从工业烟气中回收或捕获CO2的方法有很多,目前应用最广泛的是化学吸附法。考虑到实用性,最有前景的是利用烷烃胺的气体吸收工艺,通常称为胺处理工艺[4]。胺处理工艺并不是一项新的CO2捕集技术,早在1950年时,胺法捕集CO2就已经应用在工业生产当中。其中,单乙醇胺 (MEA) 吸收CO2速率快,吸收效率高,可以实现在高温下分离,低温下反应。发展至今,已成为工业生产中最常见的CO2捕集剂。但是其缺点也较为突出:易腐蚀、易降解[5]。离子液体作为一种新型的绿色CO2捕集剂,因其低挥发性以及较高的热稳定性和化学稳定性,引起了人们广泛的研究,并成为CO2捕集的一种新选择[6,7,8,9]。学者们已经开始了针对离子液体与醇胺复配溶液捕集CO2的各项研究,证明了复配溶液的优越性。Camper等[10]率先将离子液体与醇胺混合溶液应用于CO2的捕集,结果表明复配溶液最大的好处在于可以同时拥有两种吸收剂的优点并消减各自的缺点。Feng等[11]在N-甲基二乙醇胺 (MDEA) 溶液中添加离子液体 ([N1111][Gly]) 后,显示当离子液体浓度为5%~10%时,能很好改善MDEA溶液对CO2的吸收性能。最新的研究[12,13]也表明,离子液体中添加胺类物质能明显改善离子液体对CO2的吸收能力,并且混合吸收剂对CO2的吸收能力也随胺类物质的种类不同而存在比较大的差异。
腐蚀问题已成为MEA溶液吸收CO2工艺中的严重问题之一,为此学者们展开了广泛的研究。相关研究[14,15]表明,MEA溶液的浓度是影响腐蚀性的重要因素,随着MEA浓度的升高,溶液吸收CO2的能力升高,导致体系中RNH3+,HCO3-和RNHCOO-浓度升高,从而引起金属材料腐蚀速率增加。并且,MEA在吸收CO2过程中会产生氧化降解反应和热降解反应[16],从而生成多种有机酸,如醋酸、乙醇酸、草酸、甲酸、丙酸等。这些有机酸会在体系中生成热稳定性盐 (HSS) 或者胺盐,并且不能被降解一直存在于设备中[17],加速了金属材料的腐蚀[18]。其中,草酸盐对于金属腐蚀的影响最大[19,20]。离子液体具有良好的缓蚀作用[21,22],而因其独特的物化性质,其腐蚀机理可能与传统吸收剂存在一定差异。研究[23]表明,在氯化1-乙基-3-甲基咪唑离子液体中,304不锈钢的耐腐蚀性能要好于金属Ti的,因此传统的金属腐蚀防护措施可能并不适用于离子液体。另外,离子液体对金属的腐蚀还受到多种环境因素的综合影响[24,25]。例如,Perissi等[26]研究了在220 ℃的高温、敞开体系下,AISI 304钢和AISI 1018钢在离子液体中的腐蚀行为,得出溶液中的O2很有可能会造成金属材料的点蚀。Shkurankov等[27]的研究表明,含水率对于Mg和镁合金在离子液体[BMIm]CF3SO3中的腐蚀影响较大。但是,对于此种复配溶液的腐蚀性还未见深入的文献报道,对碳钢材质在饱和CO2的离子液体醇胺溶液中的腐蚀行为研究目前还较少。因此,本文选用比较常用的离子液体1-丁基-3-甲基咪唑四氟硼酸盐 ([Bmim]BF4) 与MEA组成混合溶液,研究20#钢在该体系下的腐蚀行为,可为离子液体与醇胺混合溶液对材料的腐蚀性评价提供一定的理论依据。
1 实验方法
1.1 实验材料的制备
实验材料采用CO2捕集装置中常用的20#钢,其组成 (质量分数,%) 为:C 0.24,Si 0.22,Mn 0.42,S 0.002,P 0.017,Cr 0.02,Ni 0.01,Fe余量。浸泡实验采用的试片规格为40 mm×13 mm×2 mm。电化学测试试样的尺寸为10 mm×10 mm×3 mm的方形电极,试样背面用锡焊连接Cu导线,用环氧树脂封样后,试样表面依次用水磨砂纸逐级打磨至1200#,打磨后的试样依次在去离子水、丙酮和无水乙醇中超声波辅助清洗5 min,冷风吹干后备用。实验介质由工业上常用的浓度为30% (质量分数) 的MEA溶液与不同浓度 (0%,5%,10%和20%,质量分数) 的离子液体[Bmim]BF4组成。此外,为进一步验证BF4-是否为导致点蚀加重的原因,在含饱和CO2的30%MEA溶液中添加NaBF4。NaBF4的添加量与5%,10%和20%的[Bmim]BF4中BF4-是等摩尔量的。NaBF4的添加量如表1所示。实验前向溶液中通入高纯N2除氧2 h,然后向溶液中通入CO2约2 h后放入试样,整个实验过程中持续通入CO2,以保证CO2始终处于饱和状态。实验中用水浴锅对混合溶液进行恒温加热并保温,温度设定为30和70 ℃。
