针对海洋复杂环境因素下的金属腐蚀行为,许多研究人员已经开展了研究,研究重点多关注于温度、相对湿度(RH)[5~8]和侵蚀性离子[9~11]等环境因素的影响。近年来的研究表明光照对金属腐蚀行为的影响同样不可忽视,并且已经针对不同金属在海洋环境中的光腐蚀行为进行了研究。针对Fe[12,13]、铝合金[14]和不锈钢[15~17]等金属的研究表明了紫外光照对金属腐蚀行为的抑制作用。对于Zn[18]、Cu[10,19]和耐候钢[20,21],有研究表明,紫外光照加速其阳极溶解过程更多的和其表面形成的腐蚀产物相关。在腐蚀产物层的形成过程中,其成分和结构均有可能在光照下发生改变,光照主要通过影响腐蚀产物的组成、结构和半导体特性来影响金属的腐蚀行为[22~26]。Deng等[27]研究了在3.5% (质量分数) NaCl溶液中紫外线光照射对电镀镍和铜纳米晶体的腐蚀行为,结果表明,紫外光照可提高铜箔的耐蚀性,但对镍箔的耐蚀性有相反的影响。紫外光诱导Cu2O在Cu表面形成,Cu2O比自然形成的CuO膜更稳定,更致密。与在镍箔表面自然形成的致密NiO膜相比,紫外光照后形成的Ni2O3膜更加疏松。氧化膜的变化是导致Cu和Ni纳米晶薄膜在紫外光照射下腐蚀行为发生相反变化的主要原因。
前面的分析表明,光照对金属腐蚀的影响主要与锈层的半导体特性有关,且腐蚀机理受具体腐蚀环境下形成的锈层影响。此外,锈层的半导体特性也会受到塑性拉伸应力的影响,但塑性拉伸应力与光照之间是否存在协同作用并不清楚。40Cr钢作为结构部件常用钢材,具有较高的强度,但在海洋环境下极易发生锈蚀,因此,研究塑性拉伸应力和光照共同作用下的40Cr钢腐蚀行为对于理解40Cr钢的光化学以及应力-光化学作用机理十分必要。基于以上问题,本文采用电化学技术、X射线衍射(XRD)、Raman光谱和扫描电子显微镜(SEM)等多种技术研究了锈层的成分、结构和半导体性能,结合所有实验结果,提出了相应的腐蚀机理。
1 实验方法
本研究所用材料为40Cr钢,其名义成分(质量分数,%)为:Cu 0.085,Cr 1,Ni 0.072,Mo < 0.05,Mn 0.62,Si 0.27,C 0.42,P 0.015,S < 0.001,Fe余量。本文中测试用316不锈钢分为变形和未变形两种样品,未变形样品是尺寸为15 mm × 15 mm × 5 mm的块状样品。将尺寸为60 mm × 10 mm × 0.6 mm的片状样品,根据ASTM G30标准[32],折弯成半径为5 mm的U型弯曲试样,制备实验所用的变形样品。变形样品外表面上的总应变ε = t/2R,t为厚度,R为半径。计算得到U形试样外侧顶部的应变程度约为6%,为塑性拉伸应变。实验前,将未变形40Cr钢和6%应变40Cr钢用SiC砂纸将电极表面打磨至2000#,然后用去离子水清洗。所有工作电极都用树脂密封,只露出1 cm2测试区域。浸泡实验在3.5%NaCl溶液中进行,使用循环冷却水将溶液温度保持在(25 ± 1) ℃。
