热障涂层(Thermal barrier coatings,TBCs)作为一种有效的高温热防护技术,其能够有效降低涡轮发动机热端部件的表面温度并对基体合金起到一定的保护作用[1,2]。Y2O3稳定的ZrO2(YSZ)作为传统的热障涂层材料已获得广泛的应用。然而,由于工作温度的不断提升(> 1250 ℃),YSZ面临着高温烧结、相变以及熔融硅酸盐环境沉积物(CaO-MgO-Al2O3-SiO2,CMAS)腐蚀等一系列问题[3~6],从而使涂层热导率升高,同时伴随一定的体积变化而加速涂层失效,已逐渐不能适应高温防护要求。因此,开发新型高温防护热障涂层已成为推动国家“两机”技术纵深发展的迫切需求,具有重要的战略和经济意义。
高熵陶瓷(High-entropy ceramics,HECs)是近年来发展的一种新型陶瓷材料[7,8]。由于多种半径、质量差异较大的离子随机占据晶格位置,从而使材料产生显著的高熵效应、晶格畸变效应、迟滞扩散效应以及“鸡尾酒”效应(统称“高熵效应”),这使得HECs具有比单组分材料更加优异的性能[9]。热导率作为高温隔热陶瓷材料的关键性能之一,降低热导率是发展新型TBCs技术的首要目标。近年来,国内外研究者在稀土锆酸盐高熵陶瓷(High entropy rare-earth zirconate,HE-REZ)材料的合成、热物理性能及高温稳定性等方面已取得了一些积极的成果[10]。如Zhao等[11]合成了(La0.2Ce0.2Nd0.2Sm0.2Eu0.2)2Zr2O7高熵陶瓷,由于体系内部的“迟滞扩散”效应,其晶粒生长速率远低于单一的La2Zr2O7(LZ),且室温热导率仅为0.76 W·m-1·K-1。
此外,随着航空发动机推重比的进一步提升,CMAS腐蚀已逐渐成为影响TBCs可靠性的关键因素之一[12],如何提高TBCs材料的抗CMAS腐蚀能力亦是TBCs领域的研究热点和难点。Tu等[13]制备了(La0.2Nd0.2Sm0.2Eu0.2Gd0.2)2Zr2O7高熵陶瓷和La2Zr2O7陶瓷,研究表明高熵陶瓷比单组元陶瓷具有更好的抗CMAS腐蚀性能;Yan等[14]制备的(Gd0.2Y0.2Er0.2Tm0.2Yb0.2)2Zr2O7高熵陶瓷与CMAS的反应速率缓慢,具有优异的抗CMAS腐蚀性能。Tian等[15]通过调整不同组元的含量设计出8种(LaSmGdErLu)2Zr2O7高熵陶瓷,结果显示其相组成由烧绿石相向萤石相逐渐转变,且烧绿石相和萤石相共存时,其热导率显著降低,而HE-REZ的抗CMAS腐蚀性能随萤石相含量的增加而显著提升。此外,已有研究表明大离子半径的稀土元素更容易与CMAS反应生成致密的磷灰石阻挡层,从而可以延缓CMAS的持续渗入[16,17]。为进一步明确稀土锆酸盐高熵陶瓷的CMAS腐蚀机制,本文选择大离子半径的La、Nd和小离子半径的Tm、Yb、Lu为基本组元,设计并合成一种等摩尔、双相结构的高熵陶瓷材料,表征和分析了其微观组织结构和相组成及其在1300 ℃熔融CMAS中的腐蚀行为。
1 实验方法
将纯度为99.9% (质量分数)的La2O3、Nd2O3、Tm2O3、Yb2O3、Lu2O3和ZrO2按稀土原子与Zr原子的摩尔比为1∶1进行称量,其中稀土元素的摩尔比为(La∶Nd∶Tm∶Yb∶Lu = 0.2∶0.2∶0.2∶0.2∶0.2)。以无水乙醇为溶剂,ZrO2为磨球(球料质量比为3∶1),在行星球磨机中以380 r/min球磨12 h。随后将所得浆料在120 ℃干燥10 h并研磨后在1500 ℃的马弗炉中烧结5 h得到HE-REZ前驱体粉末。将HE-REZ前驱体粉末再次研磨后装入石墨模具中,然后置于热压炉中在1600 ℃、30 MPa压力下烧结0.5 h,即可得到致密的高熵陶瓷块体。按照30 mg/cm2的负载量将预先熔融并研磨后的CMAS (33CaO-9MgO-13AlO1.5-45SiO2)粉末均匀的涂覆在HE-REZ表面,然后将覆有CMAS的HE-REZ块体置于马弗炉中,以3 ℃/min升至1300 ℃并保温不同时间后降至室温。最后,对所得样品进行相组成和微观结构表征。采用X射线衍射仪(XRD,D8 Advance)对HE-REZ样品进行物相分析,Cu靶,Kα射线,λ = 0.15406 nm,扫描速率为10 (°)/min。采用扫描电子显微镜(SEM,Gemini 300)分析和表征HE-REZ样品在CMAS腐蚀前后的微观结构和组成。
2 结果与讨论
2.1 物相分析
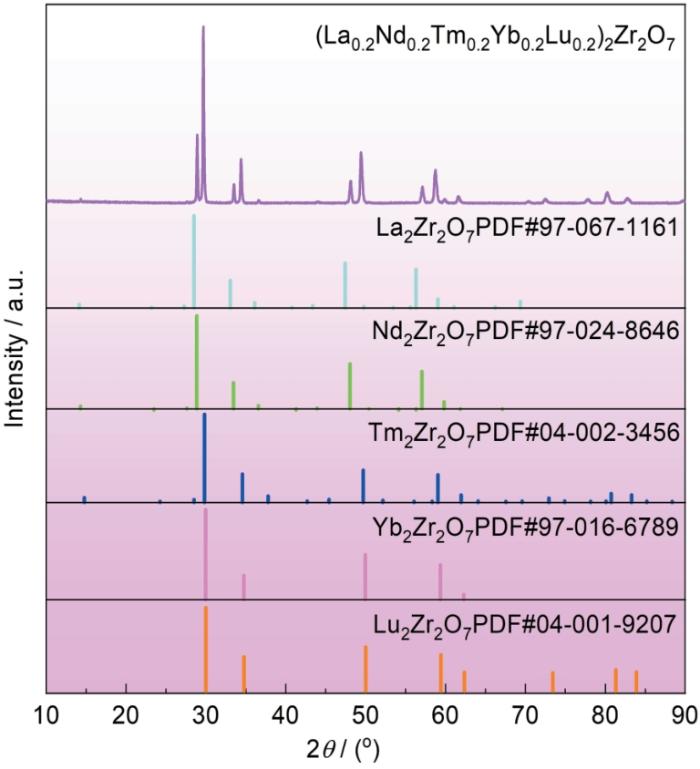