表1 NaBF4的添加量
Table 1
| Mass of mixed solution / g | [Bmim]BF4g | Mass fraction of [Bmim]BF4 / % | [Bmim]BF4mol | NaBF4g | Mass fraction of NaBF4 / % | NaBF4mol |
|---|---|---|---|---|---|---|
| 300 | 15 | 5.0 | 0.0663 | 7.2876 | 2.43% | 0.0663 |
| 300 | 30 | 10.0 | 0.1327 | 14.5752 | 4.86% | 0.1327 |
| 300 | 60 | 20.0 | 0.2654 | 29.1505 | 9.72% | 0.2654 |
1.2 腐蚀失重实验
将试样浸泡120 h后从混合溶液中取出后用去离子水冲洗,放入酸洗液 (配比为盐酸500 mL,六次甲基四胺3.5 g,加蒸馏水至1 L) 中超声波辅助清洗5 min,取出后用无水乙醇脱水,吹干后放入干燥器中,8 h后用分析天平 (精度0.1 mg) 进行称重,每个条件下对3个平行样分别称重,结果取平均值。
1.3 电化学测量
采用三电极体系,在PARSTAT 4000A型电化学工作站上进行电化学实验。工作电极为20#钢试样,参比电极为饱和甘汞电极 (SCE),辅助电极为Pt片,溶液体积为1000 mL。动电位极化曲线的扫描速率为1 mV/s,扫描电位范围为-0.8~2 V (相对于开路电位)。电化学阻抗测试的频率范围:105~5×10-2 Hz,阻抗测量信号幅值为5 mV正弦波。
1.4 腐蚀形貌分析
采用ZEISS (EVO MA15) 扫描电子显微镜 (SEM) 对试样表面形貌和元素组成进行分析,扫描电压为20 kV,并使用SEM自带的能谱仪 (EDS) 进行成分分析。
2 结果与讨论
2.1 腐蚀失重实验
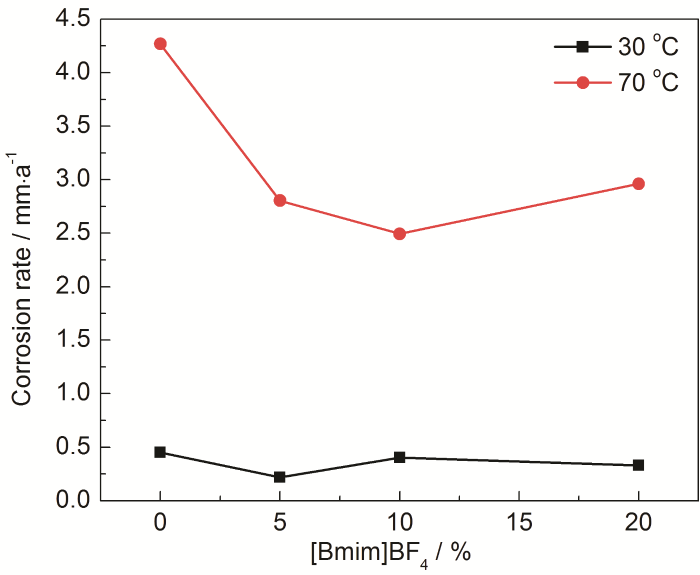
图1
图1
不同温度条件下均匀腐蚀速率随[Bmim]BF4浓度变化曲线
Fig.1
Variations of uniform corrosion rate of 20# carbon steel with the concentration of [Bmim]BF4 at 30 and 70 ℃
2.2 形貌观察与成分分析
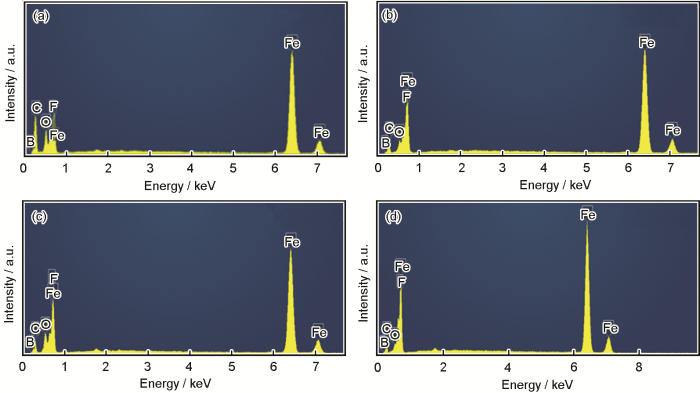
图2
图2
30%MEA+不同浓度的[Bmim]BF4条件下20#钢腐蚀产物膜微观形貌
Fig.2
SEM images of corrosion product films formed on 20# steel after immersion in the mixed solutions with different concentrations of [Bmim]BF4: (a) 30% MEA+0%[Bmim]BF4, (b) 30%MEA+5%[Bmim]BF4, (c) 30%MEA+10%[Bmim]BF4, (d) 30%MEA+20%[Bmim]BF4
图3为[Bmim]BF4浓度为0%和20%时,去除腐蚀产物膜后挂片的微观形貌。可知,[Bmim]BF4的加入,导致挂片表面的孔隙明显增多,点蚀加剧。结合腐蚀产物的形貌特征可知,[Bmim]BF4的加入会使腐蚀产物膜变得疏松多孔,加剧点蚀的产生。
图3
图3
20#钢在添加不同浓度[Bmim]BF4的30%MEA混合溶液中浸泡后去除腐蚀产物膜的表面形貌
