增材制造作为近年来快速发展的材料制备技术,因其制造流程简单、可灵活成形复杂结构等优势,在金属材料高效制备领域展现出巨大潜力[3]。目前主流的金属增材制造技术包括:选区激光熔化、电弧增材、选区电子束熔化以及电子束熔丝沉积等。其中,电子束熔丝沉积技术(EBF3)以高能电子束为热源,通过真空环境下精准熔化金属丝材并逐层堆积成形,其核心优势与钛合金特性高度适配[4]。电子束的高能量密度可快速熔化高熔点钛合金,真空环境避免钛合金高温氧化,丝材送料方式可以提升沉积效率,而快速冷却特性则有助于细化晶粒、提升成形件致密度等。因此,该技术尤其适合海洋工程中大型钛合金构件的高效制造,有望解决传统钛合金加工工艺中存在的制造成本高昂、加工流程繁琐等问题。
目前,研究人员针对电子束熔丝沉积钛合金开展了一系列研究工作[5~10]。例如,Pu等[10]采用电子束熔丝沉积制备出不同工艺参数下的单道多层NiTi沉积体,结果表明NiTi沉积体的成形质量、微观组织及性能具有很强的工艺依赖性。此外,Xu等[5]研究了电子束熔丝沉积Ti-6Al-4V合金的微观组织和力学性能,并报道了由于复杂热循环的影响,该合金内部含有片层组织、网篮组织和魏氏组织,而微观组织的差异进一步导致了显微硬度和拉伸强度的不同。相似地,Zhu等[6]研究了电子束熔丝沉积Ti-6.5Al-2Zr-Mo-V合金的梯度组织和力学性能,表明相比于中部和底部区域,具有细网篮特征的顶部区域表现出相对较好的综合力学性能。此外,Su等[7]研究了电子束熔丝沉积Ti-6Al-3Nb-2Zr-1Mo合金不同方向截面的微观组织和腐蚀行为,并揭示了该合金不同方向截面在模拟海水中均表现出自发钝化行为,而在5 mol/L HCl溶液中则表现出活性溶解行为,此外,氧化膜的差异导致了该合金不同方向截面在模拟海水中耐腐蚀性能的差异,而α相片层厚度或残余β相体积分数的差异则导致了该合金不同方向截面在5 mol/L HCl溶液中耐腐蚀性能的不同。综上所述,目前电子束熔丝沉积钛合金的研究工作涉及工艺参数优化、微观组织演变规律、力学行为与腐蚀性能。
增材制造钛合金通常需要热处理来稳定或提高其性能,由于不同的热处理制度会产生差异显著的微观组织,进而影响耐腐蚀性能,因此,选择合适的热处理制度对制备优异耐腐蚀性能的增材制造钛合金至关重要[11,12]。Delpazir等[13]研究了不同热处理制度对粉末床熔融Ti-6Al-4V合金在3.5% (质量分数) NaCl溶液中耐腐蚀性能的影响,表明在β相变点以下进行热处理可以均匀组织并改善耐腐蚀性能。Longhitano等[14]研究了热处理对增材制造Ti-6Al-4V ELI合金在PBS缓冲液中腐蚀行为的影响,揭示了耐腐蚀性能的提高源于热处理导致的β相形核与长大。Li等[15]研究了多步热处理对电弧熔丝增材制造Ti-6Al-4V合金在3.5%NaCl溶液中耐腐蚀性能的影响,阐明了多步热处理导致的α相形貌、尺寸和含量的变化是耐腐蚀性能改善的内在原因。以上研究表明,采取适宜的热处理制度调控增材制造钛合金的微观组织,进而提高合金的耐腐蚀性能是切实可行的,然而,目前针对电子束熔丝沉积钛合金热处理制度-微观组织-耐腐蚀性能的关系及作用机理尚不明晰,亟需阐明热处理制度对电子束熔丝沉积钛合金微观组织和腐蚀行为的影响机制。
基于以上分析,为填补热处理制度对电子束熔丝沉积钛合金微观组织和腐蚀行为影响的研究空白,本论文以电子束熔丝沉积Ti80合金为研究对象,然后研究不同的热处理制度对电子束熔丝沉积Ti80合金微观组织的影响规律,同时系统分析不同热处理态合金在3.5%NaCl溶液中的腐蚀行为,阐明热处理制度-微观组织演变-耐腐蚀性能的关系及影响机理,为通过调整热处理制度调控电子束熔丝沉积钛合金微观组织进而实现耐腐蚀性能的优化提供依据。
1 实验方法
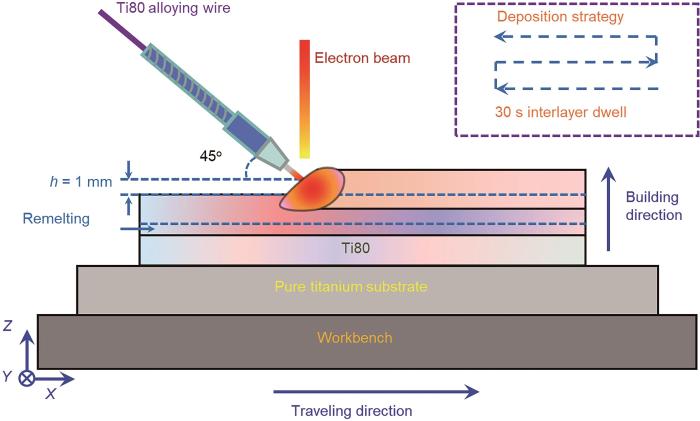
