附近的—NH2[30],说明IM缓蚀剂在金属表面有微量吸附,这也与IM缓蚀效率很低相一致。图12b为在TU缓蚀溶液金属表面的精细谱图中,检测到了403.10 eV的Fe—N键[31]和162.03 eV的Fe—S键[32],Fe—N、Fe—S是TU中的N和S原子与Fe原子配位后形成的,这进一步证实了TU缓蚀剂在垢下金属表面的吸附。从图12c中可以看出,TU/IM与Fe形成了Fe—S配位键,表明TU缓蚀剂分子扩散透过沉积物并吸附在金属表面;同时在N 1s精细谱图中,位于399.2 eV的—NH2含量高于单一的TU缓蚀剂,由此推测,电极表面的缓蚀剂吸附膜层由TU和IM共同组成。
图12
图12
SiO2垢下电极在不同缓蚀溶液中浸泡72 h后的XPS谱图
Fig.12
XPS spectra of X65 electrode covered with SiO2 after 72 h immersion in corrosion inhibition solutions containing IM (a), TU (b) and TU/IM (c)
CaCO3垢下金属在3种缓蚀溶液浸泡后表面各元素的高分辨率能谱如图13所示,在3种缓蚀体系下均检测到了—NH2,表明3种缓蚀剂均穿透CaCO3垢层吸附在试样表面。在S 2p谱图中,在TU和TU/IM缓蚀体系下,出现了较为明显的Fe—S键,表明二者在垢下金属表面的吸附能力更强。
图13
图13
CaCO3垢下电极在不同缓蚀溶液中浸泡72 h后的XPS谱图
Fig.13
XPS spectra of X65 electrode covered with CaCO3 after 72 h immersion in corrosion inhibition solutions containing IM (a), TU (b) and TU/IM (c)
2.4 讨论
使用IM、TU和TU/IM作为缓蚀剂,研究不同性质的缓蚀剂在SiO2和CaCO3惰性沉积物垢下对X65钢的缓蚀机理。3种缓蚀剂对SiO2惰性沉积物垢下金属的缓蚀效率依次为:η (TU/IM) > η (TU) > η (IM),IM加速了垢下金属的腐蚀,然而TU和TU/IM复配缓蚀剂都可以有效抑制金属基体的腐蚀行为。CaCO3惰性沉积物垢下金属在3种缓蚀溶液中的缓蚀效率顺序同上,与之不同的是IM对CaCO3垢下金属有一定的缓蚀作用,这说明缓蚀剂的缓蚀性能会受到垢层差异的影响。同时从电化学数据中可以看出,缓蚀剂分子穿透垢层扩散到垢下金属表面并发挥作用需要一定的时间,其中小分子TU缓蚀剂扩散速率最快,最先对垢下金属起缓蚀作用[33]。表面形貌图显示,TU和TU/IM缓蚀溶液中金属表面相对光滑平整,有清晰的打磨痕迹,腐蚀产物覆盖较少。XPS证实了TU和TU/IM体系的金属表面上存在Fe—S键,因为S的电负性较低,TU更容易失去电子并与金属原子形成配位键。
IM、TU和TU/IM 3种缓蚀溶液中对SiO2和CaCO3惰性沉积物垢下腐蚀的缓蚀机理如图14所示。对于IM而言,由于其油溶性特质导致扩散性、流动性较差,导致大部分IM缓蚀剂分子被垢层阻挡在外面,当少部分IM分子经过孔隙吸附在金属表面后,由于IM未能在金属基体形成完整的吸附膜,进而引起了更为严重的局部腐蚀。而TU具有良好的缓蚀作用,其分子量小、水溶性好且易于扩散,可以穿过惰性沉积物垢层到达金属表面,然后通过van der Waals力、氢键自发吸附在金属基体的Fe原子上;并且TU引入了S原子,增加了电子云密度,作为富电子区域可以与Fe原子未被填充的d轨道形成Fe—S配位键,增强了TU与表面Fe的结合能力。TU/IM复配缓蚀剂比TU、IM单独使用时缓蚀效果更好,这说明TU与IM之间存在良好的协同作用。TU与IM复配后,TU依靠良好的扩散性穿过垢层、通过良好的吸附性能在金属表面形成了第一层吸附膜,然后当IM缓蚀剂扩散到金属基体后,形成缓蚀剂吸附膜的上层;IM为吸附膜提供疏水链,进一步阻止腐蚀性离子的进入,TU具有多个吸附位、分子量小的优点,补足IM吸附膜在垢下金属表面不均匀不完整的缺陷,两者相互补充,共同形成了致密完整的吸附膜。
图14
图14
3种缓蚀剂对 SiO2 和 CaCO3 垢下X65碳钢的缓蚀机理示意图
Fig.14
Schematic diagrams of the corrosion inhibition mechanisms of IM (a), TU (b), and TU/IM (c) inhibitors on X65 carbon steel under SiO2 and CaCO3 scales
3 结论
(1) IM、TU和TU/IM对SiO2垢下金属的缓蚀效率分别为-50.49%、72.65%和90.10%,对CaCO3垢下金属的缓蚀效率分别为57.20%、92.45%和97.81%。TU和TU/IM可有效减缓腐蚀速率,它们都是混合抑制型缓蚀剂,对阴极析氢和Fe的阳极溶解反应都有抑制作用。
