目前针对稀土镁合金腐蚀的研究主要集中在微观组织方面。邓彬等[7]通过电化学方法研究了不同第二相分布的ZK60镁合金在3.5% (质量分数) NaCl溶液中的电化学腐蚀行为,结果表明第二相是引起ZK60镁合金腐蚀的主要因素,并且第二相呈分散分布的ZK60镁合金具有更好的耐蚀性能。刘雄飞等[8]研究了不同Gd含量对Mg-xGd-1Er-1Zn-0.6Zr合金的腐蚀影响,结果表明当Gd含量由7%增加至11%时,合金的耐蚀性能变差。Cao等[9]研究了热处理对轧态Mg5Gd合金腐蚀行为的影响,认为固溶处理能够溶解可引起严重微电偶腐蚀的Gd颗粒,进而提高Mg5Gd合金的耐蚀性能。部分学者也关注了镁合金在大气环境下的腐蚀行为。崔中雨等[10]研究了AZ31镁合金在西沙海洋大气环境下的腐蚀行为,结果显示AZ31镁合金在西沙海洋环境下的腐蚀产物主要为Mg(OH)2。孙硕等[11]研究了长期暴露在沈阳工业大气环境下的EW75镁合金的腐蚀行为,结果表明EW75镁合金主要发生均匀腐蚀,并且腐蚀速率在高湿高热的夏季最快。近些年,随着稀土镁合金在航空航天及国防领域的不断推广应用,贮存环境下稀土镁合金的环境适应性逐渐变为亟须解决的问题之一[12]。然而,关于镁合金在贮存环境下的腐蚀行为鲜有报道。
本文基于我国南部沿海城市环境特征,结合航空产品的寿命剖面[13],设计了沿海贮存环境下的模拟加速试验,以高强耐热的Mg-Gd-Y-Zr铸造镁合金为研究对象,通过腐蚀动力学、腐蚀形貌以及电化学分析等手段研究了其在模拟沿海贮存环境下的腐蚀行为。以期为拓宽镁合金在沿海贮存条件下的应用提供数据参考。
1 实验方法
实验材料为铸造Mg-Gd-Y-Zr合金,其化学成分为Mg-10.66Gd-0.029Y-0.48Zr (质量分数,%)。将试样通过线切割的方式分别切割为50 mm × 70 mm × 2 mm和10 mm × 10 mm × 2 mm两种尺寸,前者用于腐蚀形貌等性能测试,后者用于电化学性能表征。为了减少表面状态对实验结果的影响,实验前用砂纸依次打磨到800#,随后用无水乙醇清洗脱水,吹干后放入干燥皿备用。用游标卡尺测量记录试样的原始尺寸,试样的重量使用精度不低于0.1 mg的天平称量,为消除误差,每块试样的尺寸和重量均测量3次后求平均值。用作电化学实验的试样通过铜导线连接,并使用环氧树脂进行封样,试样工作面积为1 cm2,实验前使用砂纸依次打磨到2000#,清洗吹干后放于干燥皿中备用。
图1
图1
模拟沿海贮存环境的加速试验方法
Fig.1
Accelerated test method for simulating coastal storage environment