本文所采用的锻态Ti80合金(对照组)由西北有色金属研究院提供,实际化学成分符合Ti80合金名义成分[16]。电子束熔丝沉积所采用的原材料为T80合金丝,直径为1.6 mm,实际化学成分亦符合Ti80合金名义成分[7],所采用的基板尺寸规格为150 mm ×100 mm × 10 mm,具体工艺参数[17]:加速电压60 kV、束流密度40 mA、移动速度500 mm/min、送丝速度2 m/min。在进行电子束熔丝沉积增材制造之前,需对基板及丝材表面进行预处理:首先采用机械打磨消除表面氧化层,随后使用酒精进行超声清洗以去除污染物,最后将处理后的基板和丝材置于60 ℃的恒温干燥箱中进行3 h以上的预热处理,以降低热应力集中风险。图1为电子束熔丝沉积工艺过程的示意图。
图1
为获得不同的微观组织形态,本文选择了3种热处理制度对电子束熔丝沉积Ti80合金进行热处理。热处理制度一:升温至950 ℃保温1 h后进行炉冷(样品简称:950FC);热处理制度二:升温至1010 ℃保温1 h后进行炉冷(样品简称:1010FC);热处理制度三:升温至910 ℃保温1 h后进行水冷,再加热至 600 ℃保温4 h后进行空冷(样品简称:910WC + 600AC)。电子束熔丝沉积Ti80的热处理方案示意图如图2所示。
图2
相组成鉴定试样由线切割获取。在进行相组成鉴定之前,首先使用砂纸将其打磨至1200#,然后使用酒精超声清洗。相组成鉴定采用Empyrean智能X射线衍射仪(XRD)。
微观组织表征试样经240#、400#、600#、1000#、1500#及2000#砂纸打磨后,进行机械抛光,抛光剂为Cr2O3,抛光后的试样使用成分为10%HF + 10% HNO3 + 80%H2O (体积分数)的溶液腐蚀,腐蚀时间为10~20 s。使用JXA-8230型扫描电镜(SEM)分析试样的微观组织。
此外,使用搭载背散射探头的SUPRA55型场发射扫描电镜进行电子背散射衍射(EBSD)分析,EBSD试样按照金相试样的制备要求打磨完成后进行电解抛光。电解抛光使用直流电源,电流控制在0.7~1 A之间,时间约为2 min,使用液氮对抛光液进行冷却,温度控制在-25 ℃左右。
电化学腐蚀测试试样首先使用砂纸将其所有表面打磨干净,然后使用导电胶将一根铜导线粘贴到试样背面,最后将试样嵌入环氧树脂中制作成工作电极。电化学测试测试在3.5%NaCl溶液中进行,使用CHI660型电化学工作站,采用三电极体系,其中参比电极(RE)为饱和甘汞电极(SCE),辅助对电极(CE)采用铂片电极,工作电极(WE)则为试样。
开路电位(OCP)的测试从试样浸入电解液开始,记录间隔为1 s,到其达到一个相对稳定值为止。电化学阻抗(EIS)测试是在上一步测定的OCP值下进行,测试过程中施加的激励信号为幅值10 mV的正弦波电位信号,测试频率为105~10-2 Hz。实验测得的电化学阻抗谱使用ZSimpWin 3.10软件进行等效电路拟合分析。动电位极化(PDP)曲线测试扫描速率为0.1667 mV/s,扫描范围为-1.0~2.0 V。电化学测试中每组实验至少重复3次,以保证实验数据的准确性。
2 实验结果与分析
2.1 热处理制度对微观组织的影响
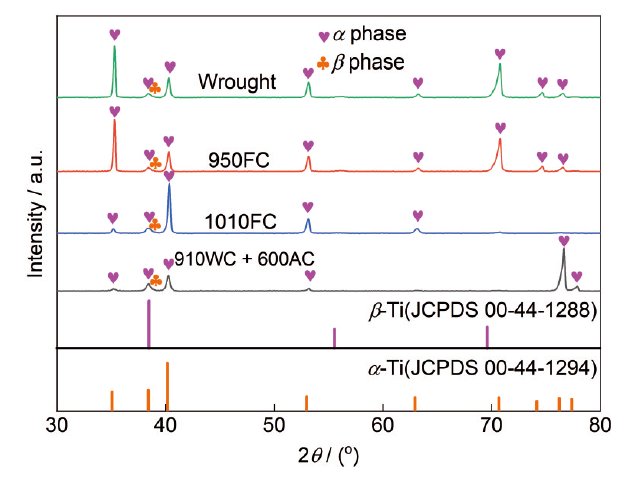
图3为锻态合金和热处理-EBF3合金的XRD图,此外,为了方便鉴别相组成,密排六方(HCP)结构的α相(JCPDS 00-44-1294)和体心立方(BCC)结构的β相(JCPDS 00-44-1288)的标准衍射峰的位置也绘制在图3中。由图3可知,锻态合金和热处理-EBF3合金均由α相和β相构成。此外,从图3中还可以看出,与α相相比,β相仅呈现一个特征衍射峰,即(110)衍射峰(2θ≈39°),且衍射峰强度相对较弱,这一现象表明,锻态合金和热处理-EBF3合金中α相均占主导地位,残留β相的体积分数显著低于α相,符合近α钛合金的典型特征。
图3
图3
锻态合金和热处理-EBF3合金的XRD图
Fig.2
XRD patterns of wrought Ti80 alloy and heat treated Ti80 alloys fabricated by EBF3
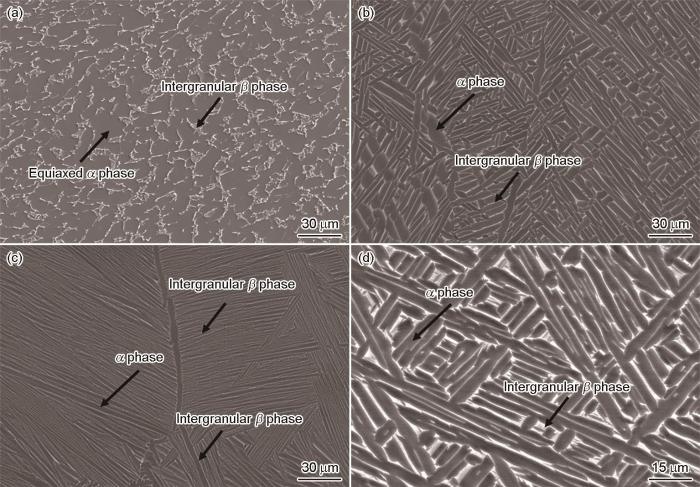