图1为稀土氧化物和ZrO2粉体在1500 ℃经高温固相反应5 h得到粉体的XRD图谱以及不同单组元稀土锆酸盐的标准XRD图谱。由图1可见,(La0.2Nd0.2Tm0.2Yb0.2Lu0.2)2Zr2O7样品为萤石型(F)和烧绿石型(P)的双相结构。这是由于A2B2O7类材料的晶体结构主要由A位与B位阳离子平均半径的比值(rratio = rA/rB)决定。当rratio > 1.46时,材料为烧绿石结构(Pyrochlore,Fd-3m 227);当rratio ≤ 1.46时为缺陷型萤石结构(Defect fluorite,Fm-3m 225)[18]。计算得到(La0.2Nd0.2Tm0.2Yb0.2Lu0.2)2Zr2O7样品中的RE3+/Zr4+为1.45,比较接近临界值1.46,故有可能为烧绿石和萤石两相共存。此外,稀土锆酸盐的相组成除与RE3+/Zr4+值有关外,还与所选择稀土离子的半径差异值密切相关[19,20]。Yang等[21]研究表明,稀土锆酸盐高熵陶瓷形成单/双相结构的临界半径差异值约为5.2%,而(La0.2Nd0.2Tm0.2Yb0.2Lu0.2)2Zr2O7样品中所选择稀土离子的半径差异值为6.5%,因此,该陶瓷为缺陷萤石和烧绿石型的双相结构。
图1
图1
(La0.2Nd0.2Tm0.2Yb0.2Lu0.2)2Zr2O7高熵陶瓷粉体的XRD图谱与单组元RE2Zr2O7 (RE = La、Nd、Tm、Yb、Lu)的XRD标准图谱
Fig.1
XRD patterns of (La0.2Nd0.2Tm0.2Yb0.2Lu0.2)2Zr2O7 high-entropy ceramic powders and XRD standard patterns of single-component RE2Zr2O7 (RE = La, Nd, Tm, Yb, Lu)
2.2 微观结构表征
图2为(La0.2Nd0.2Tm0.2Yb0.2Lu0.2)2Zr2O7高熵陶瓷粉体在1600 ℃热压0.5 h所得块体的表面微观形貌及元素面分布图。结果显示,样品出现了明显的两相区,如图2a及对应的元素面扫描分布图所示。其中,较小的晶粒(衬度较深,如图2b所示)富La和Nd,EDS能谱分析结果表明其化学组成为(La0.45Nd0.3Tm0.08Yb0.09Lu0.08)2Zr2O7;而较大的晶粒(衬度较浅)富Tm、Yb和Lu,其化学组成为(La0.15Nd0.17Tm0.23Yb0.22Lu0.23)2Zr2O7。此外,采用XRD精修计算得到样品的相组成(质量分数)为70.15%的萤石型和29.85%的烧绿石型结构,因此,图中晶粒尺寸较小的物相可能为烧绿石结构,而尺寸较大的缺陷萤石结构。此外,本文所制备的(La0.2Nd0.2Tm0.2Yb0.2Lu0.2)2Zr2O7为非均匀的双相结构,与文献[22,23]中所报道均匀的双相结构不同。
图2
图2
1600 ℃热压0.5 h制备的(La0.2Nd0.2Tm0.2Yb0.2Lu0.2)2Zr2O7高熵陶瓷的表面SEM图和对应的EDS元素面扫描图
Fig.2
SEM images (a, b) and EDS element mappings of (La0.2Nd0.2Tm0.2Yb0.2Lu0.2)2Zr2O7 high-entropy ceramic heat-pressed at 1600 ℃ for 0.5 h
2.3 CMAS腐蚀
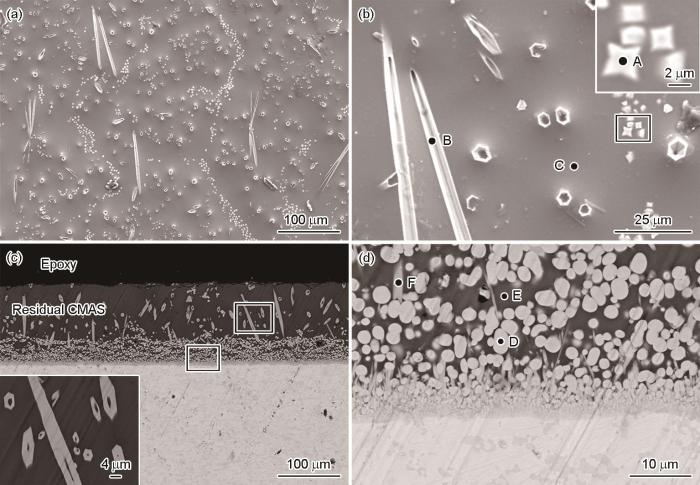
图3为(La0.2Nd0.2Tm0.2Yb0.2Lu0.2)2Zr2O7高熵陶瓷样品在1300 ℃熔融CMAS中腐蚀1 h后表面和截面的SEM图。由图可见,在CMAS的表面析出大量类球形的颗粒(A点)和少量的空心针状结构(B点)。结合表1中的EDS能谱分析结果和文献[24,25]报道,A点为稀土元素稳定的(RE, Ca)-ZrO2,如图3b插图所示,而B点为磷灰石型Ca2RE8(SiO4)6O2结构[26]。此外,在熔融CMAS中也检测到少量的稀土和Zr (C点),表明高熵陶瓷在CMAS的腐蚀作用下发生了溶解。因此,A点和B点可能为溶解的稀土和Zr在熔融CMAS表面的析出物。由图3c的截面SEM图可见,高熵陶瓷与CMAS反应速率较快,1 h后其反应层厚度已达~44 μm,但反应层较为疏松。结合表1中的EDS能谱分析结果,图中衬度较浅的类球形颗粒为(RE, Ca)-ZrO2(图3d中D点),而衬度较深的为残余CMAS (图3d中E点),除此之外,还分布有少量的磷灰石型结构(图3d中F点)。值得注意的是,残余CMAS层中亦观察到大量纵向和横向分布的空心针状磷灰石结构如图3c中的插图所示。分析其可能是由于CMAS中溶解的稀土元素与Ca和Si原位反应形成的磷灰石型Ca2RE8(SiO4)6O2结构。
图3
图3