采用Autolab PGSTAT302N工作站进行电化学测试。工作电极为待测样品,对电极和参比电极分别为铂片和饱和(KCl)甘汞电极。光学性能测试光源均为Newport 94043A太阳光模拟器,其最大功率能量密度为100 mW·cm-2,入射光波长设定为400 nm。在开路电位下进行电化学阻抗谱(EIS)测试,频率范围105~10-2 Hz,正弦扰动电位为±10 mV,EIS测量数据通过Zsimp Win软件进行分析。针对40Cr钢锈层的Mott-Schottky (M-S)测试的电压范围为-0.7 V~-0.2 V,频率为1000 Hz,扰动振幅为10 mV。针对单一40Cr钢样品的极化曲线测试无需去极化,在3.5%NaCl溶液中保持20 min,待开路电位稳定后,以20 mV/min的扫描速率在相对开路电位-0.3~+0.4 V的电位范围内进行。浸泡实验在室温25 ℃下进行,浸泡后样品采用X'Pert ProXS型X射线衍射仪进行XRD测试分析。XRD的2θ角扫描范围为10°~90°,扫描速度为4 (°)/min,并使用jade 5.0软件对结果进行分析。之后使用配备波长为633 nm激光光源的Labram Raman光谱仪进一步对锈层进行成分分析。用配备能谱分析(EDS)的扫描电子显微镜(SEM,Inspect F50,FEI)观察40Cr钢腐蚀产物形貌及检测元素分布,加速电压为25 kV,工作距离为15 mm。
2 结果及讨论
2.1 极化曲线测试
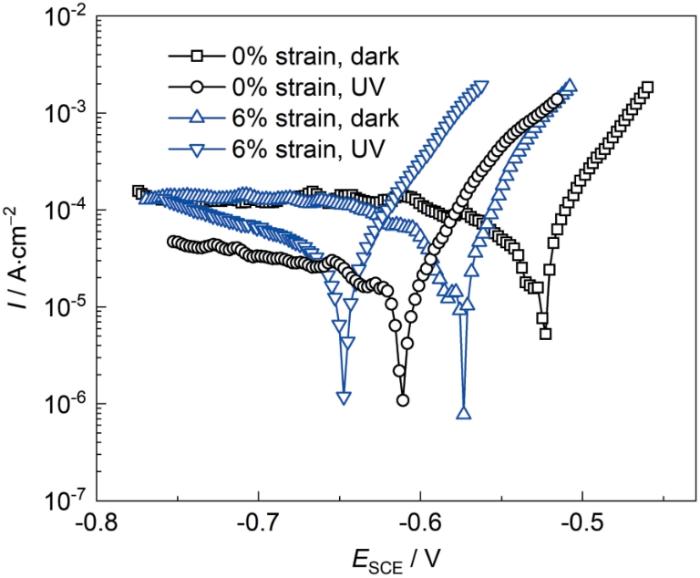
图1为未变形40Cr钢和6%应变程度40Cr钢在3.5%NaCl溶液中有无光照条件下浸泡20 min后的极化曲线。由图可知,在黑暗条件下,未变形40Cr钢的自腐蚀电位值为-0.52 V,施加6%应变后,40Cr钢的自腐蚀电位值减小至-0.57 V。在同一极化电位下,塑性变形后的40Cr钢阳极电流密度相比未变形状态明显增大,而阴极电流密度并未有明显改变。相比于黑暗条件下,开光后,未变形40Cr钢和6%应变40Cr钢的自腐蚀电位值分别负移至-0.61和-0.65 V,且光照明显影响了40Cr钢的阳极和阴极反应过程,导致同一极化电位下的40Cr钢的阳极电流密度增大,阴极电流密度减小。由此可见,紫外光照和塑性拉伸应力均能加速40Cr钢在3.5%NaCl溶液中的腐蚀,光照和塑性拉伸应力对40Cr钢电化学性能的影响可能和40Cr钢表面形成的腐蚀产物层相关。
图1
图1
0%应变和6%应变40Cr钢在3.5%NaCl溶液中有无光照条件下的极化曲线
Fig.1
Polarization curves of 0% strain and 6% strain 40Cr steel in 3.5%NaCl solution with and without illumination