图4展示了锻态合金和热处理-EBF3合金的微观组织。由图4a可知,锻态Ti80合金的微观组织由近等轴的α-相和少量晶间残留的β-相构成[16]。图4b为950FC合金的微观组织。从图4b图中可以观察到,950FC合金呈现出粗网篮组织的特征。这是因为在β相变点以下退火处理时,部分α相溶解,β相比例增加,在随后缓慢的炉冷过程中,β相内部析出片状α相,同时α相生长,从而导致整体组织显著粗化。1010FC合金的微观组织如图4c所示,具有全片层组织的特征,由交替排列的α/β大尺寸集束组成。沉积态Ti80合金在β相变点以上进行退火处理时,组织完全转变为单一β相,并在高温下经历晶粒长大。随后缓慢炉冷过程中,β相内部沿特定晶体学取向析出α片层,最终在整个β晶粒范围内形成连续分布的片层组织,残余β相以细薄长条分布于α片层之间[18]。图4d显示了910WC + 600AC合金的微观组织,以层级组织为特征,主要由网篮形貌的初生α相和β转变组织构成,β转变组织中弥散分布着次生α + β相。由于较低的固溶温度以及水冷特点,层级组织的初生α相没有得到充分生长。当钛合金处于α + β双相区固溶处理时,初生α相的形成与粗网篮组织相似,随后快速水冷导致初生α相略微长大,高温β相内析出细小的二次α′马氏体(β转变组织)。继续加热到600 ℃,β转变组织内的α′马氏体发生分解,促进形成细小的α + β组织。
图4
图4
锻态合金和热处理-EBF3合金的微观组织
Fig.4
Microstructure of wrought Ti80 alloy and heat treated Ti80 alloys fabricated by EBF3: (a) wrought alloy, (b) 950FC alloy, (c) 1010FC alloy, (d) 910WC + 600AC alloy
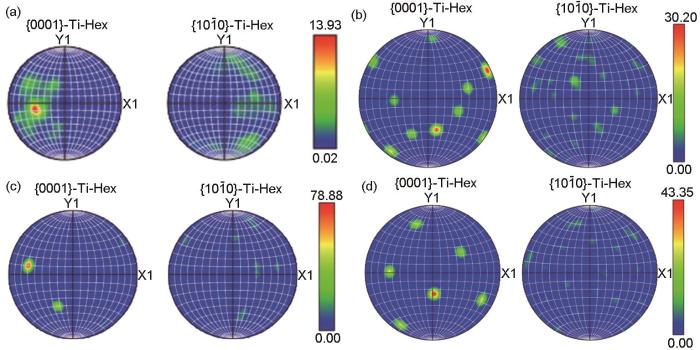
为进一步观察微观组织差异,本文进一步采集了晶粒取向信息,如图5和6所示。相比之下,全片层微观结构表现出显著的晶体取向集中现象,最大MRD (multiple of random distribution)值高达78.88,形成了亚毫米尺度上的明显微织构特征。950FC合金,即粗网篮组织和910WC + 600AC合金,即层级组织在取向分布上的集中程度相近,均出现相当强烈的取向集中。锻态α相晶粒呈现出较为均匀的取向分布特征,未观察到明显的织构集中现象。这一差异表明,不同的热处理制度对组织特征的演变具有决定性影响。通过调整热处理温度或控制冷却速率,可以实现对晶粒尺寸、形貌及取向演变行为的有效调控,进而赋予材料不同的微观组织特性与织构分布,这为实现材料性能的定向优化提供了理论基础。
图5
图6
图6
锻态合金和热处理-EBF3合金的极图
Fig.6
Pole images of wrought Ti80 alloy and heat treated Ti80 alloys fabricated by EBF3: (a) wrought alloy, (b) 910WC + 600AC alloy, (c) 1010FC alloy, (d) 950FC alloy
2.2 热处理工艺对腐蚀行为的影响
2.2.1 开路电位测试结果
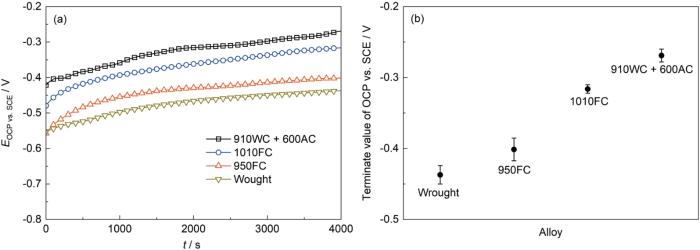
图7a为锻态合金和热处理-EBF3合金在3.5%NaCl中的OCP随浸泡时间的变化曲线。在3.5%NaCl中,锻态合金和热处理-EBF3合金的OCP均随浸泡时间的延长而逐步上升,这与合金表面氧化膜的不断生长有关[19,20]。此外,从图7a中还可以观察到一些微小的波动,这是由合金表面氧化膜的形成和溶解之间的动态竞争导致的[21]。此外,由图7b可知,在两种测试溶液中,OCP终止值按以下降序排列:910WC + 600AC合金,即层级组织>1010FC合金,即全片层组织>950FC合金,即粗网篮组织>锻态Ti80合金,即等轴组织,这表明与锻态合金相比,热处理-EBF3合金,尤其是910WC + 600AC合金(层级组织),在热力学上表现出较低的腐蚀倾向[22]。
图7
图7
锻态合金和热处理-EBF3合金的OCP测试结果
Fig.7