(La0.2Nd0.2Tm0.2Yb0.2Lu0.2)2Zr2O7高熵陶瓷在1300 ℃熔融CMAS腐蚀1 h后的表面和截面SEM图像
Fig.3
Surface (a, b) and cross sectional SEM images (c, d) of (La0.2Nd0.2Tm0.2Yb0.2Lu0.2)2Zr2O7 high-entropy ceramic after exposure to molten CMAS at 1300 ℃ for 1 h
表1 图3中标记位置的EDS能谱分析结果 (atomic fraction / %)
Table 1
| Position | La | Nd | Tm | Yb | Lu | Total Re | Zr | Ca | Mg | Al | Si |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Point A | 0.2 | 1.4 | 6.6 | 7.1 | 7.8 | 23.1 | 73.6 | 3.3 | - | - | - |
| Point B | 10.9 | 10.4 | 5.1 | 4.7 | 4.4 | 35.5 | - | 24.9 | - | - | 39.6 |
| Point C | 0.9 | 0.7 | 0.7 | 0.7 | 0.7 | 3.7 | 0.7 | 29.1 | 8.9 | 17.8 | 39.8 |
| Point D | - | 1.5 | 7.8 | 8.0 | 8.0 | 25.3 | 71.8 | 2.9 | - | - | - |
| Point E | 1.3 | - | - | - | - | 1.3 | - | 28.4 | 9.8 | 40.9 | 19.6 |
| Point F | 6.3 | 5.5 | 3.4 | 3.1 | 2.9 | 21.2 | 4.1 | 21.5 | 4.6 | 9.3 | 39.3 |
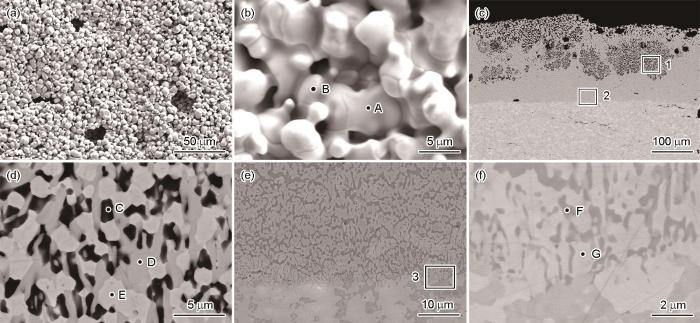
图4为(La0.2Nd0.2Tm0.2Yb0.2Lu0.2)2Zr2O7高熵陶瓷样品在1300 ℃熔融CMAS中腐蚀5 h后的表面和截面SEM图。由图4a和b可见,随着腐蚀时间的延长,残余CMAS表面析出的针状磷灰石型结构和类球形ZrO2的数量显著增加。图4c的截面SEM图表明,腐蚀层厚度亦显著增加,其中较为致密反应层的厚度约为83 μm,且残余CMAS已不连续。此外,反应层上方的残余CMAS (图4d)中出现了两种衬度的CMAS。结合EDS能谱分析(表2)结果,其中衬度较深的物相(图4d中A点)为残余CMAS,而衬度较浅的区域(图4d中B点)为溶解有一定数量稀土元素的CMAS。与腐蚀1 h后的结果类似,CMAS腐蚀5 h后的产物主要有交替分布的类球形的ZrO2和针状、空心的磷灰石型结构,且两者之间填充着一定量的CMAS,如图4e中的C点所示。此外,分析B点和C点的能谱结果可知,La和Nd在CMAS中的溶解速度略高于Tm、Yb和Lu。值得注意的是,虽然本文设计的高熵陶瓷为等摩尔,且具有萤石和烧绿石的双相结构,如图4f所示,其中衬度较深的为富La和Nd的烧绿石结构(图4f中P点),而衬度较浅的为富Tm、Yb和Lu元素的萤石结构(图4f中F点),但在腐蚀前沿并未观察到不均匀的腐蚀现象,表明(La0.2Nd0.2Tm0.2Yb0.2Lu0.2)2Zr2O7高熵陶瓷的熔融CMAS腐蚀行为与其相组成无关。
图4
图4
(La0.2Nd0.2Tm0.2Yb0.2Lu0.2)2Zr2O7高熵陶瓷在1300 ℃熔融CMAS腐蚀5 h后的表面和截面SEM图
Fig.4
Surface (a, b) and cross-sectional (c-f) SEM images of (La0.2Nd0.2Tm0.2Yb0.2Lu0.2)2Zr2O7 high-entropy ceramic after exposure to molten CMAS at 1300 ℃ for 5 h, Fig.4d and 4e were high magnification images of region 1 and region 2 in Fig.4c, respectively
表2 图4中标记区域的EDS能谱分析结果
Table 2
| Position | La | Nd | Tm | Yb | Lu | Total RE | Zr | Ca | Mg | Al | Si |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Point A | - | - | - | - | - | - | - | 37.8 | 11.0 | 16.9 | 34.3 |