2.2 表面腐蚀形貌及成分分析
图2
图2
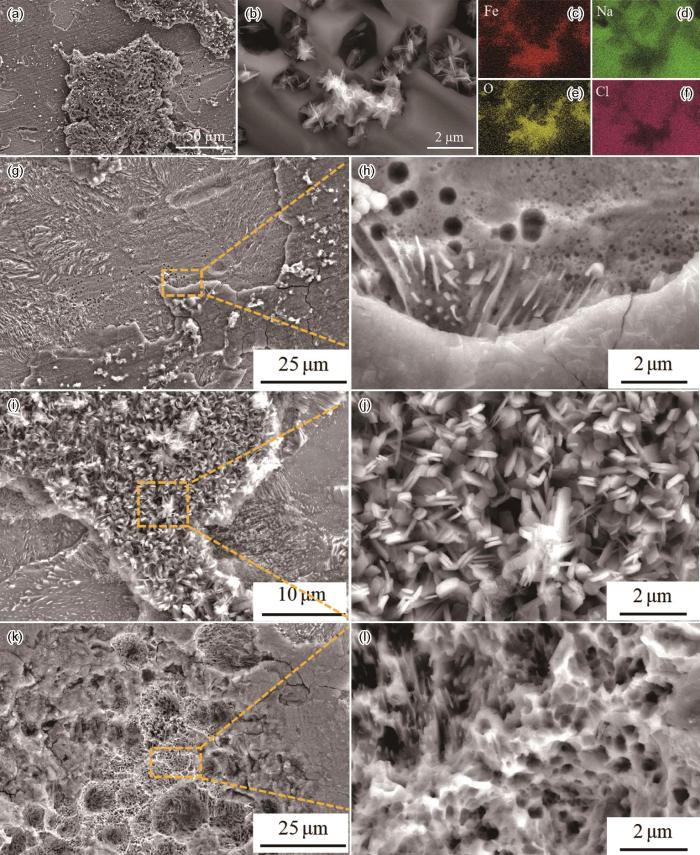
有无光照条件下0%应变和6%应变40Cr钢在3.5%NaCl溶液中浸泡120 h的表面形貌
Fig.2
Surface morphologies of 40Cr steel immersed in 3.5%NaCl solution for 120 h under different conditions: (a) 0% strain/dark, (b) 6% strain/dark, (c) 6% strain/UV
图3为有无光照条件下未变形40Cr钢在3.5% NaCl溶液中浸泡120 h后的腐蚀形貌。黑暗条件下在3.5%NaCl溶液中浸泡120 h后的未变形40Cr钢表面有明显的NaCl沉积(图3a和b),并且夹杂其中的外锈层腐蚀产物为板条状的γ-FeOOH,相应的能谱结果(图3c~f)也证明了Fe、O以及NaCl的存在。针对黑暗条件下未变形40Cr钢表面内锈层的分析如图3g所示,内锈层表面有明显的裂纹存在,从内锈层断面局部放大图(图3h)可以看出,内锈层较为致密。图3i和j为在3.5%NaCl溶液中开光条件下浸泡120 h后的未变形40Cr钢表面外锈层形貌图,其外锈层同样为板条状的γ-FeOOH,而内锈层表面除明显裂纹外还有大量的腐蚀坑存在(图3k),从腐蚀坑的局部放大图(图3l)可以看出,光照下40Cr钢表面形成的内锈层呈现蜂窝状结构。
图3
图3
不同实验条件下的40Cr钢在3.5%NaCl溶液中浸泡120 h的锈层形貌
Fig.3
Rust layer morphologies of 40Cr steel immersed in 3.5%NaCl solution for 120 h under different experimental conditions: (a-h) 0% strain/dark, (i-l) 0% strain/UV, (c-f) EDS energy spectra in Fig.3b
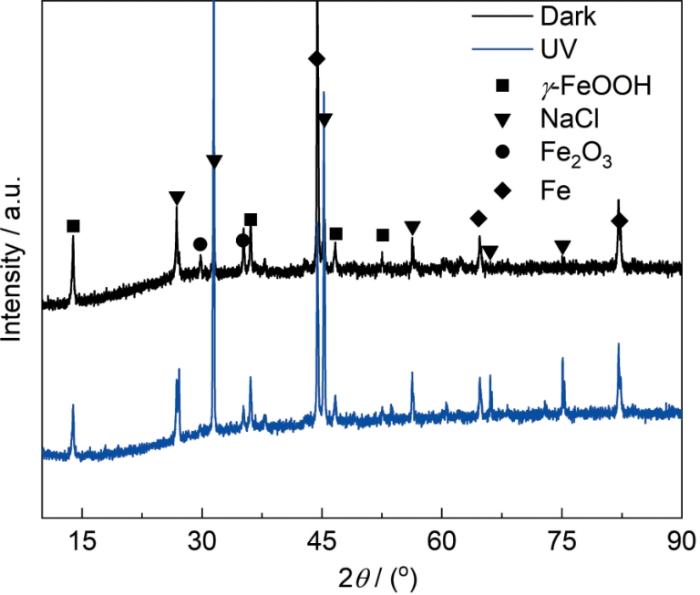
为进一步分析锈层成分,对在3.5%NaCl溶液中浸泡120 h后的40Cr钢表面腐蚀产物进行了XRD分析,结果如图4所示。未变形样品在黑暗和光照条件下形成的锈层主要成分均为γ-FeOOH和具有尖晶石结构的γ-Fe2O3,不过相比于黑暗条件,光照下40Cr钢表面锈层的XRD图中NaCl峰更加尖锐且明显,这意味着光照下形成的锈层中有更多NaCl沉积,结合前面对锈层形貌的观察可知,相比于黑暗条件,光照下40Cr钢表面更多NaCl的沉积归因于其表面形成的蜂窝状内锈层。