Results of OCP measurements: (a) Evolution of OCP with immersion time alloys, and (b) the terminate value of OCP
2.2.2 动电位极化曲线的测试结果
图8
图8
锻态合金和热处理-EBF3合金的动电位极化曲线PDP测量结果
Fig.8
Results of PDP measurements: (a) PDP curves (b) schematic illustration for the electrochemical behavior
如反应(1)和(2)所示,首先,Cl-促进了[TiCl]4-的形成,然后,[TiCl]4-转化为TiCl4,最后,TiCl4迅速水解形成TiO2氧化膜,导致了自发钝化行为的发生,从而有效抑制了合金基体与腐蚀介质的相互作用。
图8b为在3.5%NaCl溶液中测得的锻态合金和热处理-EBF3合金的PDP曲线行为的原理示意图。如图8b所示,锻态合金和热处理-EBF3合金的阴极和阳极分支在钝化区内只有一个交点,这解释了所有合金在3.5%NaCl溶液中表现出的自发钝化行为。由于所研究合金均表现出自发钝化行为,即此时的自腐蚀电位(Ecorr)处于钝化区域之内,因此自腐蚀电流密度(Icorr)近似等于钝化电流密度(Ip)[25],该值于500 mV vs. SCE处获得,用于定量衡量合金的耐腐蚀性能。由图8a中的插图可以清晰地观察到,在3.5%NaCl中,相比于锻态合金(等轴组织),热处理-EBF3合金,尤其是910WC + 600AC合金(层级组织),表现出较低的Ip,这表明910WC + 600AC合金(层级组织)具有更优异的耐腐蚀性能。
2.2.3 电化学阻抗测试结果
为进一步阐明锻态合金和热处理-EBF3合金的腐蚀行为,在3.5%NaCl溶液中进行了EIS测试,测试结果如图9所示。图9a为在3.5%NaCl溶液中测得的锻态合金和热处理-EBF3合金的Nyquist图,所有合金的Nyquist图都显示一个容抗弧,通常对应于一个时间常数。此外,从图9a可以观察到,锻态合金(等轴组织)的容抗弧直径最小,910WC + 600AC合金(层级组织)的容抗弧直径最大,这表明910WC + 600AC合金(层级组织)在3.5%NaCl溶液中的阻抗最高[26~30]。由图9b所示的Bode图可知,相位角在很大频率范围内处于80°以上,这表明了所有合金表面的近容抗响应,这种行为源于在3.5%NaCl溶液中合金表面存在一层稳定且致密的保护性氧化膜[19,31~36],同时进一步证实了一个时间常数的存在。此外,从Bode-phase中可以观察到,在高频范围内,相位角趋近于0°,即电流响应相对于电位变化而言没有滞后。Bode-magnitude图表现出两个特征区域:在高频区(~103~105 Hz),Bode-magnitude图表现为一个较低且稳定的阻抗值,这是由于3.5%NaCl溶液电阻的响应;在中低频区(~10-2~103 Hz),Bode-magnitude图为一条斜线,斜率约为-1,这是表面氧化膜的电容行为的特征响应[37]。
图9
图9
锻态合金和热处理-EBF3合金的EIS图谱
Fig.9
Nyquist (a) and Bode diagrams (b) of wrought Ti80 alloy and heat treated Ti80 alloys fabricated by EBF3
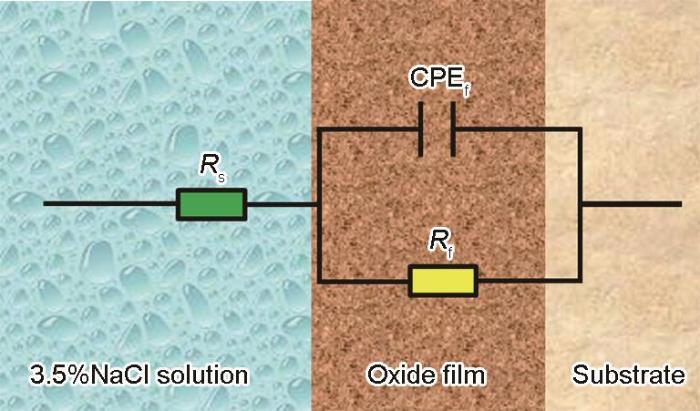
图10
其中,Q表示CPE的值,j表示虚数单位,(j =
表1 锻态合金和热处理-EBF3合金的等效电路拟合参数
Table 1
| Alloy | Rs / Ω·cm2 | CPEf / μS·s n ·cm-2 | nf | Cf / μF·cm-2 | Rf / MΩ·cm2 | χ2 / 10-4 | d / mm |
|---|---|---|---|---|---|---|---|
| Wrought | 12.33 | 39.98 | 0.899 | 53.27 | 0.41 | 9.26 | 1.08 |