| Point B | 1.8 | 1.2 | 1.2 | 1.4 | 1.2 | 6.8 | - | 19.6 | 10.6 | 22.0 | 41.0 |
| Point C | 3.7 | 3.5 | 2.2 | 1.9 | 1.7 | 13 | 3.7 | 28.5 | 6.9 | 16.1 | 31.8 |
图5为(La0.2Nd0.2Tm0.2Yb0.2Lu0.2)2Zr2O7高熵陶瓷样品在1300 ℃熔融CMAS中腐蚀10 h后的表面和截面SEM图。由图5a和b可见,腐蚀产物主要为类球形的ZrO2及少量的针状、空心磷灰石型结构,且在表面已观察不到任何的残余CMAS。由图5c的截面SEM测得反应层厚度为~132 μm。由于CMAS已经基本消耗殆尽,因此在CMAS充足的情况下,高熵陶瓷样品在1300 ℃腐蚀10 h后的反应层厚度应该远大于132 μm。此外,腐蚀产物层中针状磷灰石型产物和类球形ZrO2交替分布,两者之间衬度较深的物质经EDS能谱分析(表3)表明其主要为残余CMAS,如A点和B点所示。但腐蚀前沿的CMAS中溶解有大量的稀土元素,而反应层最外层的残余CMAS中未检测到稀土元素。这可能是由于最外层的CMAS中溶解的稀土元素已完全转化为磷灰石相结构或(RE, Ca)-ZrO2。
图5
图5
(La0.2Nd0.2Tm0.2Yb0.2Lu0.2)2Zr2O7高熵陶瓷在1300 ℃熔融CMAS腐蚀10 h后的表面和截面SEM图
Fig.5
Surface (a, b) and cross-sectional SEM images (c-f) of (La0.2Nd0.2Tm0.2Yb0.2Lu0.2)2Zr2O7 high-entropy ceramic after exposure to molten CMAS at 1300 ℃ for 10 h, Fig.5d and 5e were high magnification images of region 1 and region 2 in Fig.5c, respectively
表3 图5中标记区域的EDS能谱分析结果
Table 3
| Position | La | Nd | Tm | Yb | Lu | Total RE | Zr | Ca | Mg | Al | Si |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Point A | - | - | - | - | - | - | - | 37.4 | 8.3 | 23.2 | 31.1 |
| Point B | 9.2 | 8.5 | 4.7 | 4.3 | 3.8 | 30.5 | 6.9 | 12.5 | 6.9 | 10.1 | 33.1 |
图6为(La0.2Nd0.2Tm0.2Yb0.2Lu0.2)2Zr2O7高熵陶瓷样品在1300 ℃熔融CMAS中腐蚀20 h后的表面和截面SEM图。与CMAS腐蚀10 h后的表面SEM对比可见,20 h后表面的类球形ZrO2有一定的烧结。对图6b中所标记的点进行能谱分析如表4所示,结果显示其主要为Tm、Yb、Lu和Nd,以及少量的Ca稳定的ZrO2。然而,表面的(RE, Ca)-ZrO2中并未检测到La,可能是由于La更容易与CMAS中的Ca和Si反应形成磷灰石型结构。图6c~f为样品的横截面SEM图,测得反应层厚度约为192 μm。与CMAS腐蚀10 h相比,反应层厚度进一步增加,这是由于腐蚀前沿的磷灰石和ZrO2之间残余CMAS (图5f)的进一步渗入、反应导致的。另外,随着腐蚀时间由10 h增加到20 h,反应层致密度也进一步增加,表明在CMAS消耗殆尽的情况下,腐蚀反应层发生烧结。烧结后形成部分“葡萄串”状的产物,结合EDS能谱分析结果可以推测,衬度较深(图6d中C点)的物相为MgAl2O4尖晶石[27,28],衬度较浅(图6d中D点)的物相为磷灰石型Ca2RE8(SiO4)6O2结构,而衬度最浅的物相为(RE, Ca)-ZrO2。对比D和E点的能谱结果可以发现,La和Nd,特别是La富集于磷灰石相中,而Tm、Yb和Lu富集于ZrO2中。反应层中除“葡萄串”状的产物外,其余部分均发生烧结,如图6e和f所示。其中,衬度较深(图6f中F点)的物相为磷灰石型Ca2RE8(SiO4)6O2结构,而衬度较浅(G点)的物相为(RE, Ca)-ZrO2。稀土元素在两相中的分布规律刚好相反(图7),进一步表明大离子半径的La和Nd容易与CMAS中的Ca和Si反应形成磷灰石型结构,而小离子半径的Tm、Yb和Lu留在ZrO2中并与Ca形成了(RE, Ca)-ZrO2。这可能与稀土氧化物的光学碱度有关,据报道稀土氧化物的光学碱度随其离子半径的减小而逐渐减小,而CMAS偏碱性,故相对偏酸性的重稀土氧化物更易与其反应形成磷灰石型结构[29~31]。