图4
图4
在3.5%NaCl溶液中浸泡120 h后未变形40Cr钢表面锈层的XRD
Fig.4
XRD of surface rust layer of undeformed 40Cr steel after 120 h immersion in 3.5%NaCl solution
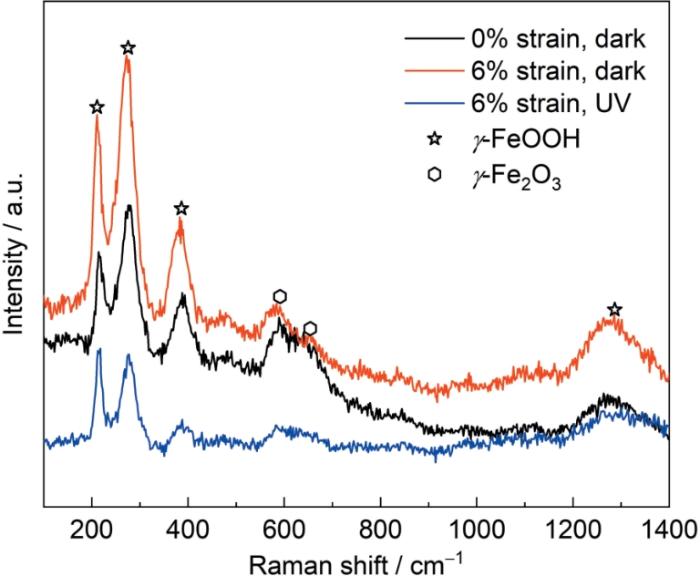
图5为初始样品以及6%应变40Cr钢在黑暗和光照条件下形成的内锈层的Raman光谱图,Raman结果表明,在此3种实验条件下形成的内锈层主要成分均为γ-FeOOH和γ-Fe2O3 。
图5
图5
在3.5%NaCl溶液中浸泡120 h后40Cr钢表面锈层的Raman图
Fig.5
Raman diagram of the surface rust layer of 40Cr steel after immersion in 3.5%NaCl solution for 120 h
2.3 电化学性能测试
为分析未变形和6%应变40Cr钢在3.5%NaCl溶液中在有无光照条件下持续浸泡120 h后的腐蚀性能,针对浸泡后的样品进行了EIS测试,结果如图6所示。EIS数据通过图7的等效电路进行拟合,其中Rs为溶液电阻,R1和Rct分别为腐蚀产物膜电阻和电荷转移电阻,Qdl为双电层,Q1用于描述腐蚀产物膜内的电荷转移过程,使用常相位角元件(CPE)代替理想电容元件[33~35]。与CPE相关的有效电容表示为Ceff = Q1/nR(1 - n)/n,其中Q和n是CPE的拟合值。表1列出了相应的阻抗拟合参数。未变形的40Cr钢在黑暗条件下的Rct值为784 Ω·cm2,在施加单一塑性拉伸应力和光照后分别降至366.9和575.7 Ω·cm2,在塑性拉伸应力和光照的协同作用下,Rct值最低,为73 Ω·cm2。由此可知,光照和塑性拉伸应力均能提高载流子的迁移速率。
图6
图6
不同实验条件下的40Cr钢在3.5%NaCl溶液中浸泡120 h后的EIS图
Fig.6
Nyquist plots (a) and Bode plots (b) of 40Cr steel after immersion in 3.5%NaCl solution for 120 h under dark without deformation, illumination without deformation, dark with deformation, and illumination with deformation conditions, respectively
表1 不同实验条件下的40Cr钢在3.5%NaCl溶液中浸泡120 h的EIS拟合结果
Table 1