| 950FC | 10.69 | 38.38 | 0.916 | 49.93 | 0.46 | 6.20 | 1.15 |
| 1010FC | 6.39 | 36.45 | 0.918 | 48.04 | 0.59 | 3.38 | 1.20 |
| 910WC + 600AC | 14.44 | 35.05 | 0.920 | 47.23 | 0.88 | 6.49 | 1.22 |
式中,Zω 为总的Faraday阻抗,ω对应于角频率(rad/s)。Zω 可以用下式来描述:
当角频率ω趋近于0时,Qf (jω) n 也趋近于0,因此,Rp的计算公式可以简化如下:
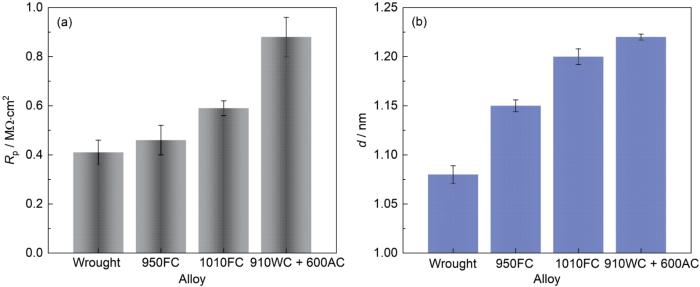
图11
图11
锻态合金和热处理-EBF3合金的Rp和d
Fig.11
Rp (a) and d (b) of wrought Ti80 alloy and heat treated Ti80 alloys fabricated by EBF3
其中,ε为氧化膜的相对介电常数 (ε = 65,对应于金红石TiO2的介电常数[51,52]),ε0为真空介电常数(8.8542 × 10-14 F·cm-1)。表1中列出了氧化膜厚度d。此外,为了更直观地对比氧化膜厚度d的差异,绘制了图11b。由表1和图11b可知,在3.5%NaCl溶液中,氧化膜厚度d按以下降序排列:910WC + 600AC合金,即层级组织> 1010FC合金,即全片层组织>950FC合金,即粗网篮组织>锻态Ti80合金,即等轴组织。由于合金自发钝化的特征,即表面存在一层致密的氧化膜,因此,氧化膜厚度的差异是导致本文中不同热处理态合金耐腐蚀性能差异的原因。钛合金的耐腐蚀性能与其表面形成的氧化膜密切相关,而在腐蚀环境中钛合金氧化膜形核优先发生在活化能较高的位置[12]。钛合金的相界一般具有较高的活化能,因此成为氧化膜形核的首选位置。由图4可知,910WC + 600AC合金的微观组织(层级组织)具有相对细小的α相片层,而细小的α相带来了更高的相界密度,Ti、Al、Nb、Zr、Mo和O优先在相界处扩散,从而为氧化膜的形成提供更多的氧化和活性位置,促进了氧化膜的形核与长大。热处理制度的不同导致α相尺寸不同,进而导致相界密度不同,因此,氧化膜的形核和生长速率不同,最终体现在氧化膜厚度(耐腐蚀性能)的差异。
3 结论
(1) 不同热处理态电子束熔丝沉积Ti80合金中α相均占据主导地位,残留β相的体积分数显著低于α相。升温至950 ℃保温1 h后进行炉冷的合金表现出粗网篮组织,典型特征为α片层明显粗化;升温至1010 ℃保温1 h后进行炉冷的合金的微观组织为全片层组织,初生β晶界清晰可见,晶粒内部由交替排列的α/β集束组成;升温至910 ℃保温1 h后进行水冷,再加热至600 ℃保温4 h后进行空冷的合金以层级组织为主,且β转变组织中弥散分布着次生α + β相;锻态合金的微观组织则主要由等轴α相以及少量残留的晶间β相构成。此外,不同微观组织的取向集中程度有着明显的差异,其中,全片层组织表现出显著的晶体取向集中现象,最大MRD值高达78.88,而锻态组织大体上呈现出较为均匀的取向分布特征。
(2) 在3.5%NaCl中,电化学测试结果均表明不同状态的Ti80合金的耐腐蚀性能按照以下降序排列:910WC + 600AC合金,即层级组织> 1010FC合金,即全片层组织> 950FC合金,即粗网篮组织>锻态Ti80合金,即等轴组织,这表明通过调整热处理制度调控合金的微观组织特征进而改善合金的耐腐蚀性能是切实可行的。
(3) 电化学测试结果表明锻态合金和热处理- EBF3合金均表现出自发钝化的现象,这与合金表面存在一层致密的氧化膜有关,而氧化膜厚度的差异在一定程度上导致了不同组织形态合金耐腐蚀性能的差异。氧化膜厚度的差异源于不同热处理态合金中α相尺寸的不同带来的氧化膜形核与长大速率的差异。
参考文献
Effect of Ru on corrosion behavior of Ti-6Al-4V alloy and its mechanism
[J].