图6
图6
(La0.2Nd0.2Tm0.2Yb0.2Lu0.2)2Zr2O7高熵陶瓷在1300 ℃熔融CMAS腐蚀20 h后的表面和截面SEM图
Fig.6
Surface (a, b) and cross-sectional SEM images (c-f) of (La0.2Nd0.2Tm0.2Yb0.2Lu0.2)2Zr2O7 high-entropy ceramic after exposure to molten CMAS at 1300 ℃ for 20 h, Fig.6d, 6e and 6f were high magnification images of region 1, region 2 in Fig.6c and region 3 in Fig.6e, respectively
表4 图6中标记区域的EDS能谱分析结果
Table 4
| Position | La | Nd | Tm | Yb | Lu | Total RE | Zr | Ca | Mg | Al | Si |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Point A | 0 | 2.0 | 8.0 | 8.8 | 8.3 | 27.1 | 69.8 | 3.1 | 0 | 0 | 0 |
| Point B | 0 | 3.0 | 8.7 | 8.4 | 7.9 | 28.0 | 67.5 | 4.5 | 0 | 0 | 0 |
| Point C | 0.6 | 0.8 | 0 | 0 | 0 | 1.4 | 0 | 2.3 | 30.2 | 66.1 | 0 |
| Point D | 15.8 | 14.4 | 6.5 | 6.0 | 5.3 | 48.0 | 0 | 14.4 | 0 | 0 | 37.6 |
| Point E | 0 | 2.1 | 9.5 | 9.8 | 9.5 | 30.9 | 66.5 | 2.6 | 0 | 0 | 0 |
| Point F | 21.5 | 16.0 | 5.6 | 5.2 | 5.4 | 53.7 | 0 | 12 | 0 | 0 | 34.3 |
| Point G | 3.3 | 6.8 | 9.1 | 12.9 | 15.9 | 48.0 | 49.9 | 2.1 | 0 | 0 | 0 |
图7
图7
5种稀土元素在磷灰石型结构和ZrO2中的含量
Fig.7
Contents of La, Nd, Tm, Yb and Lu in apatite type phase and ZrO2
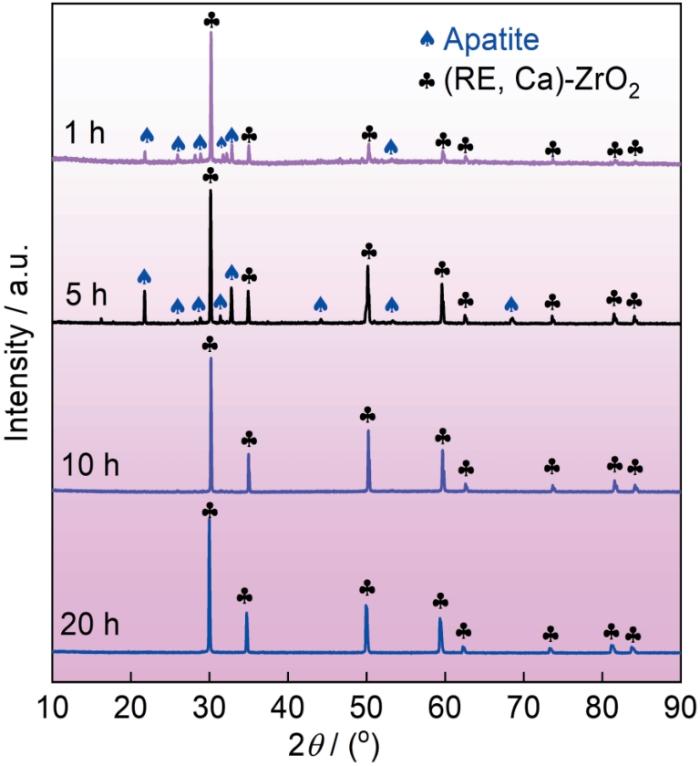
图8为(La0.2Nd0.2Tm0.2Yb0.2Lu0.2)2Zr2O7高熵陶瓷在1300 ℃熔融CMAS中腐蚀不同时间后的表面XRD图谱。结果显示,CMAS腐蚀1 h后样品表面主要由磷灰石型Ca2RE8(SiO4)6O2结构和(RE, Ca)-ZrO2。随腐蚀时间延长至5 h,表面依然由磷灰石型Ca2RE8(SiO4)6O2结构和(RE, Ca)-ZrO2组成。但是磷灰石型结构的峰强度有所增加,表明其数量较1 h更多。然而,腐蚀10和20 h后,样品表面均仅检测到(RE, Ca)-ZrO2。上述检测结果与前面的SEM结果相一致,进一步表明(La0.2Nd0.2Tm0.2Yb0.2Lu0.2)2Zr2O7高熵陶瓷在1300 ℃与熔融CMAS反应后的腐蚀产物为磷灰石型Ca2RE8(SiO4)6O2结构和(RE, Ca)-ZrO2。
图8
图8
(La0.2Nd0.2Tm0.2Yb0.2Lu0.2)2Zr2O7高熵陶瓷在1300 ℃熔融CMAS中腐蚀不同时间后的表面XRD图谱
Fig.8
XRD patterns of (La0.2Nd0.2Tm0.2Yb0.2Lu0.2)2Zr2O7 high-entropy ceramic after exposure to molten CMAS at 1300 ℃ for different time
2.4 CMAS腐蚀机理分析
由于本文制备的(La0.2Nd0.2Tm0.2Yb0.2Lu0.2)2Zr2O7高熵陶瓷块体的致密度大于98%,故熔融CMAS向陶瓷内部的直接渗透基本可以忽略。但上述研究表明高熵陶瓷在1300 ℃的熔融CMAS中发生了严重的腐蚀溶解,故其腐蚀机制可能为高熵陶瓷与腐蚀介质(即CMAS中的活性组分)间的化学反应,其详细腐蚀机制可总结如下:
首先,当体系的温度高于CMAS的熔点(约1250 ℃)时,CMAS会完全铺展于高熵陶瓷表面[32]。同时,高熵陶瓷中的部分稀土和少量的Zr溶解在CMAS中(图3中C点)。溶解的部分稀土元素与CMAS中的Ca和Si反应形成针状、空心的磷灰石型Ca2RE8(SiO4)6O2结构(图3中B和F点);由于表面氧势较高,另一部分溶解在CMAS中的稀土和Zr与其中少量的Ca反应形成形状规则的稀土与Ca共同稳定的(RE, Ca)-ZrO2 (图3中A点)。由于高熵陶瓷中的部分稀土元素和少量Zr溶解在CMAS中,剩余的稀土元素则留在ZrO2中并与CMAS中的Ca反应形成稀土与Ca共同稳定的类球形(RE, Ca)-ZrO2 (图3中D点)。
随腐蚀时间进一步延长至10 h,表面的熔融CMAS基本消耗殆尽,其最终的腐蚀产物为稀土与Ca共同稳定的类球形(RE, Ca)-ZrO2及少量的针状磷灰石型Ca2RE8(SiO4)6O2结构(图5a和b)。然而,腐蚀前沿的磷灰石和ZrO2之间还存在少量的CMAS(图5中B点),其会继续沿磷灰石和ZrO2间的空隙向内渗透,使高熵陶瓷发生溶解。因此,随腐蚀时间的延长,反应层厚度略有增加(从10 h的132 μm增加到20 h的192 μm)。然而,当反应层中所有的CMAS均消耗完全(主要为Ca和Si)时,磷灰石型结构和ZrO2之间逐渐烧结致密化(图6b~e)。同时,由于CMAS中Ca和Si的消耗,剩余的Mg和Al在高温烧结形成MgAl2O4尖晶石(图6中C点)[27],并分布于磷灰石和ZrO2之间(图6d),形成类似“葡萄串”状的结构(图6c)。
此外,由于(La0.2Nd0.2Tm0.2Yb0.2Lu0.2)2Zr2O7高熵陶瓷在熔融CMAS中的腐蚀产物为磷灰石型Ca2RE8(SiO4)6O2结构和(RE, Ca)-ZrO2,故可推测磷灰石型Ca2RE8(SiO4)6O2结构的形成是CMAS腐蚀的驱动力。另外,图7所示高熵陶瓷中的稀土元素在磷灰石型结构和ZrO2中的分布结果表明,大离子半径的La和Nd容易与CMAS中的Ca和Si反应形成磷灰石型结构,而小离子半径的Tm、Yb和Lu留在ZrO2中并与Ca形成了(RE, Ca)-ZrO2。为此,进一步推测以小离子半径的稀土元素为主元的锆酸盐高熵陶瓷,较大离子半径为主元的高熵陶瓷具有更加优异的CMAS腐蚀性能。因此,未来在稀土锆酸盐高熵陶瓷热障涂层的陶瓷顶层设计时,应尽可能选择小离子半径的重稀土元素作为主元,从而使其具有较好的抗熔融CMAS腐蚀性能。
3 结论
(1) (La 0.2 Nd 0.2 Tm 0.2 Yb 0.2 Lu 0.2 )2Zr2O7高熵陶瓷由70.15%的缺陷萤石相和29.85%的烧绿石相结构组成,且为非均匀双相结构。在1300 ℃的CMAS中会发生持续、快速的溶解。最终产物为稀土与Ca共同稳定的(RE, Ca)-ZrO2和针状、空心的磷灰石型Ca2RE8(SiO4)6O2结构。
(2) 当CMAS消耗完全时,磷灰石型结构和ZrO2逐渐烧结致密化。同时,CMAS中剩余的Mg和Al在高温烧结形成MgAl2O4尖晶石。
(3) 大离子半径的La和Nd更容易与CMAS中的Ca和Si反应形成磷灰石型结构,而小离子半径的Tm、Yb和Lu则更倾向于留在高熵陶瓷中并与少量Ca反应形成(RE, Ca)-ZrO2。因此,选择小离子半径的重稀土元素作为主元的稀土锆酸盐高熵陶瓷可具有更好的抗熔融CMAS腐蚀性能。
参考文献
Thermal barrier coatings for gas-turbine engine applications
[J].Hundreds of different types of coatings are used to protect a variety of structural engineering materials from corrosion, wear, and erosion, and to provide lubrication and thermal insulation. Of all these, thermal barrier coatings (TBCs) have the most complex structure and must operate in the most demanding high-temperature environment of aircraft and industrial gas-turbine engines. TBCs, which comprise metal and ceramic multilayers, insulate turbine