(2) IM扩散性和流动性较差,导致大部分IM分子被垢层阻挡在外面,IM未能在金属表面形成完整的吸附膜。TU和TU/IM可通过化学吸附作用在金属表面形成保护性吸附膜;TU中的S可以与垢下金属表面Fe原子富电子区的空d轨道形成配位化学键;TU/IM在垢下金属表面形成了双层吸附膜,下层为优先穿过垢层扩散到垢下区域并通过Fe—S配位键吸附到金属表面的TU,上层为通过长碳链形成疏水层的IM。
参考文献
Research progress on CO2 corrosion and protective countermeasures for oil casing
[J].
油套管CO2腐蚀和防护研究进展
[J].
Under-deposit corrosion of carbon steel beneath full coverage of CaCO3 deposit layer under different atmospheres
[J].The under-deposit corrosion of carbon steel beneath the full coverage of CaCO3 deposit layer in 3.5 wt.% NaCl solution under different atmospheres has been investigated by electrochemical tests, wire beam electrode technique and morphology characterization. The results indicate that dissolved O-2 and CO2 can affect the corrosion of carbon steel under the CaCO3 deposit layer by influencing the cathodic reactions, with the oxygen reduction reaction and reduction reaction of H2CO3 being the dominant cathodic reactions for the systems without and with CO2 injection, respectively. Dissolved O-2 can enhance the localized under-deposit corrosion tendency by forming oxygen concentration cells beneath the deposit layer, while its effect on the general under-deposit corrosion rate depends on the main cathodic reactions. Under atmospheres without CO2 injection, increased concentration of dissolved O-2 can accelerate the general corrosion rate by promoting the oxygen reduction reaction, and it can decrease the general corrosion rate under CO2-purged atmospheres by decreasing the amounts of H2CO3 for the cathodic reaction.
Corrosion analysis of shale gas downhole tubing under the synergistic action of multiple factors
[J].
Galvanic corrosion behavior of deposit-covered and uncovered carbon steel
[J].
Theoretical insight into an empirical rule about organic corrosion inhibitors containing nitrogen, oxygen, and sulfur atoms
[J].
Synergistic inhibition effect of imidazoline ammonium salt and three cationic surfactants in H2S/CO2 brine solution
[J].
CO2/H2S腐蚀体系中咪唑啉季铵盐与3种阳离子表面活性剂间的缓蚀协同效应
[J].
Inhibition of imidazolines on CO2 induced corrosion of carbon steel in oil and water alternatively wetting conditions
[J].