Ru对Ti-6Al-4V合金腐蚀行为的影响及机理研究
[J].
Annealed microstructure dependent corrosion behavior of Ti-6Al-3Nb-2Zr-1Mo alloy
[J].Corrosion resistance of titanium (Ti) alloys is closely connected with their microstructure which can be adjusted and controlled via different annealing schemes. Herein, we systematically investigate the specific effects of annealing on the corrosion performance of Ti-6Al-3Nb-2Zr-1Mo (Ti80) alloy in 3.5 wt.% NaCl and 5 M HCl solutions, respectively, based on open circuit potential (OCP), potentiodynamic polarization, electrochemical impedance spectroscopy (EIS), static immersion tests and surface analysis. Results indicate that increasing annealing temperature endows Ti80 alloy with a higher volume fraction of β phase and finer α phase, which in turn improves its corrosion resistance. Surface characterization demonstrates that β phase is more resistant to corrosion than α phase owing to a higher content of Nb, Mo, and Zr in the former; additionally, the decreased thickness of α phase alleviates segregation of elements to further restrain the micro-galvanic couple effects between α and β phases. Meanwhile, the influential mechanisms of environmental conditions on corrosion of Ti80 alloy are discussed in detail. As the formation of a highly compact and stable oxide film on surface, annealed Ti80 alloys exhibit a low corrosion current density (10-6 A/cm2) and high polarization impedance (106 Ω? cm2) in 3.5 wt.% NaCl solution. However, they suffer severe corrosion in 5 M HCl solution, resulting from the breakdown of native oxide films (the conversion of TiO2 to aqueous Ti3+), active dissolution of substrate Ti to aqueous Ti3+ and existence of micro-galvanic couple effects. Those findings could provide new insights to designing Ti alloys with high-corrosion resistance through microstructural optimization.
The effect of hot isostatic pressure on the corrosion performance of Ti-6Al-4 V produced by an electron-beam melting additive manufacturing process
[J].
Elevated temperature characterization of electron beam freeform fabricated Ti-6Al-4V and dispersion strengthened Ti-8Al-1Er
[J].
Microstructure and mechanical properties of Ti-6Al-4V alloy fabricated using electron beam freeform fabrication
[J].
Insights into the gradient microstructure and mechanical properties of Ti-6.5Al-2Zr-Mo-V alloy manufactured by electron beam freeform fabrication
[J].
Tuning microstructure and improving the corrosion resistance of Ti-6Al-3Nb-2Zr-1Mo alloy using the electron beam freeform fabrication
[J].
Effect of α texture on the tensile deformation behavior of Ti-6Al-4V alloy produced via electron beam rapid manufacturing
[J].
Role of trace boron in the microstructure modification and the anisotropy of mechanical and wear properties of the Ti6Al4V alloy produced by electron beam freeform fabrication
[J].
Evolution of transformation behavior and tensile functional properties with process parameters for electron beam wire-feed additive manufactured NiTi shape memory alloys
[J].
Corrosion behavior of additively manufactured Ti-6Al-4V parts and the effect of post annealing
[J].
Effect of build-up direction and annealing on corrosion properties of selected laser melting Ti6Al4V alloy
[J].
成型方向及热处理对选区激光熔化Ti6Al4V合金腐蚀性能的影响
[J].研究选区激光熔化(Selective laser melting,SLM) Ti6Al4V合金的成型方向及热处理对其在Hanks体液中腐蚀性能的影响。针对不同状态SLM-Ti6Al4V合金的微观组织结构,借助电化学方法跟踪其在Hanks体液中的腐蚀钝化行为,从α/α′相含量、尺寸和取向分布等因素揭示不同状态合金在医用环境中耐腐蚀性能差异的机理。结果表明,成型方向和后续热处理对SLM-Ti6Al4V合金微观组织结构的影响导致了其腐蚀行为的差异。800 ℃以下热处理合金的XZ面耐腐蚀性优于XY面,表现出明显的各向异性,且随热处理温度的升高α/α′相尺寸增大,耐腐蚀性逐渐下降,各向异性减弱。800 ℃及以下热处理SLM-Ti6Al4V合金的腐蚀以点蚀为主,900 ℃及以上热处理合金的腐蚀机制为沿晶界腐蚀。800 ℃热处理合金的α/α′相尺寸及界面密度与成型态相当,表现出良好的耐腐蚀能力。
Microstructure and corrosion behavior of differently heat-treated Ti-6Al-4V alloy processed by laser powder bed fusion of hydride-dehydride powder
[J].