Fig.3
SEM images of 20# carbon steel after removal of corrosion product films formed during immersion in the mixed solutions with different concentrations of [Bmim]BF4: (a) 30%MEA+0%[Bmim]BF4, (b) 30%MEA+20%[Bmim]BF4
图4
图4
20#钢在含不同浓度[Bmim]BF4的混合溶液中外层腐蚀产物膜的EDS分析结果
Fig.4
EDS results of the outer layers of corrosion product films formed on 20# steel during immersion in the mixed solutions with different concentrations of [Bmim]BF4: (a) area I in Fig.2a, (b) area II in Fig.2b, (c) area III in Fig.2c, (d) area IV in Fig.2d
表2 Fe的EDS分析结果
Table 2
| Corcentration of [Bmim]BF4 / % | Mass fraction % | Atomic fraction % |
|---|---|---|
| 0 | 81.59 | 49.90 |
| 5 | 93.48 | 77.28 |
| 10 | 90.56 | 70.31 |
| 20 | 95.40 | 83.51 |
2.3 电化学测试
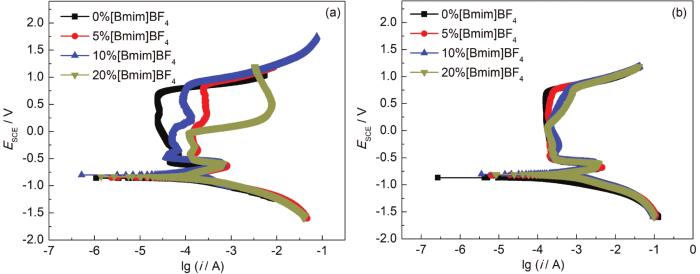
图5为20#碳钢在30和70 ℃下添加不同浓度[Bmim]BF4的30%MEA溶液中的极化曲线 (含钝化区)。30 ℃时,随着[Bmim]BF4浓度的增加,钝化区电位范围越来越小。加入[Bmim]BF4后,20#钢在较低电位时就发生了点蚀现象,表明[Bmim]BF4的加入会诱发点蚀的发生,这与清除腐蚀产物后的微观形貌观察结果相吻合。在[Bmim]BF4浓度为5%与10%的情况下,钝化膜被击穿后,随着电位的上升,阳极过程再次受阻出现再钝化现象,蚀点被修复。[Bmim]BF4浓度为20%时,也出现了再钝化的现象,但出现时间相对较晚。以上结果表明,随着[Bmim]BF4浓度的增加对钝化膜不稳定性的影响会越来越大。在70 ℃下,[Bmim]BF4浓度为5%时,钝化膜与不含[Bmim]BF4体系的状况相似,即钝化膜较为稳定,但维钝电流密度要略高于不含[Bmim]BF4的情况。而在[Bmim]BF4浓度为10%与20%情况下,其钝化膜在电位分别为约0.07和-0.01 V时就被击穿,钝化电位范围小了很多,使得点蚀更容易发生。
图5
图5
不同温度下极化曲线随MEA和[Bmim]BF4浓度的变化规律
Fig.5
Polarization curves of 20# carbon steel in the mixed solutions with different concentrations of [Bmim]BF4 at 30 ℃ (a) and 70 ℃ (b)
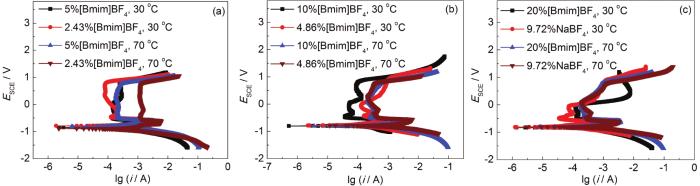
通过EDS分析可知,20#钢在含有离子液体[Bmim]BF4的混合液中浸泡后,表面腐蚀产物中存在F。为进一步验证BF4-是否为导致点蚀加重的原因,通过极化曲线来判断BF4-是否是导致钝化区范围变小,促进点蚀的原因。
图6为含相同摩尔质量的NaBF4与相对应的[Bmim]BF4的极化曲线对比图。可看出,NaBF4的极化曲线在阳极钝化区与[Bmim]BF4大致相同,有近乎相同的击穿电位。随着BF4-的增加,钝化区逐渐变窄,充分证明BF4-是在含30%MEA饱和CO2体系下加剧20#钢点蚀的重要原因。
图6
图6