| Environment | Rs / Ω·cm2 | Ceff, 1 / μF·cm-2 | n1 | R1 / Ω·cm2 | Ceff, dl / μF·cm-2 | ndl | Rct / Ω·cm2 |
|---|---|---|---|---|---|---|---|
| 0% strain, dark | 4.08 | 117.6 | 0.6 | 0.24 | 326 | 0.92 | 784 |
| 0% strain, UV | 9.17 | 28.1 | 0.59 | 0.52 | 411 | 0.85 | 575.7 |
| 6% strain, dark | 2.83 | 876.7 | 0.77 | 229.3 | 13620 | 0.81 | 366.9 |
| 6% strain, UV | 3.19 | 853.1 | 0.8 | 476.1 | 14501 | 0.87 | 73 |
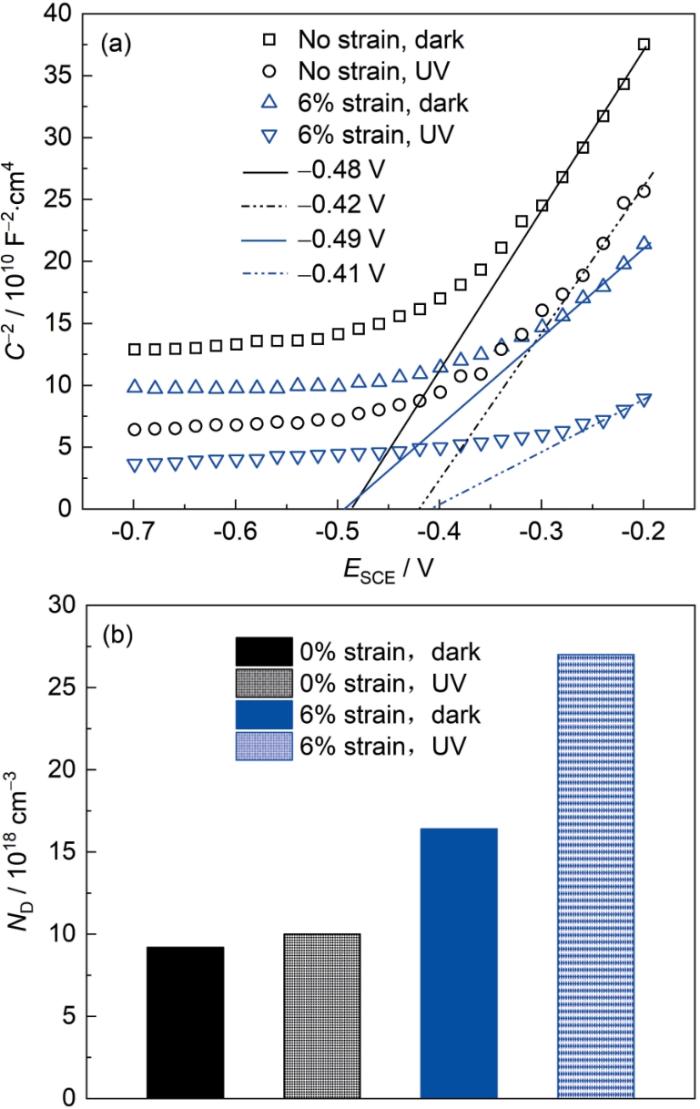
对在3.5%NaCl溶液中浸泡120 h的40Cr钢进行了M-S曲线测试,以分析40Cr钢表面锈层的半导体特性。根据Mott-Schottky基理论,n型半导体的空间电荷电容与电势之间的关系可由
其中,ε0是真空介电常数,ε是半导体的相对介电常数,ND是受体密度,e是单电子电量(1.6 × 10-19 C),Efb是平带电势,k是Boltzmann常数,T是绝对温度。
如图8a所示,0%应力40Cr钢和6%应变40Cr钢在3.5%NaCl溶液中浸泡120 h形成锈层的M-S图的线性部分均显示出正斜率,表明锈层具有n型半导体特性。根据
图8
图8
不同实验条件下的40Cr钢在3.5%NaCl溶液中浸泡120 h后的M-S图
Fig.8
M-S plots of 40Cr steel after immersion in 3.5%NaCl solution for 120 h under dark without deformation, illumination without deformation, dark with deformation, and illumination with deformation conditions, respectively (a) and the calculated donor carrier density (b)
为探索塑性拉伸应力对40Cr钢光电性能的影响,对黑暗条件下0%应力和6%应变40Cr钢在3.5%NaCl溶液中浸泡120 h形成的锈层进行光致OCP测试,结果如图9所示,开光后,0%应力和6%应变40Cr钢电位均发生正移,150 s循环光照期间0%应力和6%应变40Cr钢电位变化幅度分别为8和11 mV,这表明相对应的腐蚀产物的光伏打效应分别能够产生8和11 mV的正光电压,正的光电压表明光照能够加速40Cr钢的阳极溶解过程。此外,从实验结果可以看出,相比于未变形样品,塑性拉伸应力下40Cr钢表面形成的锈层具有更好的光电响应。
