钢材在储存、使用过程中表面极易腐蚀,因金属腐蚀而造成的经济损失约占GDP总量的5%。冷轧钢广泛运用在汽车、建筑等行业的零件制造中,长期暴露在环境中易被锈蚀,使用酸剂可以有效的除去冷轧钢表面的锈蚀物,但会对冷轧钢基体产生破坏,添加缓蚀剂是减缓在酸洗过程中金属腐蚀的重要方法[2]。
表面活性剂具有低毒、低成本等优点,通常被用作腐蚀抑制剂。其结构具有双亲性,能附着在金属表面表现出良好的缓蚀性能,延缓不同金属在各种腐蚀性介质中的腐蚀。研究表明,其吸附和缓蚀作用的水平与金属或金属氧化物和周围溶液环境的相互作用有关[4]。关于非离子表面活性剂作为缓蚀剂的研究主要集中在硫酸或者盐酸介质中对钢的缓蚀性能,研究表明十二烷基酚聚氧乙烯醚[5],吐温-40[6]、吐温-60[7]、吐温-80[8],聚乙二醇月桂酸单酯希夫碱基[9]等非离子表面活性剂在硫酸或盐酸介质中对钢具有良好的缓蚀性能,但此类表面活性剂作为缓蚀剂在其他酸洗介质中的缓蚀性能鲜有报道。且作为缓蚀剂的作用机理尚需深入研究,特别是吐温系列所表现出的独特缓蚀效果及其作用机制等需进一步探究。
1 实验方法
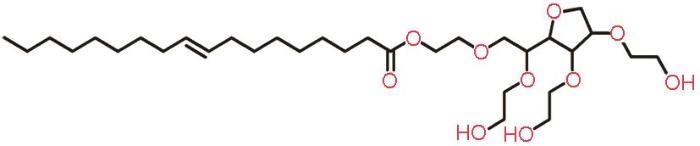
本实验采用的冷轧钢(CRS)试样主要成分(质量分数,%)为:C 0.06、Si 0.02、Mn 0.21、P 0.012、S 0.01,Fe余量。吐温-80 (C24H44O6(C2H4O)n)为化学纯,NH2SO3H为分析纯。吐温-80结构式如图1所示。
图1
用磨砂纸将厚度为0.5 mm的CRS试样逐级从60目打磨至2000目,清洗表面灰尘、用脱脂棉蘸取丙酮擦洗以除去油脂,吹干;再将其裁剪成为25 mm × 20 mm大小并储存在真空干燥的环境中。称取钢片质量,分别将其置于含和不含吐温-80的0.10 mol/L NH2SO3H介质中浸泡12 h。取样,除去表面产物,烘干称量;各温度下的实验平行3组,取其质量差平均值。根据式
式中,W为反应前后钢片质量差(g),S为钢片反应面积即其表面积(m2),t为反应时间(h);v0和v是在不含和含有吐温-80的0.10 mol/L NH2SO3H介质中的腐蚀速率(g/(m2·h))。
把冷轧钢加工成Φ10 mm × 10 mm的规格尺寸,将铜导线焊接在其顶面,使用环氧树脂将其包裹完全,并保证侧表面与环氧树脂接触处无气泡。留下底面10 mm × 10 mm的冷轧钢暴露区域作为测试区域。每次测试前用磨砂纸(直至2000目)进行打磨至光滑。然后依次使用无水乙醇和丙酮擦洗表面以除去油脂,再进行风干,以备使用。
电化学测试在CHI660E型电化学工作站进行,使用传统三电极系统进行测试。电化学实验在30℃下进行,先在待测溶液中测试30 min开路电位,之后在相对于开路电位-250 mV~+250 mV的电位范围内以1 mV/s的扫描速率进行动电位极化测试。
将钢片至于含有不同浓度吐温-80的0.10 mol/L NH2SO3H介质中浸泡12 h,清洗,吹干,再分别使用Thermo Scientific K-Alpha + 型X射线光电子能谱仪(XPS)和Bruker Dimension Icon型原子力显微镜(AFM)进行表面分析测试。
将缓蚀前后的介质溶液和只加入不同浓度吐温-80的溶液采用环法(铂环)在JYW-200A自动表面张力仪上进行表面张力测试;用PE38型电导率仪对各梯度浓度的溶液进行3组平行测试。