油水交替环境中咪唑啉对CO2腐蚀的抑制作用研究
[J].通过添加两类典型油溶性和水溶性咪唑啉缓蚀剂,检验了其在油水动态润湿环境中对碳钢CO<sub>2</sub>腐蚀的抑制效果。研究表明,水溶性咪唑啉的缓蚀效果优于油溶性咪唑啉,其缓蚀效果差异主要源于油水界面行为的改变。油溶性咪唑啉分子通过增强油相中自修复作用来提高缓蚀效果;水溶性咪唑啉分子可以有效抑制油水界面水滴的形成和长大等过程,减弱CO<sub>2</sub>对油膜的破坏作用,增强动态润湿缓蚀效果。
Reactivity properties of derivatives of 2-imidazoline: An ab initio DFT study
[J].
Effect of thiourea imidazoline quaternary ammonium salt corrosion inhibitor on corrosion of X80 pipeline steel
[J].
硫脲基咪唑啉季铵盐缓蚀剂对X80管线钢腐蚀的影响
[J].考察硫脲基咪唑啉季铵盐 (IM-S1) 缓蚀剂对X80管线钢在3种不同pH的模拟油田水溶液中的缓蚀性能。采用电化学极化曲线、电化学阻抗分析、SVET、表面形貌分析等方法,研究缓蚀剂在不同pH、不同温度的模拟油田水溶液对X80管线钢的缓蚀性能。极化曲线测试显示,pH7.2环境中的腐蚀电流密度最小,其次是pH10.5,在pH3.5环境中的腐蚀电流密度最大;并随温度升高,腐蚀电流密度均有所升高。电化学阻抗的测试表明,在pH7.2模拟溶液条件下,所显示的容抗弧直径最大,且拟合结果中R<sub>ct</sub>明显高于其他两种测试条件。SVET分析显示,在pH7.2的测试条件下,管线钢表面吸附成膜性要优于其他两种测试条件;且离子电流密度随时间呈下降趋势,说明缓蚀剂粒子更适宜在此pH值条件下吸附成膜。根据SEM分析,可以明显看出,IM-S1缓蚀剂在中性条件的缓蚀效果要优于pH3.5和pH10.5条件的缓蚀作用效果。IM-S1缓蚀剂更适宜在中性条件下使用,并且在中低温 (40~60 ℃) 条件下具有良好的缓蚀效果。该类缓蚀剂在中性溶液条件的吸附成膜性要优于酸性和碱性条件的成膜性,并且吸附成膜降低离子电流密度,从而有效降低腐蚀反应速率。
Corrosion protection of imidazoline corrosion inhibitors with different carbon chain length in CO2 driving oil environment
[J].
不同碳链长度咪唑啉缓蚀剂在CO2驱采油环境中的腐蚀防护作用
[J].
Penetration of imidazoline derivatives through deposited scale for inhibiting the under-deposit corrosion of pipeline steel
[J].
Development of standard test method for investigation of under-deposit corrosion in carbon dioxide environment and its application in oil and gas industry
[A].
Electrochemical evaluation of under-deposit corrosion and its inhibition using the wire beam electrode method
[J].
Performance of corrosion inhibitor with sand deposit in CO2 environment
[J].
Synergistic effects of deposits and sulfate reducing bacteria on the corrosion of carbon steel
[J].
Impact of mineral deposits on CO2 corrosion of carbon steel
[A].
Evaluation of corrosion inhibition at sand-deposited carbon steel in CO2-saturated brine
[J].
Research progress on corrosion inhibitor and inhibition mechanism against CO2 corrosion of carbon steel
[J].
碳钢CO2腐蚀的缓蚀剂策略及缓蚀行为研究进展
[J].
Corrosion of artificial rock layer covered steel electrodes in a CO2 environment: The influence of permeability
[J].
The role of corrosion inhibition in the mitigation of CaCO3 scaling on steel surface
[J].
Comparison of the corrosion behavior of pure titanium and its alloys in fluoride-containing sulfuric acid
[J].