实验前对试样的金相组织进行表征,采用4%(质量分数)硝酸酒精侵蚀后通过体视显微镜获得试样的金相组织形貌;使用Quanta 250扫描电子显微镜(SEM)对实验前后的试样表面和截面进行微观形貌分析,表面形貌采用二次电子成像模式以获得腐蚀产物的清晰形貌,截面采用背散射电子成像模式以区分产物层中的元素差异。同时结合搭载的能量色散X射线谱仪(EDS)进行元素分析。使用Ultima IV型X射线衍射(XRD)对实验前后的试样进行物相分析,扫描范围为10°~90°,扫描速率为4°/min。使用Parstat4000电化学工作站,采用甘汞电极作参比电极,铂电极作对电极,经过腐蚀实验后的镁合金试样作为工作电极,电解质溶液采用1% (质量分数) NaCl溶液,在三电极体系中分别进行开路电位(OCP)测试,电化学阻抗谱(EIS)测试和动电位极化曲线测试。OCP测试时间为1800 s,EIS测试范围为105~10-2 Hz,动电位极化曲线测试范围为-0.5~+0.5 V,扫速为0.5 mV/s。参照 GB/T 16545-2015,选用除锈液(200 g三氧化铬+ 10 g硝酸银+790 mL去离子水)去除样品表面锈层,并计算材料的腐蚀失重。
2 结果与讨论
2.1 微观结构及物相分析
图2
图2
Mg-Gd-Y-Zr合金的微观形貌及物相组成
Fig.2
Microscopic morphology and phase composition of Mg-Gd-Y-Zr alloy: (a) metallographic structure morphology, (b) BSE morphology, (c) XRD pattern
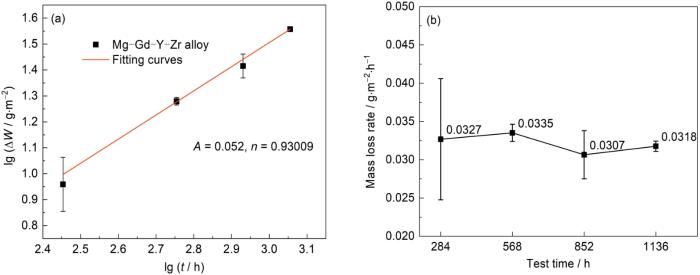
2.2 腐蚀动力学
图3
图3
Mg-Gd-Y-Zr合金在模拟沿海贮存环境实验后的腐蚀失重曲线
Fig.3
Corrosion mass loss (a) and mass loss rate (b) curves of Mg-Gd-Y-Zr alloy after simulating coastal storage envir-onment experiment
2.3 腐蚀产物形貌及元素分析
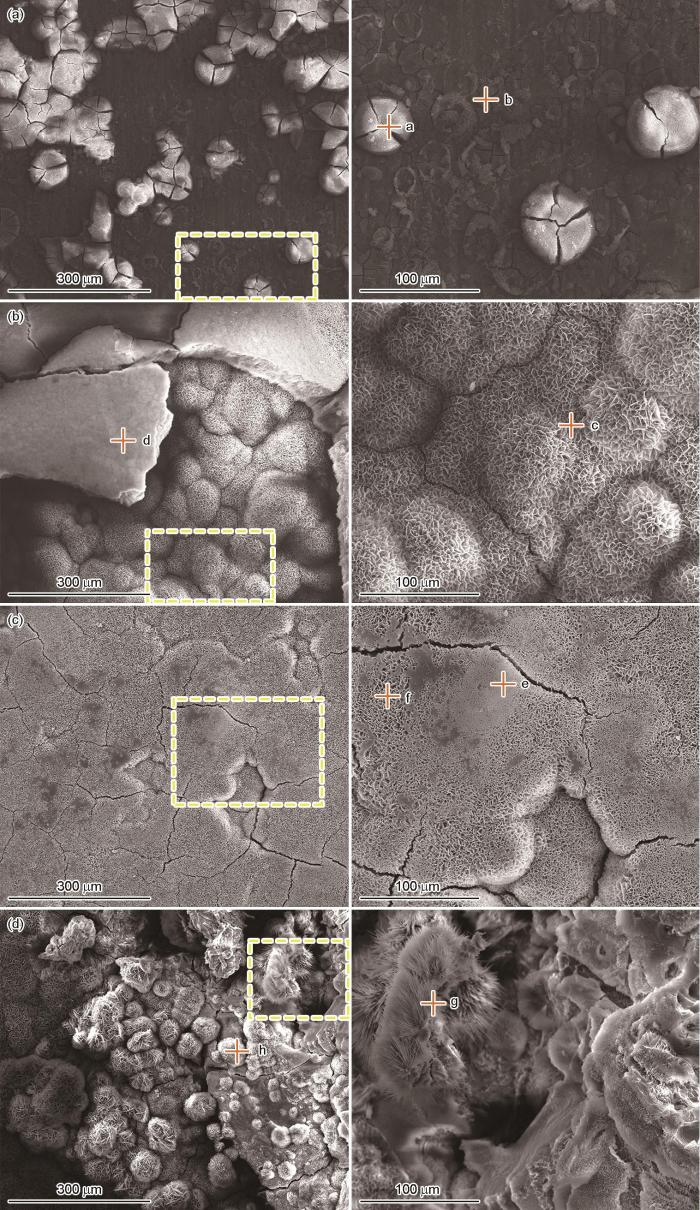
Mg-Gd-Y-Zr合金加速实验后的SEM形貌如图4所示,EDS测试结果如表1所示。可以看出,在实验284 h后,试样表面大多为龟裂块状腐蚀产物,经EDS分析可知,腐蚀产物中含有C、O、Mg和Cl,而点b处还含有较多的Mg和Gd,表明此处刚开始发生腐蚀;568 h后,试样表面腐蚀产物增多,腐蚀产物以蜂窝状和块状形态为主,EDS结果表明,两种腐蚀产物的元素成分均为C、O和Mg,推测可能为Mg(OH)2或MgO;852 h实验后,试样表面覆盖一层蜂窝状的腐蚀产物,其元素成分与568 h相比无明显差异;1136 h实验后,试样表面的腐蚀产物层被破坏,可以看到块状、蜂窝状和纤维状腐蚀产物,EDS表明纤维状腐蚀产物含有较高的Cl,说明在实验过程中可能生成了含Cl的腐蚀产物。