Heat treatments effects on functionalization and corrosion behavior of Ti-6Al-4V ELI alloy made by additive manufacturing
[J].
Microstructural evolution and corrosion resistance of additively manufactured Ti-6Al-4V alloy annular sha-ped components using multistage heat treatment
[J].
The corrosion behavior of Ti-6Al-3Nb-2Zr-1Mo alloy: Effects of HCl concentration and temperature
[J].Investigation about the corrosion behavior of Ti alloys in different ambient environment is of great significance for their practical application. Herein, we systematically investigate the corrosion behavior of a newfound Ti-6Al-3Nb-2Zr-1Mo (Ti80) alloy in hydrochloric acid (HCl) ranging from 1.37 to 7 M, and temperature ranging from 25 to 55 ℃, by means of electrochemical measurements, static immersion tests and surface analysis. Results manifest that increasing either HCl concentration or temperature can accelerate the corrosion of Ti80 alloy via promoting the breakdown of native protective oxide film and then further facilitating the active dissolution of Ti80 matrix. According to potentiodynamic polarization curves, Ti80 alloy displays a spontaneous passive behavior in 1.37 M HCl at 25 ℃, compared to a typical active-passive behavior under the other conditions. As indicated by cathodic Tafel slope, the rate determining step for cathodic hydrogen evolution reaction is likely the discharge reaction step. The apparent activation energies obtained from corrosion current density and maximum anodic current density for Ti80 alloy in 5 M HCl solution are 62.4 and 55.6 kJ mol-1, respectively, which signifies that the rate determining step in the corrosion process of Ti80 alloy is mainly determined by surface-chemical reaction rather than diffusion. Besides, the electrochemical impedance spectroscopy tests demonstrate that a stable and compact oxide film exists in 1.37 M HCl at 25 ℃, whereas a porous corrosion product film forms under the other conditions. Overall, the critical HCl concentration at which Ti80 alloy can maintain passivation at 25 ℃ can be determined as a value between 1.37 and 3 M. Furthermore, the corroded surface morphology characterization reveals that equiaxed α phase is more susceptible to corrosion compared to intergranular β phase due to a lower content of Nb, Mo, and Zr in the former.
Deciphering the microstructural development and excellent ductility in electron beam wire-fed additive manufacturing of Ti-6Al-3Nb-2Zr-1Mo alloys based on high deposition rate
[J].
Interdependent slip and twinning behaviors for improving cryogenic mechanical properties in Ti-6Al-3Nb-2Zr-1Mo alloy additively manufactured by electron beam wire-fed
[J].
The effect of fluoride ions on the corrosion behavior of pure titanium in 0.05 M sulfuric acid
[J].
Formation mechanism of residue water stains on TC4 Ti-alloy surface and conter-measures
[J].
钛合金表面碱洗及水洗留痕形成机制与腐蚀过程控制
[J].航空发动机用TC4合金在碱洗及随后的高温水洗步骤清洗积碳后,表面出现大量水洗痕迹,从而对后续荧光分析产生严重影响。本研究以TC4合金为实验对象,通过分析TC4合金在10% (质量分数) NaOH溶液中的电化学腐蚀行为分析TC4合金碱洗出现留痕的机制。实验结果表明随碱洗温度升高,TC4合金的腐蚀速率加快。显微观察及能谱分析表明,水洗痕迹源于材料自身的钝化膜由于残留碱液浓度升高而发生腐蚀。去离子水与自来水清洗对比实验说明,随温度升高,试片均出现水洗痕迹,但采用去离子水清洗,可有效避免水洗留痕的产生。
Improved corrosion behaviour of electron beam melted Ti-6Al-4V alloy in phosphate buffered saline
[J].
Effects of grain size on the corrosion resistance of wrought magnesium alloys containing neodymium
[J].
Corrosion performance of additively manufactured bimetallic aluminum alloys
[J].
Distinction in electrochemical behaviour of Ti6Al4V alloy produced by direct energy deposition and forging
[J].
Synergistic effects of fluoride and chloride on general corrosion behavior of AISI 316 stainless steel and pure titanium in H2SO4 solutions
[J].
A review of impedance plot methods used for corrosion performance analysis of painted metals
[J].
The role of nickel in mechanical performance and corrosion behaviour of nickel-aluminium bronze in 3.5 wt.%NaCl solution
[J].
Effect of Ce on the localized corrosion behavior of non-equiatomic high-entropy alloy Fe40Mn20Cr20Ni20 in 0.5 M H2SO4 solution
[J].
Corrosion and passive behavior of Al x CrFeNi3- x (x = 0.6, 0.8, 1.0) eutectic high entropy alloys in chloride environment
[J].
Corrosion behaviors and mechanism of CrFeNi2 based high-entropy alloys
[J].
Comparison of the corrosion behavior of pure titanium and its alloys in fluoride-containing sulfuric acid
[J].