2 结果与讨论
2.1 吐温-80的缓蚀性能
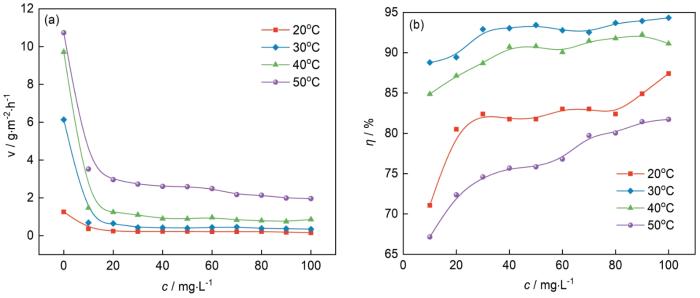
0.10 mol/L NH2SO3H溶液中不同温度下冷轧钢的腐蚀速率与吐温-80浓度的关系曲线如图2a所示。由图可知,腐蚀速率随着温度的升高而增大。当加入吐温-80后,腐蚀速率急剧下降,且随着浓度的增大腐蚀速率也随之减小。图2b为不同温度下冷轧钢的缓蚀率与吐温-80浓度的关系曲线。由图可知,随着吐温-80的浓度的增加,缓蚀率逐渐增大;当浓度为100 mg/L时,缓蚀率达到最高,说明高浓度的吐温-80会在冷轧钢表面形成更稳定的吸附。不同温度下,吐温-80对钢的最大缓蚀率表现为:30℃ > 40℃ > 20℃ > 50℃;当温度为30和40℃,吐温-80的浓度大于70 mg/L时,缓蚀率增长缓慢、趋于平缓,这可能是由于它在冷轧钢上的吸附达到饱和所致。温度为30℃时,缓蚀率最高可达94.33%。这可能是因为温度较低,吐温-80不能稳定的吸附在钢片表面;温度较高,钢片表面被酸腐蚀的速率加快,吐温-80的吸附也由于钢片表面H2的析出而产生了脱附,从而导致了缓蚀率的降低。这说明吐温-80的缓蚀性能应当在一个适宜的温度条件下才能发挥出较好的缓蚀效果。
图2
图2
冷轧钢在不同温度下0.10 mol/L NH2SO3H溶液中的腐蚀速率和缓蚀率随吐温-80浓度的变化
Fig.2
Corrosion rates (a) and corrosion inhibition rates (b) of cold-rolled steel in 0.10 mol/L NH2SO3H solutions at different temperatures as a function of the concentration of Tween-80
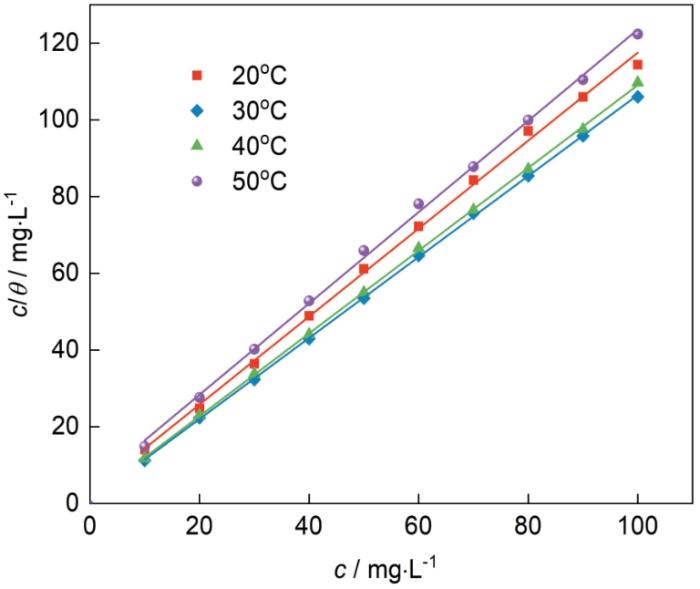
2.2 吐温-80在钢片表面的吸附等温式
Langmuir吸附等温模型是一种理想的单分子吸附模型,用其来对失重法数据进行拟合[15]:
式中:c为吐温-80的浓度,θ为表面覆盖度(θ ≈ ηw ),K是吸附平衡常数[16]。
根据
式中:ρsolvent为溶剂的浓度,在本体系中溶剂为水且为稀溶液,故水的浓度为1.0 × 106 mg/L;R为气体常数(8.314 J/(mol·K))。
图3
图3
不同温度下冷轧钢的c/θ-c拟合直线
Fig.3
Fitting straight lines of c/θ-c curves of cold-rolled steel at different temperatures
表1 c/θ-c 线性拟合参数
Table 1
| T / oC | R2 | Slope | Intercept | K mg·L-1 | ΔG kJ·mol-1 |
|---|---|---|---|---|---|
| 20 | 0.99811 | 1.15 | 2.89182 | 0.35 | -31.07 |
| 30 | 0.99985 | 1.06 | 0.94615 | 1.06 | -33.79 |
| 40 | 0.99981 | 1.08 | 1.22155 | 0.82 | -33.17 |