20#碳钢在含相同摩尔质量BF4-溶液中的极化曲线对比图
Fig.6
Polarization curves of 20# carbon steel in the solutions containing the same molar mass of BF4-: (a) 30%MEA+5%[Bmim]BF4 (or 2.43%NaBF4), (b) 30%MEA+10%[Bmim]BF4 (or 4.86%NaBF4), (c) 30%MEA+20%[Bmim]BF4 (or 9.72%NaBF4)
图7是20#钢在30和70 ℃下添加不同浓度[Bmim]BF4的30%MEA饱和CO2溶液中浸泡0.5 h后所测试阻抗谱的拟合曲线。由于Faraday电流密度比电极反应的交换电流密度大得多,电极表面附近反应物的浓度与溶液本体中的浓度会有差别[31]。图7反映了在浸泡0.5 h后,阻抗谱低频区在含有[Bmim]BF4的混合溶液中表现出了扩散尾,而高频区出现表征极化电阻的容抗弧,且在含[Bmim]BF4混合体系的明显大于仅有MEA溶液体系的。由此可知,在浸泡0.5 h的状态下,含有[Bmim]BF4的混合溶液中,电极反应产物相对于仅含有醇胺溶液中更容易产生浓度差,反应产物在仅含有MEA溶液中扩散速度较大。并且在两种实验温度下,随着[Bmim]BF4浓度的增加,容抗弧变大,说明工作电极表面活化状态逐渐降低,体系的腐蚀性降低,这与腐蚀失重实验结果一致。
图7
图7
20#钢在含不同浓度[Bmim]BF4的混合溶液中浸泡0.5 h后不同温度条件下的EIS曲线
Fig.7
EIS curves of 20# steel immersed in the mixed solution for 0.5 h at 30 ℃ (a) and 70 ℃ (b)
图8是20#钢在不同温度下的含不同浓度 [Bmim]BF4的混合溶液中浸泡0.5 h的阻抗谱等效电路。表3是20#钢在30和70 ℃下添加不同浓度[Bmim]BF4的含饱和CO2的30%MEA中浸泡0.5 h后的阻抗拟合参数。可知,在相同温度下,溶液电阻Rs随着[Bmim]BF4含量增大而增大,其原因是溶液中的相当一部分H2O被[Bmim]BF4所替代[32],而H2O是氧化剂的主要来源,H2O的减少使溶液中带电粒子传质速度变慢。由于添加了[Bmim]BF4的溶液粘度远大于MEA溶液的,这在一定程度上阻碍了氧化剂在溶液中的扩散[21],且随着温度的升高粘度降低。在温度一定时,随着[Bmim]BF4浓度的增加,Rs会升高,导致腐蚀速率下降;在[Bmim]BF4浓度一定时,随着温度的增加,Rs会有所降低,腐蚀速率增加。
图8
图8
20#钢在混合溶液中浸泡0.5 h后电化学阻抗谱等效电路
Fig.8
Equivalent circuits for fitting EIS of 20# steel immersed in the mixed solutions without (a) and with (b) [Bmim]BF4 for 0.5 h
表3 20#钢在30和70 ℃下含不同浓度[Bmim]BF4的混合溶液中浸泡0.5 h后阻抗拟合参数
Table 3
| Temperature / ℃ | Mass fraction of [Bmim]BF4 / % | Rs / Ω·cm2 | CPE1-T / Ω-1·cm-2·s-1 | CPE1-P | Rp / Ω·cm2 | Rt / Ω·cm2 | CPE2-T / Ω-1·cm-2·s-1 |
|---|---|---|---|---|---|---|---|
| 30 | 0 | 3.556 | 4.38×10-4 | 0.78328 | 450.80 | --- | --- |
| 5 | 4.106 | 4.17×10-4 | 0.79523 | --- | 563.8 | 2.1548×10-2 | |
| 10 | 4.800 | 3.18×10-4 | 0.77613 | --- | 1101 | 7.0330×10-3 | |