and combustor engine components from the hot gas stream, and improve the durability and energy efficiency of these engines. Improvements in TBCs will require a better understanding of the complex changes in their structure and properties that occur under operating conditions that lead to their failure. The structure, properties, and failure mechanisms of TBCs are herein reviewed, together with a discussion of current limitations and future opportunities.
Preparation and properties comparison of two novel rare earth modified ceramic ingots used for thermal barrier coatings
[J].
两种稀土掺杂改性热障涂层陶瓷靶材制备及其性能比较分析研究
[J].
Phase evolution upon aging of air-plasma sprayed t′-zirconia coatings: I—Synchrotron X-Ray diffraction
[J].
High-temperature corrosion and protection of thermal barrier coatings for aeroengines and gas turbines
[J].
航空发动机及燃气轮机热障涂层高温腐蚀与防护
[J].
Phase stability and thermal insulation of YSZ and erbia-yttria co-doped zirconia EB-PVD thermal barrier coating systems
[J].
Study on molten salt corrosion resistance of YSZ thermal barrier coating prepared by PS-PVD
[J].
PS-PVD制备YSZ热障涂层抗熔盐腐蚀研究
[J].
Entropy-stabilized oxides
[J].Rost, Christina M.; Sachet, Edward; Borman, Trent; Moballegh, Ali; Dickey, Elizabeth C.; Hou, Dong; Jones, Jacob L.; Maria, Jon-Paul N Carolina State Univ, Dept Mat Sci & Engn, Raleigh, NC 27695 USA. Curtarolo, Stefano Duke Univ, Dept Mech Engn & Mat Sci, Ctr Mat Genom, Durham, NC 27708 USA.
Preparation and electrical properties study of non-equimolar Sr (Ti, Zr, Y, Sn, Hf)O3- σ high-entropy perovskite oxide
[J].
非等摩尔比Sr (Ti, Zr, Y, Sn, Hf)O3- σ 高熵钙钛矿氧化物的制备及电学性能研究
[J].
Design and experimental investigation of potential low-thermal-conductivity high-entropy rare-earth zirconates
[J].
Structural evolution and thermal conductivities of (Gd1- x Yb x )2Zr2O7 (x = 0, 0.02, 0.04, 0.06, 0.08, 0.1) ceramics for thermal barrier coatings
[J].
(La0.2Ce0.2Nd0.2Sm0.2Eu0.2)2Zr2O7: a novel high-entropy ceramic with low thermal conductivity and sluggish grain growth rate
[J].