| 50 | 0.99876 | 1.19 | 4.59349 | 0.22 | -29.94 |
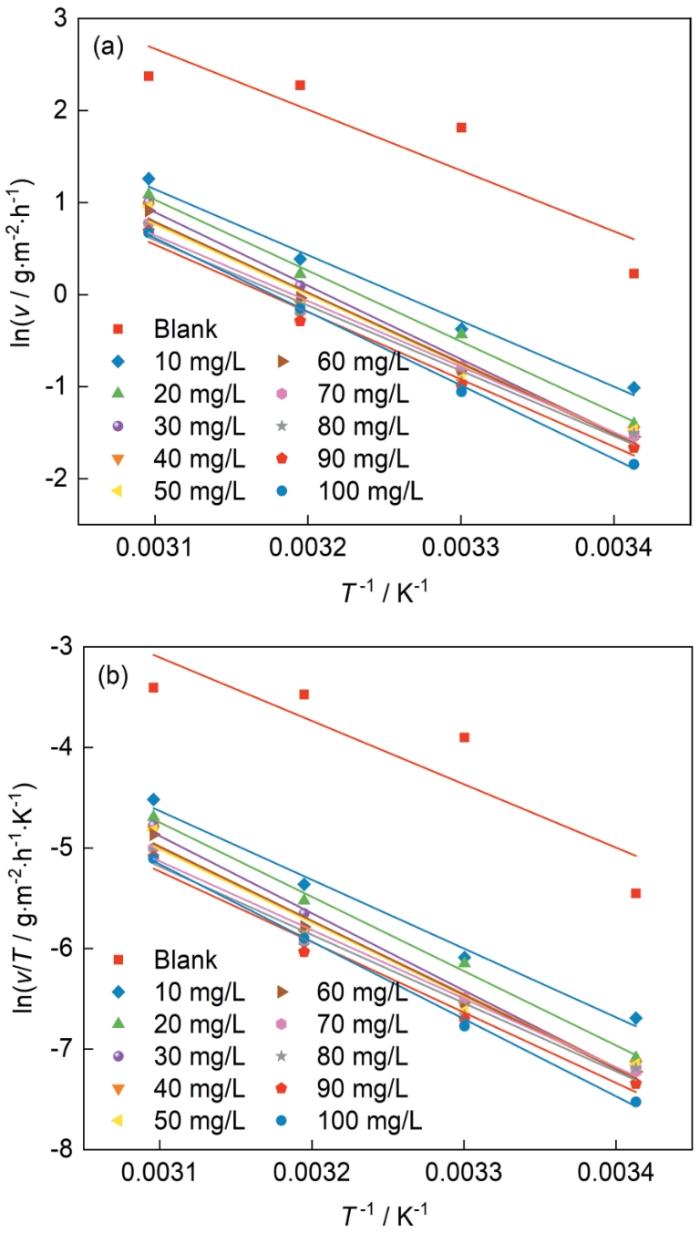
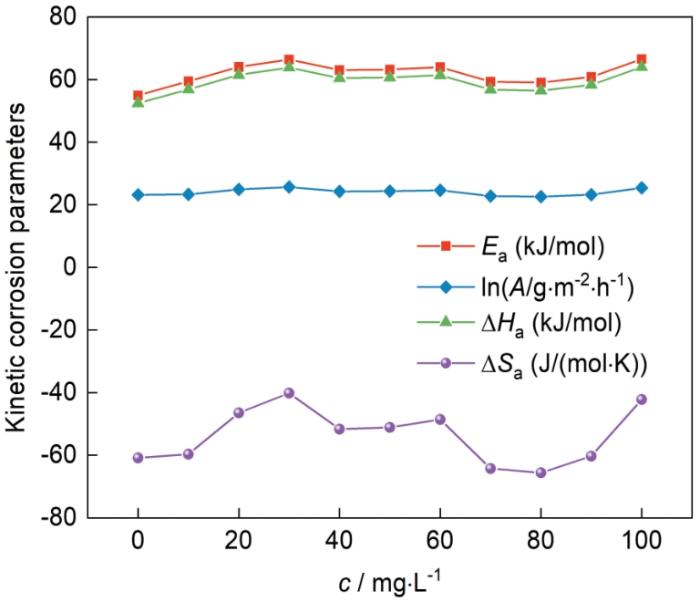
2.3 吐温-80在钢片表面的腐蚀动力学参数
图4为Arrhenius方程和过渡态理论方程的拟合图。由图可知,R2接近1,说明其具有良好的线性关系,并且冷轧钢片的腐蚀速率符合Arrhenius方程和过渡态理论方程。通过
图4
图4
冷轧钢在含有不同浓度的吐温-80的0.10 mol/L NH2SO3H中的lnv-T-1和ln(v/T)-T-1曲线的线性拟合
Fig.4
Fitting straight lines of lnv-T-1 (a) and ln (v/T)-T-1 (b) curves of cold-rolled steel in 0.10 mol/L NH2SO3H solutions containing different concen-trations of Tween-80
图5
图5
吐温-80浓度对冷轧钢的腐蚀动力学参数的影响
Fig.5
Effects of the concentration of Tween-80 on the corrosion kinetic parameters of cold-rolled steel
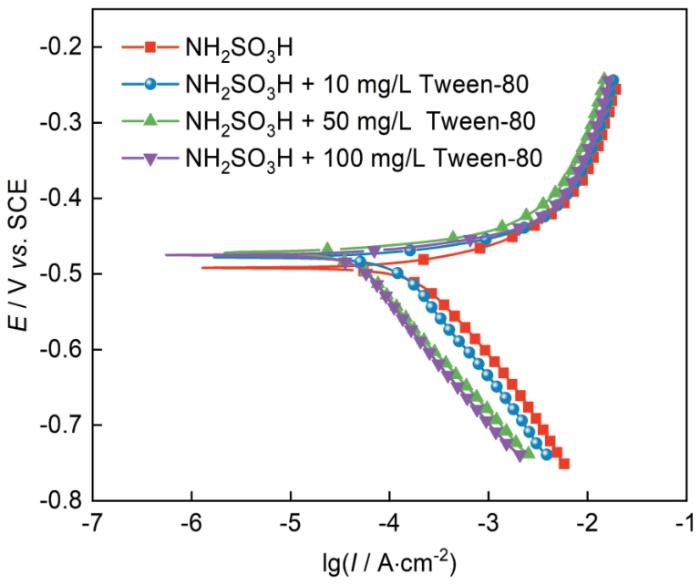
2.4 冷轧钢在含吐温-80的NH2SO3H溶液的极化曲线
图6
图6
冷轧钢在30℃下含不同浓度吐温-80的0.10 mol/L NH2SO3H溶液中的动电位极化曲线
Fig.6
Dynamic polarization curves of cold-rolled steel in 0.10 mol/LNH2SO3H solutions containing different concentrations of Tween-80 at 30oC
采用Tafel外推法[24]拟合实验数据,极化曲线的缓蚀率计算如下:
式中,Icorr(0)和Icorr(inh)是分别是在不添加和添加不同浓度吐温-80的条件下所测试出来的冷轧钢的腐蚀电流密度(μA/cm2)。
表2 冷轧钢的极化曲线拟合参数
Table 2
c mg·L-1 | Ecorr mV vs. SCE | Icorr μA·cm-2 | bc mV/dec | ba mV/dec | ηp |
|---|---|---|---|---|---|
0 10 | -492 -476 | 226.1 101.2 | -169.4 -150.4 | 44.3 24.5 | - 55.2% |
| 50 | -472 | 35.1 | -144.6 | 14.2 | 84.5% |
| 100 | -475 | 33.1 | -147.3 | 13.7 | 85.4% |
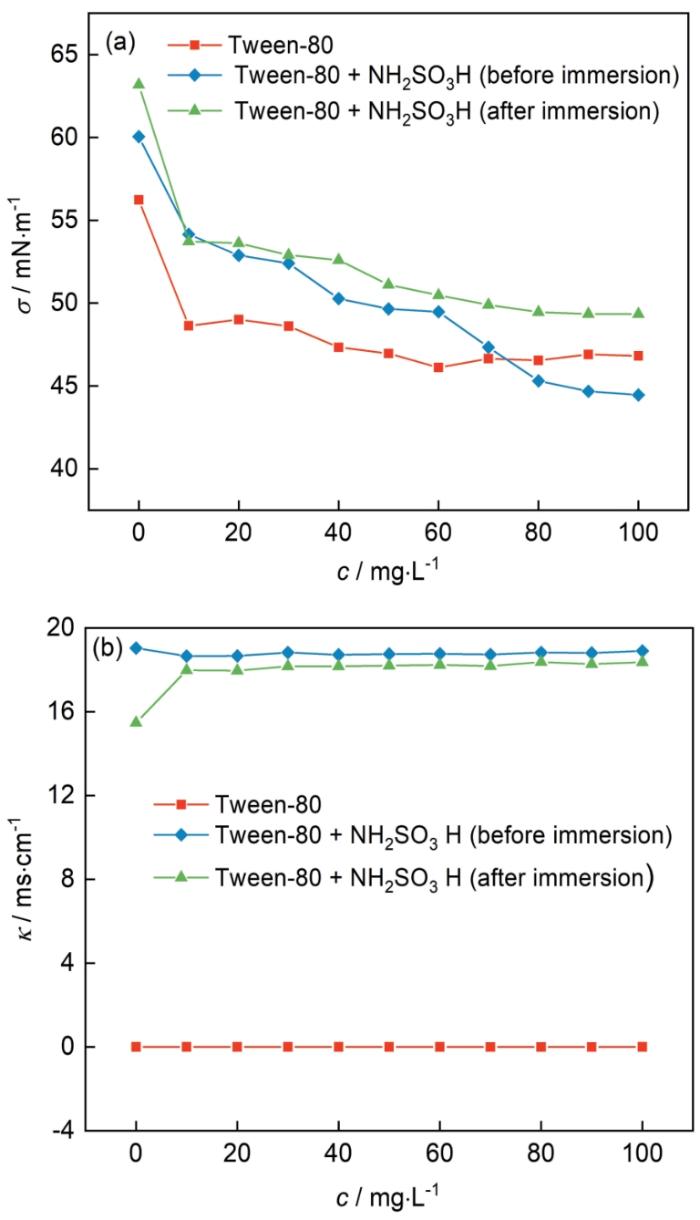
2.5 缓蚀剂溶液表面张力以及电导率