Evaluation of the dissolved oxygen-related electrochemical behavior of pure titanium in acidic fluoride-containing solutions
[J].
Progress on research of thiourea and derivatives as corrosion inhibitor
[J].
硫脲及其衍生物的缓蚀行为研究进展
[J].从研究方法、缓蚀作用和促进作用、浓度极值现象、 加速渗H作用、吸附类型及作用机理等方面综述了国内外对硫脲及其衍生物缓蚀行为的研究 进展.硫脲及其衍生物作为吸附型酸洗缓蚀剂,材料、介质、温度、缓蚀剂浓度等因素对其 作用行为有较大影响.
Progress in the development of sour corrosion inhibitors: Past, present, and future perspectives
[J].Metallic pipelines and gathering tanks play a vital role during oil and gas exploration, production, transmission, and processing. These facilities are usually attacked by corrosion. The use of corrosion inhibitors is one of the most economical and reliable approaches to control the corrosion of oil and gas metallic facilities. This paper looks at the progress made in the development of sour corrosion inhibitors from early 1900 to date. Scientific literatures were reviewed. The review identified four classes of organic corrosion inhibitors for sour environments, namely, amine-based, imidazoline-based, polymer-based, and Gemini-surfactant-based inhibitors. The strengths and weaknesses of these inhibitors were highlighted. The review revealed that the patronage of amine-based chemistries has declined, and the current technology is based on imidazoline and quaternary salt chemistries. The existing knowledge gap and the future research direction in the area of sour corrosion inhibitors development have been highlighted. (C) 2019 The Korean Society of Industrial and Engineering Chemistry. Published by Elsevier B.V.
CO2 corrosion inhibitors performance at deposit-covered carbon steel and their adsorption on different deposits
[J].The efficiency of inhibitors to prevent under deposit corrosion of carbon steel and their adsorption on aluminum oxide, calcium carbonate, and silica sand deposits have been evaluated using electrochemical measurements and UV-visible spectroscopy. 2-Mercaptopyrimidine provided the highest corrosion protection on both bare and deposit-covered steels. In contrast, 1-Dodecylpiridinium chloride had minimal adsorption on all deposits, but it exhibited insufficient performance. Inhibitors adsorption tended to be related to the inhibitor type and not notably to the physical properties of the deposits. Deposit porosity, layers thickness, and depletion of the inhibitor by adsorption on deposits could not be linked entirely to corrosivity and inhibitors performance.
Corrosion behaviors and physical properties of polypyrrole-molybdate coating electropolymerized on carbon steel
[J].
On the localised corrosion of carbon steel induced by the in-situ local damage of porous corrosion products
[J].The effect of in-situ local damage of uniform porous corrosion products on the localised corrosion of carbon steel is investigated using the wire beam electrode technique (WBE) combined with morphology characterisation and electrochemical tests. The WBE measurements demonstrate that the localised corrosion is enhanced by the in-situ local removal of porous corrosion products, supported by the morphology characterisation and electrochemical tests. The enhanced localised corrosion does not originate from the damaged wire in WBE where the corrosion products are removed but from the other undamaged wires, which is reported for the first time. A mechanism is proposed that the intensive anodic polarisation effect of the damaged wire on the undamaged wires could account for the enhanced localised corrosion, which is due to the protective corrosion products newly formed on the damaged surface and the increase in the potential of damaged wire.
Key parameters affecting sweet and sour corrosion: Impact on corrosion risk assessment and inhibition
[J].
Relaxation energies in XPS and XAES of solid sulfur compounds
[J].
Polar group substituted imidazolium zwitterions as eco-friendly corrosion inhibitors for mild steel in acid solution
[J].
Tetrahydroacridines as corrosion inhibitor for X80 steel corrosion in simulated acidic oilfield water
[J].
Electrochemical and quantum chemical studies of some sulphur-containing organic compounds as inhibitors for the acid corrosion of mild steel
[J].