| 20 | 5.289 | 3.66×10-4 | 0.86637 | --- | 1513 | 8.0210×10-3 | |
| 70 | 0 | 2.329 | 8.22×10-4 | 0.76553 | 80.93 | --- | --- |
| 5 | 2.475 | 6.05×10-4 | 0.76703 | --- | 88.31 | 5.4759×10-1 | |
| 10 | 2.533 | 6.34×10-4 | 0.75338 | --- | 94.37 | 1.6909×10-1 | |
| 20 | 2.769 | 6.61×10-4 | 0.76547 | --- | 135.7 | 6.7300×10-1 |
对于仅有30%MEA饱和CO2溶液体系,Fraday阻抗为极化电阻Rp,其含义是单位面积电极表面电阻;而在含有[Bmim]BF4的体系中,Faraday阻抗由扩散阻抗和电极反应阻抗即电荷转移电阻Rt共同组成。由表3可知,即使不考虑扩散阻抗,仅用Rt与Rp作比较可见,在温度一定时,随着[Bmim]BF4浓度的增加,Faraday阻抗会升高;在[Bmim]BF4浓度一定时,随着温度的增加,Faraday阻抗会有所降低。表明,[Bmim]BF4的加入会阻止电极极化过程,减缓了腐蚀过程的进行,这与腐蚀失重实验结果一致。
3 结论
(1) 在MEA溶液中添加[Bmim]BF4对20#钢的腐蚀具有一定的缓蚀作用,会阻止电极极化过程,抑制均匀腐蚀过程的进行,导致20#钢的均匀腐蚀速率减小。但是,[Bmim]BF4浓度的增加带来的腐蚀抑制效果并不明显,且会加剧点蚀的产生。
(2) 20#钢在含饱和CO2的离子液体醇胺溶液的腐蚀极化过程中,随着[Bmim]BF4浓度的升高,极化曲线钝化区范围变窄,对钝化膜不稳定性的影响会越来越大。
(3) 在MEA与[Bmim]BF4组成的混合溶液体系中,BF4-的存在是点蚀产生的主要原因;随着[Bmim]BF4浓度的增加,20#钢表面的点蚀坑增多。
参考文献
Determining the corrosive potential of CO2 transport pipeline in high pCO2-water environments
[J].
Transmission of CO2-safety and economic considerations
[A].
Techno-economic evaluation of the 2-amino-2-methyl-1-propanol (AMP) process for CO2 capture from natural gas combined cycle power plant
[J].
Novel non-aqueous MEA solutions for CO2 capture
[J].
Post-combustion CO2 capture with chemical absorption: A state-of-the-art review
[J].Global concentration of CO2 in the atmosphere is increasing rapidly. CO2 emissions have an impact on global climate change. Effective CO2 emission abatement strategies such as Carbon Capture and Storage (CCS) are required to combat this trend. There are three major approaches for CCS: post-combustion capture, pre-combustion capture and oxyfuel process. Post-combustion capture offers some