在30℃条件下,钢片在吐温-80水溶液和添加吐温-80的0.10 mol/L NH2SO3H溶液中浸泡前后的表面张力如图7a所示。可知,当加入吐温-80后,表面张力均急剧下降,并随着浓度的增大而继续减小至趋于平缓。当添加的吐温-80浓度相同时,反应前的表面张力值明显低于反应后的。这可能是因为反应后溶液中吐温-80的含量由于部分吸附在钢片表面而减少,导致了表面张力的上升。图7b为浸泡钢片前后溶液的电导率。由图可知,加入吐温-80后的电导率值随浓度变化不大,可能是因为吐温-80是非离子型表面活性剂,在水中的电离程度很小,导电能力较差;加入吐温-80的NH2SO3H溶液在未浸泡钢片时的电导率随浓度的变化不大,但是比只添加缓蚀剂的要更大,这是因为NH2SO3H能够在水中电离出NH2SO3-和H+,故电导率值较大;浸泡钢片后,溶液的电导率略下降,在加入吐温-80后电导率急剧上升后变为平缓,这可能是在没有缓蚀剂的作用下,酸与钢片反应,导致电离的NH2SO3-和H+减少,导致电导率值较低;加入缓蚀剂后的溶液的电导率比未浸泡的略低,即钢表面的腐蚀反应程度较小,H+的消耗较少,说明吐温-80对钢具有较好的缓蚀作用。
图7
图7
30℃时吐温-80浓度对冷轧钢在不同溶液中浸泡前后的表面张力(σ)及电导率(к)的影响
Fig.7
Variations of Tween-80 concentration at 30oC on the surface tension (a) and conductivity (b) of cold-rolled steel before and after soaking in different solutions
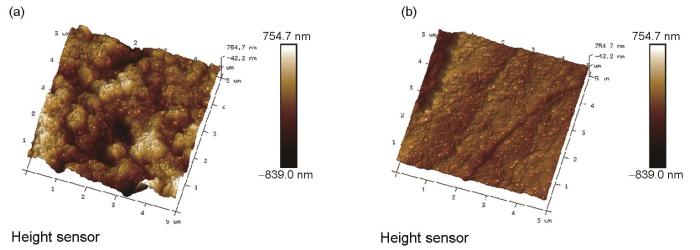
2.6 AFM微观形貌
图8
图8
冷轧钢在30℃下0.10 mol/L NH2SO3H溶液中浸泡12 h后的AFM表面形貌
Fig.8
AFM surface microtopographies of cold-rolled steel after immersion at 30oC for 12 h in 0.10 mol/L NH2SO3H solution without (a) and with (b) 100 mg/L Tween-80
表3是AFM的表面粗糙度参数。由表可知,在未添加吐温-80的溶液中浸泡12 h后的各个参数均比在添加吐温-80后的大,且Rmax的值在添加吐温-80后从1866 nm降低到了530 nm,说明吐温-80的加入能够减缓NH2SO3H在钢片表面的腐蚀。
表3 冷轧钢表面的AFM粗糙度参数
Table 3
| Inhibitor | Rq / nm | Ra / nm | Rmax / nm |
|---|---|---|---|
| NH2SO3H | 245 | 189 | 1866 |
| NH2SO3H + Tween-80 | 74.2 | 51.2 | 530 |
2.7 XPS表面分析
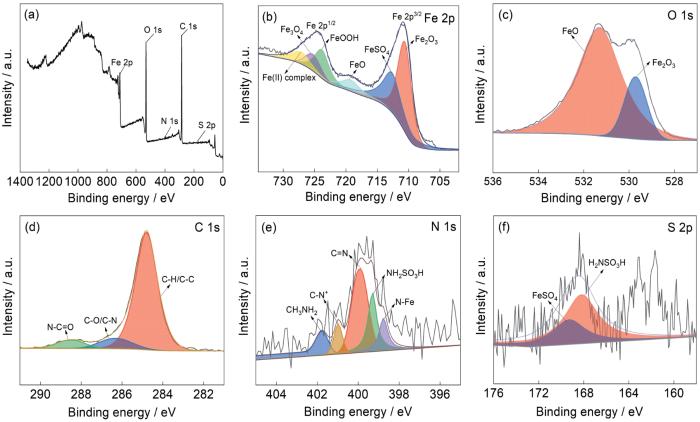