图4
图4
Mg-Gd-Y-Zr合金在模拟沿海贮存环境实验不同时间后的表面腐蚀产物形貌
Fig.4
Surface corrosion product morphologies of Mg-Gd-Y-Zr alloy after simulating coastal storage environment experiment for 284 h (a), 568 h (b), 852 h (c) and 1136 h (d)
表1 表面腐蚀产物的EDS能谱 (mass fraction / %)
Table 1
| Point | C | O | Mg | Cl | Gd |
|---|---|---|---|---|---|
| a | 10.9 | 38.8 | 49.8 | 0.5 | - |
| b | 7.7 | 17.6 | 64.2 | - | 10.5 |
| c | 11.4 | 42.8 | 45.8 | - | - |
| d | 10.6 | 43.4 | 46.0 | - | - |
| e | 10.9 | 37.7 | 51.4 | - | - |
| f | 11.4 | 41.2 | 47.5 | - | - |
| g | 14.5 | 44.7 | 28.2 | 12.6 | - |
| h | 15.3 | 54.1 | 30.6 | - | - |
图5显示了Mg-Gd-Y-Zr合金在模拟沿海贮存环境实验后的腐蚀产物截面形貌。可以看出,试样的腐蚀产物层大致分为两层,较暗的腐蚀产物外层的结构较为疏松,可以看到存在较多的孔隙,这可能与蜂窝状的腐蚀产物相对应,较亮的腐蚀产物内层相对致密,这可能存在Gd、Y和Zr的富集。随着实验的进行,腐蚀产物层呈变厚的趋势,一般而言,变厚的腐蚀产物对侵蚀性离子的阻挡作用增强。但可以看到,在各周期的腐蚀产物层中均存在贯穿至基体的裂纹,这可能为腐蚀性介质提供侵入的通道。
图5
图5
Mg-Gd-Y-Zr合金在模拟沿海贮存环境实验后的截面锈层形貌
Fig.5
Morphologies of rust layer on the cross-section of Mg-Gd-Y-Zr alloy after simulating coastal storage environment experiment for 284 h (a), 568 h (b), 852 h (c) and 1136 h (d)
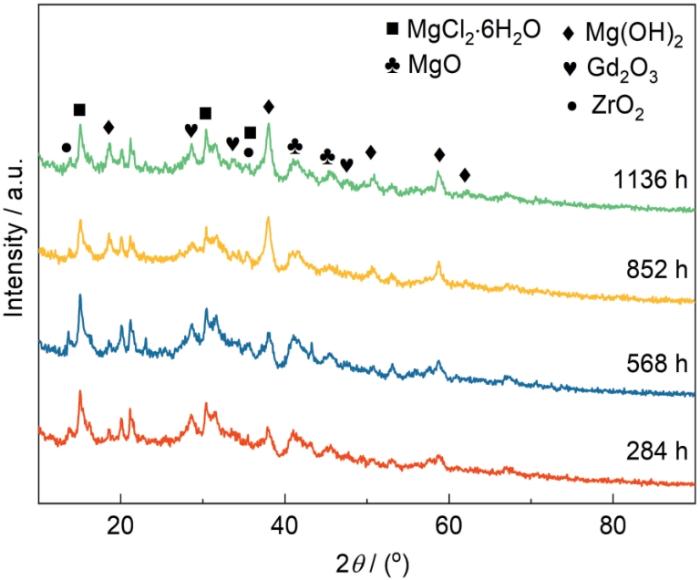
2.4 腐蚀产物组成分析
图6
图6
模拟沿海贮存环境实验不同时间后腐蚀产物的XRD谱
Fig.6
XRD patterns of corrosion products after different cycles of simulating coastal storage environment experiment