图9
图9
0%应变和6%应变40Cr钢在循环光照下的光生E-t曲线
Fig.9
photogenerated E-t curves of 0% strain and 6% strain 40Cr steels under chopped illumination
2.4 40Cr钢在塑性拉伸应力和光照共同作用下的腐蚀机理
塑性拉伸应变和光照共同作用下的40Cr钢腐蚀示意图如图10所示。光照对40Cr钢腐蚀行为的影响和其表面形成的半导体腐蚀产物的结构和性能相关,光照下外锈层的致密化能够使得单位面积内更多的半导体锈层受光激发而产生光生电子和空穴(图2),相比于黑暗条件,光照下锈层更小的电荷转移电阻表明光生电子和空穴在锈层内的迁移速率更快(表1),而光照下正的光电压的形成则表明光生空穴向金属/锈层界面迁移(图9),加速40Cr钢阳极溶解过程。此外,光照下腐蚀产物层的Fermi能级发生正移(图8),这使得40Cr钢基体阳极溶解产生的电子更多的向腐蚀产物层迁移,同时光照下40Cr钢表面形成的内锈层呈蜂窝状结构,使得更多的侵蚀性离子(Cl-)进入锈层(图4),加速基体的阳极溶解。
图10
图10
塑性应变和光照共同作用下的40Cr钢腐蚀示意图
Fig.10
Schematic diagram of corrosion of 40Cr steel under the combined effect of plastic strain and illumination
除上述讨论的光照的影响外,施加塑性拉伸应力同样能够加速40Cr钢的阳极溶解过程,这被认为更多的和其应力下基体表面电化学活性的增强有关[28]。此外,塑性拉伸应力也能够增强腐蚀产物的光电响应,进而增强光照对40Cr钢腐蚀行为的影响。因此,在塑性拉伸应力和光照共同作用下的40Cr钢具有更高的载流子浓度和更低的电荷转移电阻。
3 结论
(1) 光照下40Cr钢表面蜂窝状内锈层的形成加速了锈层内NaCl的沉积,单一光照下锈层电荷转移电阻的降低(575.7 Ω·cm2)以及锈层平带电位值的增大(-0.41 V)使得光生空穴更多的向金属/腐蚀产物界面迁移,加速40Cr钢阳极溶解。
(2) 塑性拉伸应力能够增强40Cr钢表面锈层光电响应,进而强化光腐蚀行为。在紫外光照和塑性拉伸应力共同作用下,40Cr钢表面锈层电荷转移电阻降低(73 Ω·cm2)且锈层平带电位值增大(-0.41 V),这导致其具有更高的载流子浓度(27 × 1018 cm-3)。
参考文献
Fatigue behavior of high-strength steel S135 under coupling multi-factor in complex environments
[J].