Microstructure, wear resistance, and corrosion performance of Ti35Zr28Nb alloy fabricated by powder metallurgy for orthopedic applications
[J].A ternary Ti35Zr28Nb alloy was fabricated by powder metallurgy (PM) from pre-alloyed powder. The microstructure, hardness, corrosion behavior, and wear response of the produced alloy were investigated systematically. The results show that nearly full dense Ti35Zr28Nb alloy (relative density is 98.1 ± 1.2 %) can be fabricated by PM. The microstructure was dominated with uniform β phase. The Ti35Zr28Nb alloy displayed spontaneous passivity in a naturally aerated simulated body fluid (SBF) solution at 37 ± 0.5 °C. The Ti35Zr28Nb alloy exhibited the highest corrosion resistance as compared to as-cast Ti6Al4V and pure Ti because of the formation of a protective passive film containing TiO2, Nb2O5, and ZrO2, including the highest corrosion potential (-0.22 ± 0.01 V), the lowest corrosion current density (57.45 ± 1.88 nA), the lowest passive potential (0.05 ± 0.01 V) and the widest passivation range (1.29 ± 0.09 V). Under the same wear condition, the wear rate of the Ti35Zr28Nb alloy (0.0021 ± 0.0002 mm3/m·N) was lower than that of the CP Ti (0.0029 ± 0.0004 mm3/m·N) and close to that of the Ti6Al4V (0.0020 ± 0.0003 mm3/m·N). The wear mechanism of the Ti35Zr28Nb alloy was mainly dominated by abrasive wear, accompanied by adhesive wear. The highest corrosion resistance together with the adequate wear resistance makes the PM-fabricated Ti35Zr28Nb alloy an attractive candidate for orthopedic implant materials.
Effect of fluoride on the corrosion behavior of nanostructured Ti-24Nb-4Zr-8Sn alloy in acidulated artificial saliva
[J].The surface of titanium dental implants is highly susceptible to aggressive fluoride ions in the oral environment. Nanotechnology has proven an effective approach to improve the stability and corrosion resistance of titanium by applying a passive film. In this study, we investigated the effects of fluoride on the corrosion behavior of nanostructured (NS) Ti-24Nb-4Zr-8Sn (Ti2448) alloy in acidulated artificial saliva (AAS) at 37 °C, and then conducted comparisons with its coarse grained (CG) counterpart. Electrochemical techniques, such as potentiodynamic polarization and electrochemical impedance spectroscopy (EIS), as well as surface analysis including X-ray photoelectron spectroscopy (XPS) with argon ion sputtering, and scanning electronic microscopy (SEM) were employed to evaluate the effects of fluoride on sensitivity to pitting and the tolerance of Ti2448 to fluoride in AAS solution. The results demonstrate that corrosion current density increased with F- concentration. In all respects, the NS Ti2448 alloy presented corrosion resistance superior to that of its coarse grained (CG) counterpart at low F- concentrations (≤0.1%). Furthermore, a high content of F- (1%) was shown to promote the active dissolution of both alloys by increasing the rate of corrosion. Following immersion in the fluoridated AAS solution for 60 days, a tissue-friendly compound, Ca3(PO4)2, was detected on the surface of the NS when F- = 0.01% and Na2TiF6 was identified as the main component in the corrosion products of the CG as well as NS Ti2448 alloys when F- = 1%. High concentrations of F- produced pitting corrosion on the CG alloy, whereas NS Ti2448 alloy presented general corrosion in the form of lamellar separation under the same conditions. These findings demonstrate the superior corrosion resistance of the NS Ti2448 alloy as well as lower pitting sensitivity and higher tolerance to fluoride due mainly to grain refinement.
Effect of surface treatment on electrochemical behavior of CP Ti, Ti-6Al-4V and Ti-13Nb-13Zr alloys in simulated human body fluid
[J].
Electrochemical impedance spectroscopy study of the passive oxide film on titanium for implant application
[J].
Electrochemical impedance spectroscopy investigation of the electrochemical behaviour of copper coated with artificial patina layers and submitted to wet and dry cycles
[J].
Study of the corrosion behavior of titanium and some of its alloys for biomedical and dental implant applications
[J].
The anisotropic corrosion behaviour of wire arc additive manufactured Ti-6Al-4V alloy in 3.5% NaCl solution
[J].
Microstructure, microhardness and corrosion resistance of remelted TiG2 and Ti6Al4V by a high power diode laser
[J].
Building direction dependence of corrosion resistance property of Ti-6Al-4V alloy fabricated by electron beam melting
[J].
Effects of Mo content on corrosion and tribocorrosion behaviours of Ti-Mo orthopaedic alloys fabricated by powder metallurgy
[J].
Electrochemical impedance spectroscopy (EIS) of corrosion processes on inhomogeneous surfaces
[J].
Determination of effective capacitance and film thickness from constant-phase-element parameters
[J].
Establishment of equivalent circuits from electrochemical impedance spectroscopy study of corrosion inhibition of steel by pyrazine in sulphuric acidic solution
[J].
Corrosion behaviour of a quenched and partitioned medium carbon steel in 3.5wt.%NaCl solution
[J].
Effect of Cu on the passivity of Ti-xCu (x = 0, 3 and 5wt%) alloy in phosphate-buffered saline solution within the framework of PDM-II
[J].
Is niobium more corrosion-resistant than commercially pure titanium in fluoride-containing artificial saliva?
[J].
Fluoride induced corrosion of Ti-45Nb in sulfuric acid solutions
[J].
Technical note: concerning the conversion of the constant phase element parameter Y into a capacitance
[J].
Understanding the effect of fluoride on corrosion behavior of pure titanium in different acids
[J].
Electrochemical and XPS studies of titanium for biomaterial applications with respect to the effect of hydrogen peroxide
[J].