2.5 电化学性能
为了研究Mg-Gd-Y-Zr合金在模拟沿海贮存环境加速实验过程中的腐蚀电化学特性,对各周期实验后的试样进行了极化曲线测试,结果如图7所示。可以看出,在4个实验周期内,Mg-Gd-Y-Zr合金均表现出阳极溶解和阴极析氢特征。图7b给出了Mg-Gd-Y-Zr合金实验过程中的自腐蚀电位(Ecorr)和腐蚀电流密度(Icorr)的变化曲线。一般而言,Ecorr值可以反映材料腐蚀的热力学倾向,当Ecorr更正时,表明试样表面的腐蚀产物层更稳定,基体更不容易发生腐蚀[17];而Icorr可以反映材料腐蚀的动力学过程,Icorr值越大,材料腐蚀越快。可以看出,在实验852 h内,试样的Ecorr值逐渐正移,这表明腐蚀产物层逐渐向稳定的状态转变;而Icorr值也随之迅速降低,由33.2 μA·cm-2降低至3.2 μA·cm-2,这说明腐蚀产物层明显减缓了基体的腐蚀速率。实验1136 h后,试样的Ecorr值略微负移,而Icorr值也略微减小,这表明此时的腐蚀产物层已达到稳定状态。
图7
图7
Mg-Gd-Y-Zr合金在模拟沿海贮存环境实验后的极化曲线及拟合参数
Fig.7
Polarization curves (a) and fitting parameters (b) of Mg-Gd-Y-Zr alloy after simulating coastal storage environment experiment
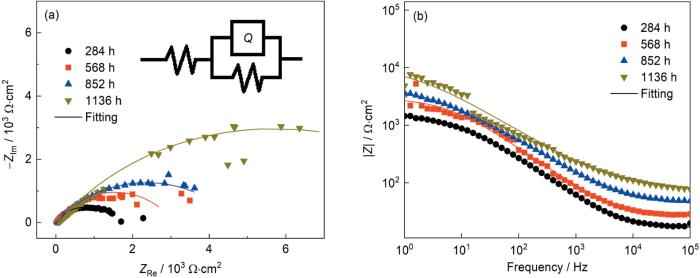
采用EIS进一步理解Mg-Gd-Y-Zr合金腐蚀产物层的结构特点,如图8所示,由于Mg的基体反应剧烈,低频范围内的测试结果过于紊乱,因此仅绘制了1~105 Hz范围内的EIS测试结果。可以看出,随着实验的进行,腐蚀产物层的容抗弧逐渐增大。容抗弧的半径大小与膜层的保护性有关,这表明了腐蚀产物层的保护性能逐渐升高[15]。采用如图8a所示的等效电路进行拟合,拟合参数如表2所示。可以看出,与Mg-Gd-Y-Zr合金腐蚀产物层有关的Rf值随着实验时间的延长不断升高。因此,随着实验时间的延长,由于腐蚀产物层的不断发展,一定程度上增大了腐蚀性介质侵入基体的阻力,进而降低了Mg-Gd-Y-Zr合金的腐蚀发展速度。
图8
图8
Mg-Gd-Y-Zr合金在模拟沿海贮存环境实验后的电化学阻抗谱
Fig.8
Nyquist (a) and Bode modulus (b) plots of Mg-Gd-Y-Zr alloy after simulating coastal storage environment experiment
表2 电化学阻抗谱图的拟合参数
Table 2