advantages as existing combustion technologies can still be used without radical changes on them. This makes post-combustion capture easier to implement as a retrofit option (to existing power plants) compared to the other two approaches. Therefore, post-combustion capture is probably the first technology that will be deployed. This paper aims to provide a state-of-the-art assessment of the research work carried out so far in post-combustion capture with chemical absorption. The technology will be introduced first, followed by required preparation of flue gas from power plants to use this technology. The important research programmes worldwide and the experimental studies based on pilot plants will be reviewed. This is followed by an overview of various studies based on modelling and simulation. Then the focus is turned to review development of different solvents and process intensification. Based on these, we try to predict challenges and potential new developments from different aspects such as new solvents, pilot plants, process heat integration (to improve efficiency), modelling and simulation, process intensification and government policy impact. (C) 2010 The Institution of Chemical Engineers. Published by Elsevier B.V.
Ionic liquids for CO2 capture and emission reduction
[J].
A process simulation study of CO2 capture by ionic liquids
[J].
Relative chemical stability of imidazolium-based alkaline anion exchange polymerized ionic liquids
[J].
Ionic liquids of superior thermal stability
[J].
Room-temperature ionic liquid-amine solutions: Tunable solvents for efficient and reversible capture of CO2
[J].
Study on the absorption of carbon dioxide in high concentrated MDEA and ILs solutions
[J].