The corrosion behavior of 316 stainless steel under the cooperative effect of plastic stress and UV illumination in 3.5wt%NaCl solution
[J].
Synergistic acceleration effects of ultraviolet and salt spray on the degradation and failure of electromagnetic wave-absorbing coatings
[J].
Effects of chloride and oxygen on stress corrosion cracking of cold worked 316/316L austenitic stainless steel in high temperature water
[J].
Adsorption of water on copper, nickel, and iron
[J].In atmospheric corrosion of copper, nickel, and iron, adsorption of water affects corrosion rates. Knowledge of water adsorption and metal oxyhydroxide formation is important in understanding the atmospheric corrosion process. The quartz-crystal microbalance (QCM) technique was used to measure mass changes of copper, nickel, and iron at 0% to 100% relative humidity (RH) and 7°C to 90°C under nitrogen (N2) and air environments. Less water was adsorbed on copper, nickel, and iron that formed oxides than on gold. The amount of water adsorption was similar on copper, nickel, and iron under N2 and air carrier gases. Shapes of isotherms suggested physical adsorption, capillary condensation, and pore filling occurred on all metals and were more significant at higher temperatures. Adsorption isotherms were Type III and Type IV according to the Brunauer-Emmett-Teller (BET) classification.
The evolution of the adsorbed solution layer during atmospheric corrosion and its effects on the corrosion rate of copper
[J].
Electrochemical measurements of time-of-wetness and atmospheric corrosion rates
[J].An atmospheric corrosion monitor has been developed which allows monitoring of time-of-wetness and corrosion behavior in outdoor exposure. This device is being used in exposure studies in Southern California and has also been applied to laboratory studies of atmospheric corrosion, in which the effects of salt particles, gaseous pollutants, impurities in rust, temperature, and relative humidity are evaluated.
A study of the wetting of metal surfaces in order to understand the processes controlling atmospheric corrosion
[J].
Comparative study on corrosion behavior of Cu and Sn under UV light illumination in chloride-containing borate buffer solution
[J].
Atmospheric corrosion of Cu during constant deposition of NaCl
[J].
Corrosion characteristics of carbon steel in simulated marine atmospheres
[J].
基于海洋大气环境因素影响下的碳钢腐蚀特征研究
[J].
Illumination effects on the stability of the passive film on iron
[J].
Hole annihilation vs. induced convection: breakdown of different contributions to the photocorrosion mechanism of oxide-covered iron
[J].
Uniform and pitting corrosion events induced by SCN- anions on Al alloys surfaces and the effect of UV light
[J].
Influence of uv light on passive behavior of the 304 stainless steel in acid solution
[J].
Photo-inhibition of pitting corrosion on types 304 and 316 stainless steels in chloride-containing solutions
[J].
Influence of uv light on the passive behaviour of SS316-effect of prior illumination
[J].
Effect of UV illumination on the NaCl-induced atmospheric corrosion of pure zinc
[J].
Study on the mechanism of the photoelectrochemical effect on the initial NaCl-induced atmospheric corrosion process of pure copper exposed in humidified pure air
[J].
The role of UV illumination on the initial atmospheric corrosion of 09CuPCrNi weathering steel in the presence of NaCl particles
[J].
The role of the photovoltaic effect of γ-FeOOH and β-FeOOH on the corrosion of 09CuPCrNi weathering steel under visible light
[J].
Physicochemical and photocatalytic activity of needle-like γ-FeOOH/Halloysite
[J].
Effect of γ-FeOOH and γ-Fe2O3 on the corrosion of Q235 carbon steel under visible light
[J].