对冷轧钢表面缓蚀膜进行XPS测试,图9a为加入吐温-80后钢片表面的总XPS图谱。谱图中有Fe 2p、O 1s、N 1s、C 1s、S 2p峰的存在,证明了吐温-80在钢表面的吸附。图9b是Fe 2p的谱图,可以清晰的观察到两个明显的峰,710.9 eV为Fe 2p3/2 XPS光谱,724.5 eV为Fe 2p1/2 XPS光谱。在结合能分别为710.7、712.9、719.4、724.0、725.6和727.5 eV的谱峰中,分别对应Fe2O3、FeSO4、FeO、FeOOH、Fe3O4和Fe(Ⅱ)络合物[26,27]。其中,Fe2O3、FeO、FeOOH和Fe3O4等可能是冷轧钢表面与水和氧气结合产生的物质;FeSO4可能是氨基磺酸对冷轧钢的腐蚀产物。图9c为O 1s的谱图,在结合能为529.7和531.3 eV的谱峰中,所对应的化合物分别为Fe2O3和FeO,均为冷轧钢的氧化产物。图9d为C 1s的谱图,在结合能为284.8、286.3和288.4 eV的谱峰中,所对应的分别是C-H/C-C,C-O/C-N和N-C=O。图9e为N 1s的谱图,在结合能为398.7、399.2、399.9、400.9和401.7 eV的谱峰中,所对应的物质分别是N-Fe、NH2SO3H、C=N、C-N+和CH3NH2,其中N-Fe和CH3NH2可能是冷轧钢与吐温-80缓蚀而产生的物质。图9f为S 2p的谱图,在结合能为168.1和169.2 eV的谱峰中,所对应的物质分别是NH2SO3H和FeSO4[29,30]。XPS的测试结果表明在冷轧钢表面的主要成分是酸的腐蚀产物、氧化产物和缓蚀剂与Fe形成的配合物。
图9
图9
冷轧钢在30℃下含100 mg/L 吐温-80的0.10 mol/L NH2SO3H溶液中浸泡12 h后的XPS分析
Fig.9
XPS analysis results of cold-rolled steel immersed in 0.10 mol/L NH2SO3H solution containing 100 mg/L Tween-80 for 12 h at 30oC: (a) survey, (b) Fe 2p, (c) O 1s, (d) C 1s, (e) N 1s, (f) S 2p
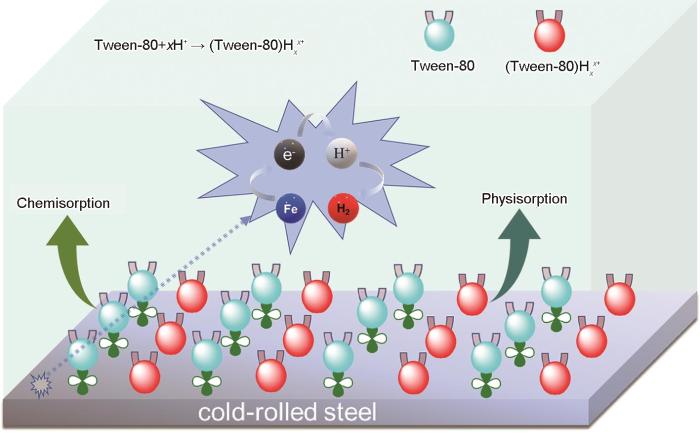
2.8 吐温-80对钢的缓蚀机理
图10
图10
吐温-80在冷轧钢表面的缓蚀机理
Fig.10
Schematic illustration of corrosion inhibition mechanism of Tween-80 on cold-rolled steel
吐温-80分子中的疏水基团为结合油酸酯的聚氧乙烯侧链[32],亲水基团为未结合的侧链,亲水基团易吸附在钢表面,疏水基团在外排列可形成一层疏水吸附膜。另一方面,由于吐温-80分子中含氧基团与H+结合而质子化,紧密的吸附在冷轧钢表面,所以阻碍了体系中NH2SO3-和H+与钢片的接触,减缓了腐蚀,该行为是物理吸附。吐温-80分子中的-COOH、-OH以及五元杂环上的O都含有孤对电子,能够与钢片表面的Fe上的大量空轨道相结合形成配合物,从而减缓腐蚀,该行为是化学吸附。
3 结论
吐温-80在氨基磺酸介质中对冷轧钢有良好的缓蚀性能。在30℃,吐温-80浓度为100 mg/L时,缓蚀性能最佳。吐温-80在冷轧钢表面是混合吸附,服从Langmuir吸附等温式,且为放热吸附;腐蚀动力学参数均比在空白溶液中的大,总体呈上升趋势。吐温-80是一种以抑制阴极反应为主的混合型抑制剂。在缓蚀体系浸泡前后表面张力下降,而电导率上升;AFM和XPS的表征说明吐温-80会在冷轧钢表面形成一层缓蚀膜层来达到缓蚀效果。