| Test time / h | Rs / Ω·cm2 | Q | Rf / Ω·cm2 | |
|---|---|---|---|---|
| Y0 / 10-6 Ω-1·cm-2·s n | n | |||
| 282 | 15.9 | 35.4 | 0.7182 | 1551 |
| 568 | 25.8 | 23.8 | 0.7195 | 3012 |
| 852 | 41.9 | 30.4 | 0.6351 | 4633 |
| 1136 | 74.7 | 23.7 | 0.6171 | 11280 |
2.6 腐蚀机理分析
海洋环境中的Cl-能够加速材料的腐蚀过程,并且在含Cl的环境中难以形成具有保护性的腐蚀产物层。在含Cl的大气环境中,暴露的镁合金可能发生以下反应[18]:
镁溶解反应:
阳极:
阴极:
由于Mg比较活泼,其与水可直接发生反应,Mg基体作为阳极发生溶解,并在周围的镁基体表面析出H2。
氢氧化物生成:
Mg2+与OH-反应生成氢氧化物产物膜。
部分氢氧化物脱水:
氢氧化物产物膜不稳定,难以有效保护基体。根据Pilling-Bedworth理论,Gd2O3的P-B比介于1~2之间,因此含Gd2O3的氧化膜具有较好的稳定性[19]。结合极化曲线和电化学阻抗的变化,不难看出试样表面的腐蚀产物膜在不断的向氧化物形态转变,而在模拟沿海贮存环境试验1136 h后的腐蚀电位的波动变化则表明此时已形成较为稳定的腐蚀产物膜。
实验过程中试样截面的腐蚀产物分两层,内层较为致密,外层较为疏松,并且致密的内层存在较大原子序数元素的富集。由于疏松多孔的结构,外层无法阻碍腐蚀性介质的侵入,而致密的内层进一步提升了对腐蚀性介质的阻碍作用,进而减缓了基体的腐蚀。
3 结论
(1) Mg-Gd-Y-Zr合金在模拟沿海贮存环境中的腐蚀产物为Mg(OH)2、MgCl2·6H2O、MgO、Gd2O3以及少量的ZrO2,Gd2O3增强了腐蚀产物层的保护性能。
(2) Mg-Gd-Y-Zr合金的腐蚀产物分两层,外层结构较为疏松,内层结构较为致密。
(3) 在模拟沿海贮存环境中,Mg-Gd-Y-Zr合金的腐蚀产物层对基体具有较好的保护作用。
参考文献
Research on aluminum alloy materials and application technology for automobile lightweight
[J].
汽车轻量化用铝合金材料及应用技术的研究
[J].
Research on material lightweight and shape design of automobile wheel hub
[J].
汽车轮毂材料轻量化与造型设计研究
[J].
Superhydrophobic and corrosion-resistant nickel-based composite coating on magnesium alloy
[J].