Research progress on plasma spray-physical vapor deposition protective coatings and their failure mechanisms
[J].
等离子喷涂-物理气相沉积防护涂层及其失效机理研究进展
[J].
Graceful behavior during CMAS corrosion of a high-entropy rare-earth zirconate for thermal barrier coating material
[J].
Mechanical, thermal and CM-AS resistance properties of high-entropy (Gd0.2Y0.2Er0.2Tm0.2Yb0.2)2-Zr2O7 ceramics
[J].
Improved thermal properties and CMAS corrosion resistance of high-entropy RE zirconates by tuning fluorite-pyrochlore structure
[J].
Effects of cation substitution and temperature on the interaction between thermal barrier oxides and molten CMAS
[J].
Calcium-magnesium-alumina-silicate (CMAS) resistant high entropy ceramic (Y0.2Gd0.2Er0.2Yb0.2Lu0.2)2-Zr2O7 for thermal barrier coatings
[J].
Preparation and phase evolution of high-entropy oxides A2B2O7 with multiple elements at A and B sites
[J].
Rare-earth zirconate Ln2Zr2O7 (Ln: La, Nd, Gd, and Dy) powders, xerogels, and aerogels: preparation, structure, and properties
[J].The physicochemical properties of rare-earth zirconates can be tuned by the rational modification of their structures and phase compositions. In the present work, La-, Nd-, Gd-, and Dy-zirconate nanostructured materials were prepared by different synthetic protocols, leading to powders, xerogels, and, for the first time, monolithic aerogels. Powders were synthesized by the co-precipitation method, while xerogels and aerogels were synthesized by the sol-gel technique, followed by ambient and supercritical drying, respectively. Their microstructures, thermogravimetric profiles, textural properties, and crystallographic structures are reported. The co-precipitation method led to dense powders (< 1 m g), while the sol-gel technique resulted in large surface area xerogels ( = 144 m g) and aerogels ( = 168 m g). In addition, the incorporation of lanthanide ions into the zirconia lattice altered the crystal structures of the powders, xerogels, and aerogels. Single-phase pyrochlores were obtained for LaZrO and NdZrO powders and xerogels, while defect fluorite structures formed in the case of GdZrO and DyZrO. All aerogels contain a mixture of cubic and tetragonal ZrO phases. Thus, a direct effect is shown between the drying conditions and the resulting crystalline phases of the nanostructured rare-earth zirconates.
Size disorder: a descriptor for predicting the single- or dual-phase formation in multi-component rare earth zirconates
[J].
Single-phase forming ability of high-entropy ceramics from a size disorder perspective: a case study of (La0.2Eu0.2Gd0.2Y0.2Yb0.2)2Zr2O7
[J].
Preparation and thermophysical properties of a novel dual-phase and single-phase rare-earth-zirconate high-entropy ceramics
[J].
Dual-phase rare-earth-zirconate high-entropy ceramics with glass-like thermal conductivity
[J].
Hot corrosion behaviour of La2(Zr0.7Ce0.3)2O7 thermal barrier coating ceramics exposed to molten calcium magnesium aluminosilicate at different temperatures
[J].
Investigation on improving corrosion resistance of rare earth pyrosilicates by high-entropy design with RE-doping
[J].
Calcium magnesium aluminosilicate (CMAS) corrosion behaviors of apatite Ca2La8(SiO4)6O2 thermal barrier coating material
[J].
Corrosion resistance of nonstoichiometric gadolinium zirconate coatings against CaO-MgO-Al2O3-SiO2 silicate
[J].
CMAS corrosion behavior of a novel high entropy (Nd0.2Gd0.2Y0.2Er0.2Yb0.2)2Zr2O7 thermal barrier coating materials
[J].
Electronic polarizability and optical basicity properties of oxide glasses through average electronegativity
[J]. J.
Basic properties of rare earth oxides
[J].
Interaction between Y-Al-Si-O glass-ceramics for environmental barrier coating materials and Ca-Mg-Al-Si-O melts
[J].
Spreading and corrosion behavior of CMAS melt on different materials for thermal barrier coating
[J].
CMAS熔体在不同热障涂层用材料表面的铺展和腐蚀行为
[J].选取了5种热障涂层用陶瓷材料 (Y<sub>2</sub>O<sub>3</sub>、La<sub>2</sub>Ce<sub>2</sub>O<sub>7</sub>、Gd<sub>2</sub>Zr<sub>2</sub>O<sub>7</sub>、Al<sub>2</sub>O<sub>3</sub>、12YSHf),对其进行了CMAS熔体铺展和腐蚀行为的分析,并与常用的陶瓷材料7YSZ进行了对比。研究表明,12YSHf和Al<sub>2</sub>O<sub>3</sub>在减缓CMAS熔体铺展方面具有较好的效果;Al<sub>2</sub>O<sub>3</sub>和La<sub>2</sub>Ce<sub>2</sub>O<sub>7</sub>高温下与CMAS反应后界面层厚度较小,具有较好的抗CMAS腐蚀性能。综上,Al<sub>2</sub>O<sub>3</sub>在延缓CMAS熔体铺展和抗CMAS腐蚀方面的综合效果最为突出。