参考文献
Primers based on tara and quebracho tannins for poorly prepared steel surfaces
[J].Steel panels must be protected during storage and transportation because they can corrode. This protection used to be afforded by chromate primers, but nowadays tannin-based primers are being studied. These primers are not only used as protection but also as adhesion promoter if the steel surface was not well prepared (presence of oxides or low roughness). The objective of this work was to prepare lanthanum "tannate", employing tara and quebracho tannins. The "tannates" were incorporated in primers formulation and applied on steel SAE 1010 panels (previously exposed to the humidity chamber or with 6 pm of roughness depth). These primed panels were exposed to humidity chamber where corrosion and blistering degrees were evaluated. Adhesion test were done. Electrochemical assays such as ionic resistance and corrosion potential measurements were carried out. Results showed that the adhesion and the barrier properties of the primers were good. Besides, on low roughness steel, the primers diminished the corrosion current. Results were compared with traditional zinc tetroxychromate primer.
The cost of corrosion in China
[J].
Study of corrosion inhibitors in acid Pickling
[J].
酸洗过程中的缓蚀剂
[J].
A review of surfactants as corrosion inhibitors and associated modeling
[J].
The synergistic effects of Dodecane Phenol Polyethylene and potassium iodide on the inhibition of A3 steel in hydrochloric
[D].
盐酸介质中十二烷基酚聚氧乙烯醚与碘化钾对A3钢缓蚀协同效应的研究
[D].
Tween-40 as corrosion inhibitor for cold rolled steel in sulphuric acid: weight loss study, electrochemical characterization, and AFM
[J].
Synergistic inhibition between tween 60 and NaCl on the corrosion of cold rolled steel in 0. 5M sulfuric acid
[J].
Inhibitory effect of Tween-80 on carbon steel in sulfuric acid
[J].
吐温-80对碳钢在硫酸溶液中的缓蚀作用研究
[J].
Synthesis and inhibition performance of Polyethylene Glycol(Peg) Lauric Acid Monoester Schiff Bases of non-ionic surface active ggent
[J].
聚乙二醇月桂酸单酯席夫碱基非离子型表面活性剂的合成及缓蚀性能
[J].
Application of amino sulfonic acid in supercritical boiler cleaning
[J].
氨基磺酸在超临界锅炉清洗中的应用
[J].
Corrosion Inhibition effect of Mikania Micrantha Kunth Extract on cold rolled steel in Sulfamic Acid solution
[J].
薇甘菊提取物对冷轧钢在氨基磺酸溶液中的缓蚀效果
[J].
The corrosion inhibition of alkyl imidazoline on carbon steel in amidosulphuric acid solution
[J].
氨基磺酸溶液中烷基咪唑啉对碳钢的缓蚀作用
[J].采用失重实验、电化学和扫描电镜等方法研究了2-十一烷基-N-羧甲基-N-羟乙基咪唑啉(UHCI) 在8 mass%氨基磺酸溶液中对碳钢的缓蚀行为。失重实验表明,该缓蚀剂在氨基磺酸溶液中能够有效地抑制碳钢腐蚀,当缓蚀剂的质量分数为0.4 mass%时,碳钢腐蚀速率为0.6370 g/(m<sup>2</sup>•h),缓蚀效率达到90.12%。极化曲线测试结果表明,该缓蚀剂为混合型缓蚀剂。该缓蚀剂的吸附行为符合Langmuir吸附等温式,吸附机理是一种物理-化学混合吸附。扫描电镜结果也证明 UHCI可有效地抑制氨基磺酸对碳钢的腐蚀。
Surface adsorption and corrosion inhibition behaviors of N-Cinnamylidene Methylamine Schiff Base on Q235 Steel in Sulfamic Acid
[J].