The evolution of the corrosion mechanism of structural steel exposed to the urban industrial atmosphere for seven years
[J].The corrosion mechanism and characteristics of steel in typical atmospheric environments directly affect the rationality of corrosion protection methods. This study investigates the corrosion evolution law of Q235 steel that has been exposed to the urban industrial atmosphere for seven years. The mass loss is used for corrosion dynamics analysis. The rust layers have been characterized by SEM, EDS, and XRD. Finally, the corrosion mechanism was analyzed through a combination of electrochemical methods, corrosion kinetics, and rust layer characteristics. The mass loss results indicate that a two-stage corrosion power function law can still effectively describe the corrosion rate of a seven-year exposure that complies with the power function law. The short-term corrosion results fail to fully reflect the corrosion performance of Q235 steel. The typical morphological structures of γ-FeOOH and α-FeOOH are identified, and the rust layers change from a loose and flat form to a granular and, finally, compact into a smooth surface. The crystalline phases of the rust layers include α-FeOOH, γ-FeOOH, Fe3O4/γ-Fe2O3 and α-Fe2O3. Corrosion products in the initial period are mainly γ-FeOOH, followed by α-FeOOH, and a small amount of Fe3O4/γ-Fe2O3. With the increase in exposure time, α-FeOOH and Fe3O4/γ-Fe2O3 in the rust layer increase. SO2 and Fe3O4/γ-Fe2O3 are the primary factors accelerating steel corrosion. During the first three years of atmospheric corrosion, the primary corrosion mechanism was governed by the acid cycle reaction mechanism. However, from the fifth year of atmospheric corrosion, oxygen-absorbing corrosion began to gradually dominate, specifically oxygen-absorbing corrosion.
The influence of copper and chromium on the semiconducting behaviour of passive films formed on weathering steels
[J].
The function of Cr on the rust formed on weathering steel performed in a simulated tropical marine atmosphere environment
[J].
Influence of UV light irradiation on the corrosion behavior of electrodeposited Ni and Cu nanocrystalline foils
[J].Influence of ultraviolet (UV) light irradiation on the corrosion behavior of electrodeposited Ni and Cu nanocrystalline foils in 3.5% NaCl solution was studied by means of electrochemical methods, electron work function (EWF) analysis, and characterization with atomic force microscopy (AFM) and X-ray photoelectron spectroscopy (XPS). It was demonstrated that the influence of solar light on corrosion of the metals was non-negligible, which could be very different for different materials. The UV light irradiation resulted in an increase in corrosion resistance of the Cu foil but showed an opposite influence on that of the Ni foil. Based on surface state analysis, it was concluded that the UV irradiation altered the surface oxide films. The UV light induced the formation of Cu2O on Cu, which is more stable and compacted than naturally formed CuO film. However, the UV light accelerated the formation of Ni2O3, which is loose, porous and brittle, compared to naturally formed NiO on Ni. The changes in oxide films were responsible for the opposite variations in the corrosion behavior of the Cu and Ni nanocrystalline foils caused by the UV light irradiation.
Corrosion of high-strength steel in 3.5%NaCl solution under hydrostatic pressure: understanding electrochemical corrosion with tensile stress coupling
[J].
Corrosion of high-strength steel in 3.5%NaCl solution under hydrostatic pressure: Initial corrosion with tensile stress coupling
[J].
Research progress on irradiation assisted stress corrosion cracking behavior and mechanism of austenitic steel
[J].
奥氏体钢辐照促进应力腐蚀开裂行为机制的研究进展
[J].奥氏体不锈钢是压水堆堆内构件的主要材料,服役过程中在中子辐照、拉应力和一回路高温高压水的联合作用下可发生辐照促进应力腐蚀开裂(IASCC),并导致部件断裂失效,从而影响堆芯构件结构完整性,已经成为影响核电站安全、经济运行的关键问题之一。本文通过调研总结,综述了辐照致IASCC裂纹萌生、扩展及敏感性变化的行为规律,分析了局部变形、晶界氧化、辐照硬化、辐照诱导偏析(RIS)、辐照肿胀及辐照蠕变、辐照诱导相变等主要IASCC机制,总结了辐照后退火(PIA)在IASCC机制研究中的应用,并讨论了IASCC行为机理研究的发展趋势,以期为中子辐照IASCC的行为机理研究提供参考。
Rusting behavior of a deformed 450 MPa-grade weathering steel in 5wt.%NaCl salt spray
[J].
Influence of outer rust layers on corrosion of carbon steel and weathering steel during wet-dry cycles
[J].
The atmospheric corrosion kinetics of low carbon steel in a tropical marine environment
[J].
The role of rusts in corrosion and corrosion protection of iron and steel
[J].