CO2 capture with hybrid absorbents of low viscosity imidazolium-based ionic liquids and amine
[J].
Study of CO2 removal in natural gas process using mixture of ionic liquid and MEA through process simulation
[J].
Corrosion behavior of carbon steel in the CO2 absorption process using aqueous amine solutions
[J].
Research progress on corrosion of carbon steel during process of capture CO2 with monoethanolamine solution
[J].
MEA捕集CO2腐蚀研究进展
[J].2过程中的腐蚀机理,以及O2浓度、MEA浓度、溶液温度、CO2浓度、热稳定盐对溶液腐蚀性的影响。结果表明,碳钢在MEA—CO2溶液中存在电化学反应,阳极反应为铁失电子生成铁离子,阴极反应为游离水、水合氢离子、碳酸氢根离子以及氧得电子。腐蚀产物主要为FeCO3、Fe(OH)2和Fe(OH)3。O2浓度升高、MEA浓度升高、溶液温度升高、CO2浓度增大、热稳定盐浓度升高等均可使溶液腐蚀性增强。不同种类热稳定盐对溶液腐蚀性影响不同,其中草酸盐对溶液腐蚀影响最大。CO2捕集过程中的腐蚀问题常通过添加缓蚀剂来得到缓解。无机缓蚀剂腐蚀抑制效果好,但毒性大;有机缓蚀剂毒性较低,但腐蚀抑制效果较差。]]>
Pathways for the formation of products of the oxidative degradation of CO2-loaded concentrated aqueous monoethanolamine solutions during CO2 absorption from flue gases
[J].
Part 3: Corrosion and prevention in post-combustion CO2 capture systems
[J].Chintana Saiwan*(1), Teeradet Supap(2), Raphael O Idem(2) & Paitoon TontiwachwuthikuI(2,3) This article is part 3 of the review series on 'Recent progress and new development of post-combustion carbon capture technology using reactive solvents'. This review focuses on alkanolamine absorption during post-combustion CO2 capture from coal-fired flue gas, looking at a range of absorbents, including those that are commonly used, as well as blended and new solvents. The effects on corrosion of blended and new absorbents and process parameters (e.g., amine concentration, CO2 loading, oxygen concentration, SO2 concentration and temperature) are reviewed. Also reviewed is the effect of corrosion on the formation of heat-stable salts in the absorbent stream, as well as the presence of impurities in flue gas. Finally, corrosion mechanisms are discussed and corrosion inhibition approaches are reviewed.
Understanding corrosion in alkanolamine gas treating plants: Part 1
[J].
Electrochemical investigation on the effect of heat-stable salts on corrosion in CO2 capture plants using aqueous solution of MEA
[J].
Effect of MEA's degradation products on corrosion at CO2 capture plants
[J].
Prospects of using room-temperature ionic liquids as corrosion inhibitors in aqueous ethanolamine-based CO2 capture solvents
[J].
Understanding the corrosion behavior of carbon steel in amino-functionalized ionic liquids for CO2 capture assisted by weight loss and electrochemical techniques
[J].
Corrosion behaviors of materials in aluminum chloride-1-ethyl-3-methylimidazolium chloride ionic liquid
[J].
Corrosion characteristics of nickel, copper, and stainless steel in a Lewis neutral chloroaluminate ionic liquid
[J].
High temperature corrosion properties of ionic liquids
[J].
Ionic liquids as diathermic fluids for solar trough collectors’ technology: A corrosion study
[J].
AFM-assisted investigation of the corrosion behaviour of magnesium and AZ91 alloys in an ionic liquid with varying water content
[J].
RP0775-2005 Preparation, installation, analysis, and interpretation of corrosion coupons in oilfield operations
[S].
Corrosion evaluation of MEA solutions by SEM-EDS, ICP-MS and XRD
[J].
Time-dependent electrochemical behavior of carbon steel in MEA-based CO2 capture process
[J].