AZ31镁合金表面超疏水耐腐蚀镍基复合涂层
[J].结合化学沉积和电沉积技术,以动态氢气泡为模板,在AZ31镁合金表面制备了一种超疏水耐腐蚀的镍基复合涂层。涂层形貌、结构、组成、润湿性和腐蚀防护性能的表征结果表明,电沉积溶液中添加ZnO纳米粒子会改变多孔镍层的表面形貌,影响疏水能力。静态水接触角 (WCA) 测试表明,电沉积溶液中ZnO纳米粒子的浓度为5.0 g‧L<sup>-1</sup>时获得的电沉积镀层经硬脂酸改性后,具有最大的WCA值,达到160.8°±2.8°。相较于裸镁合金,该复合涂层腐蚀电位显著正移,腐蚀电流密度和电荷转移电阻分别降低和提升两个数量级以上,说明复合涂层对镁合金基底具备良好的腐蚀保护能力。
Research progress in the preparation of anti-corrosion superhydrophobic coatings on magnesium alloys
[J].
镁合金表面防腐蚀超疏水涂层制备研究进展
[J].
Research progress on microstructure and mechanical properties of medical rare-earth magnesium alloys
[J].
Effect of rare earth La on microstructure and corrosion resistance of AZ91D magnesium alloy
[J].
稀土镧对AZ91D镁合金组织及耐蚀性的影响
[J].
Effect of the second phase on the corrosion behavior of Mg-Zn-Zr alloy
[J].
第二相对Mg-Zn-Zr合金腐蚀行为的影响
[J].
Effect of Gd on microstructure and corrosion behavior of Mg-xGd-1Er-1Zn-0.6Zr alloys
[J].
Gd对Mg-xGd-1Er-1Zn-0.6Zr合金显微组织和腐蚀行为的影响
[J].采用重力铸造法制备Gd含量分别为7%(质量分数,下同),9%和11%的Mg-xGd-1Er-1Zn-0.6Zr合金,利用光学显微镜、扫描电镜和X射线衍射仪等研究合金的显微组织,通过开路电位、动电位极化和电化学阻抗测试等方法研究合金在3.5%NaCl溶液中的腐蚀行为。结果表明:当Gd含量从7%增至11%时,开路电位峰值时间从1609 s降为851 s,电荷转移电阻从588.5 Ω降至31.9 Ω,腐蚀电流密度从2.21×10<sup>-5</sup> A/cm<sup>2</sup>增至3.97×10<sup>-5</sup> A/cm<sup>2</sup>,说明随着Gd含量的增加,合金耐蚀性下降,这主要归因于第二相的微电偶腐蚀效应和腐蚀屏障效应共同作用。当Gd含量从7%增至11%时,(Mg, Zn)<sub>3</sub>(Gd, Er)相体积分数从1.9%增至5.2%,并从沿晶界不连续分布转变为半连续分布,层片状LPSO相体积分数从11.7%增至26.7%,并沿着晶界贯穿晶粒内部,(Mg, Zn)<sub>3</sub>(Gd, Er)相和层片状LPSO相体积分数的增加导致合金耐腐蚀性能下降,但大量细小层状LPSO相也能阻止腐蚀扩展,使得Gd含量为11%的合金在8~24 h内腐蚀速率增长减缓。
Influence of heat treatment on corrosion behavior of hot rolled Mg5Gd alloys
[J].
Long-term corrosion behavior of AZ31 magnesium alloy in Xisha marine atmosphere
[J].
西沙严酷海洋大气环境下AZ31镁合金的长周期腐蚀行为
[J].
Corrosion behavior of extruded EW75 Mg-alloy in Shenyang industrial atmosphere
[J].
挤压态EW75稀土镁合金在沈阳工业大气环境中的腐蚀行为研究
[J].
Research on the impact of storage environment on missile reliability
[J].
贮存环境对导弹可靠性影响研究
[J].
Analysis for carrier-based aerial ammunition storage environment
[J].
舰载航空弹药贮存环境分析
[J].