氨基磺酸中肉桂醛缩甲胺席夫碱对Q235钢的缓蚀吸附行为
[J].
Inhibition of Q235 Steel in 1 mol/L HCl solution by a new efficient Imidazolium Schiff Base corrosion inhibitor
[J].
新型高效咪唑希夫碱缓蚀剂对Q235钢在1 mol/L HCl溶液中的缓蚀作用
[J].
Inhibition effect of Brainea Insignis extract against carbon steel corrosion in HCl solution
[J].
铁蕨提取物对碳钢在盐酸中的缓蚀行为研究
[J].
Inhibition action of Hexadecylpyridinium bromide on cold rolled steel in Cl3CCOOH solution
[J].
溴代十六烷基吡啶对冷轧钢在三氯乙酸中的缓蚀性能
[J].
The inhibition of the corrosion of Armco iron in HCl solutions in the presence of surfactants of the type of N-alkyl quaternary ammonium salts
[J].
A new insight into corrosion inhibition mechanism of copper in aerated 3.5 wt.% NaCl solution by eco-friendly Imidazopyrimidine Dye: experimental and theoretical approach
[J].
Study on corrosion inhibition behavior of Photinia Leaf Extracts for Q235 steel in HCl medium
[J].
石楠叶提取物在盐酸中对Q235钢的缓蚀行为研究
[J].
New organic compounds based on siloxane moiety as corrosion inhibitors for carbon steel in HCl solution: weight loss, electrochemical and surface studies
[J].
Corrosion inhibition of Aluminum in Hydrochloric Acid solution by Alkylimidazolium Ionic liquids
[J].
Gravimetric, electrochemical and quantum chemical studies of some pyridazine derivatives as corrosion inhibitors for mild steel in 1 M HCl solution
[J].
Preparation of Zinc Phytate and its effect on corrosion behavior of carbon steel
[J].
植酸锌的制备及其对Q235钢腐蚀行为的影响
[J].采用磷酸、植酸、氯化锌制备磷酸锌和植酸锌,通过扫描电子显微镜(SEM)、能谱分析(EDS)、红外光谱(FT-IR)和热重(TG)等手段对制备的磷酸锌和植酸锌进行表征,通过滴定实验分析磷酸锌和植酸锌在水溶液中的溶解度。根据Tafel极化法、电化学阻抗法和腐蚀浸泡实验分析了磷酸锌和植酸锌浸出液中Q235钢的腐蚀特性。结果表明:制备的磷酸锌为非均匀大小的微米片状结构,厚度在0.5~1 μm之间,植酸锌为团聚状粉末状颗粒,粒径在2~5 μm之间;植酸锌在浸出2 h以后,溶液中植酸根含量达到饱和,对Q235钢的缓蚀效率约在90%,表现出良好的缓蚀性能。
Corrosion inhibition of Navel Orange Peel extract to stainless steel in acidic medium
[J].
在酸性介质中脐橙皮提取物对不锈钢的缓蚀作用
[J].
Preparation and corrosion inhibition of super hydrophobic adsorption film of lotus leaf extract on mild steel
[J].
Q235钢表面的超疏水吸附层形成与缓蚀研究
[J].用新鲜荷叶作为研究对象,经过简便的乙醇回流萃取取得提取物。室温条件下,荷叶提取物能够在THF/HCl水溶液的混合溶液 (体积比为1/1,1.0 mol/L HCl溶液) 中产生聚集。傅立叶变换红外光谱以及X射线光电子能谱的结果证明了荷叶提取物在Q235钢样品表面发生化学作用,能够形成超疏水的吸附层。电化学结果表明荷叶提取物对碳钢在HCl溶液中具备良好的缓蚀性能,在0.4 g/L浓度下,最大缓蚀效率达到93.14%。
Eupatorium Adenophora (Spreng.) leaves extract as a highly efficient eco-friendly inhibitor for steel corrosion in Trichloroacetic acid solution
[J].
Nonionic surfactant of coconut Diethanolamide as a novel corrosion inhibitor for cold rolled steel in both HCl and H2SO4 solutions
[J]. J.
Inhibition mechanism of Fagopyrum Esculentum Moench. Extract on steel in HCl media
[J].
荞麦提取物对钢在HCl介质中的缓蚀机理
[J].
Study on the corrosion inhibitor of carbon steel in sulfamic acid
[D].
氨基磺酸中碳钢腐蚀缓蚀剂研究
[D].
Alternanthera philoxeroides extract as a corrosion inhibitor for steel in Cl3CCOOH solution
[J].
The synergistic effect of anions and the ammonium cation on the inhibition of iron corrosion in acid solution
[J].