Spatial distribution and concentrations of salt fogs in a coastal urban environment: a case study in Zhuhai city
[J].
Research on corrosion behavior of truck body steel in chlorine-containing sulfuric acid environment
[J].
Pitting corrosion of a rare earth Mg alloy GW93
[J].Pitting corrosion of magnesium (Mg) alloys is greatly associated with their microstructure, especially second phases. The second phases in traditional Mg alloys such as AZ91 are electrochemically nobler than Mg matrix, while the second phases in Rare earth (RE) Mg alloy GW93 are more active than Mg matrix. As a result, the pitting corrosion mechanism of Mg alloy GW93 is different from the traditional ones. This paper aims to clarify the pitting corrosion mechanism of Mg alloy GW93 through the studies of Volta potential by Scanning Kelvin Probe Force Microscopy (SKPFM), corrosion morphology by Scanning Electron Microscope (SEM), and corrosion resistance by electrochemical tests. Results reveal that the pitting corrosion of GW93 includes three stages, first, dissolution of the second phases, followed by corrosion of Mg matrix adjacent to the dissolved second phases, and finally, propagation of corrosion pits along the depth direction of the dissolved second phases. Anodic second phases and enrichment of Cl- in the thick corrosion product films dominate the propagation of pitting corrosion.
Atmospheric corrosion of field-exposed AZ31 magnesium in a tropical marine environment
[J].
The influence of yttrium (Y) on the corrosion of Mg-Y binary alloys
[J].
Comparative in vitro study on binary Mg-RE (Sc, Y, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb and Lu) alloy systems
[J].Correct selection of alloying elements is important for developing novel biodegradable magnesium alloys with superior mechanical and biological performances. In contrast to various reports on nutrient elements (Ca, Zn, Sr, etc.) as alloying elements of biomedical magnesium alloys, there is limited information about how to choose the right rare earth elements (REEs) as alloying elements of magnesium. In this work, 16 kinds of REEs were individually added into Mg, including Sc, Y, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Du, Ho, Er, Tm, Yb and Lu, to fabricate binary Mg-RE model alloys with different composition points. Under the same working history, comparative studies were undertaken and the impact of each kind of rare earth element on the microstructure, mechanical property, corrosion behavior and biocompatibility of Mg were investigated. The corresponding influence level for the 16 kinds of REEs were ranked. The results showed that the second phases were detected in some Mg-RE alloys, which were mainly composed of MgRE. By adding different REEs into Mg with proper contents, the mechanical properties of resulting Mg-RE binary alloys could be adjusted in wide range. The corrosion resistance of Mg-light REE alloys was generally better than Mg-heavy REE alloys. As for biocompatibility, Mg-RE model alloys showed no cytotoxic effect on MC3T3-E1 cells. The hemolysis rates of all experimental Mg-RE model alloys were lower than 5% except for Mg-Lu alloy model. In general, the addition of different REEs into Mg could improve its performance from different aspects. This work provides a better understanding on suitable REEs as alloying elements for magnesium, and the future R&D direction on biomedical Mg-RE alloys was proposed. STATEMENT OF SIGNIFICANCE: In contrast to various reports on nutrient elements (Ca, Zn, Sr, etc.) as alloying elements of biomedical magnesium alloys, until now there is limited information about how to choose the right rare earth elements (REEs) as alloying elements of magnesium. In this work, comparative studies were undertaken by individually adding 16 kinds of REEs, including Sc, Y, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Du, Ho, Er, Tm, Yb and Lu, into Mg to fabricate binary Mg-RE model alloys, with different composition points, then the impact of each kind of rare earth element on the microstructure, mechanical property, corrosion behavior and biocompatibility of Mg under the same working history were investigated, and the corresponding influence level for the 16 kinds of REEs were ranked. This work provides a better understanding on suitable REEs as alloying elements for magnesium, and the future R&D direction on biomedical Mg-RE alloys was proposed.Copyright © 2019 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.