高熵合金(HEAs)因其高熵效应、缓慢扩散效应、晶格畸变效应和“鸡尾酒效应”,使其具有合金元素更难扩散、更易形成固溶体、良好的力学性能和耐腐蚀性等特征[12~16],有望在LFR中获得应用。然而,HEAs在LBE环境下的腐蚀研究刚刚起步,但是研究者对HEAs在其它介质中的腐蚀已经开展了一些研究。Al-Cr-Fe-Ni-Co高熵合金因表现出良好的力学性能和耐腐蚀性能[17,18],受到研究者的广泛关注,然而由于Co的中子吸收和中子活化问题[15],在LFR中应用需要将其用其它元素替代。Shi等[19]研究了添加Cu,Ti和Nb对AlCrFeNi基高熵合金在液态Pb中腐蚀行为的影响,结果表明,添加Nb的高熵合金表现出良好的耐腐蚀性。Xu等[20]通过理论计算,发现Nb和Mo可抑制马氏体钢在无氧(或低氧浓度)的液态Pb和Bi中的溶解腐蚀。同时Mo和Nb在液态Pb的溶解度较低,尤其是Mo的溶解度比Ni、Cr和Fe低至少一个数量级[4]。综上,可以考虑在无Co的Al-Cr-Fe-Ni体系基础上,添加Mo和Nb来提高其抗腐蚀性能。
本文制备了Fe34Cr30Mo15Ni15Nb3Al3高熵合金(简称HEA-15),并研究了其在500℃、氧浓度为10-6%(质量分数)的LBE中的腐蚀行为,以期为耐铅铋腐蚀的高熵合金研发提供建议。
1 实验方法
采用真空感应熔炼技术制备了高熵合金母合金,所用的原材料纯度均为99.99%。熔炼后将其加工成尺寸为10 mm × 10 mm × 5 mm的块状样品,然后分别使用SiC砂纸打磨和金刚石悬浮液进行抛光处理,最后使用酒精清洗后干燥备用。
静态铅铋腐蚀实验是在500℃、氧含量为10-6%的液态铅铋合金中进行,腐蚀时间分别为1000、1500和2000 h。腐蚀实验结束后,对于表面分析样品,首先将其置于冰乙酸、过氧化氢和无水乙醇体积比为1∶1∶1的混合液中,清洗其表面粘附的残余铅铋合金;再使用丙酮和无水乙醇进行清洗吹干,然后进行组织观察。对于横截面分析样品,采用冷镶后进行打磨、抛光和清洗。
使用X’Pert Pro MPD型X射线衍射仪(XRD)对腐蚀前后的样品进行相结构分析,靶材为Cu靶,工作电压40 kV,电流40 mA,扫描角度为20°~90°,扫描时间20 min。使用配有X射线能谱仪(EDS)的ΣIGMA型场发射扫描电子显微镜(FE-SEM)对腐蚀前后的样品表面和截面进行微观形貌以及元素分布分析。对每个截面样品选择5个典型区域进行线扫描,来测量每个腐蚀样品氧化层的厚度。
2 结果与分析
2.1 腐蚀前微观组织分析
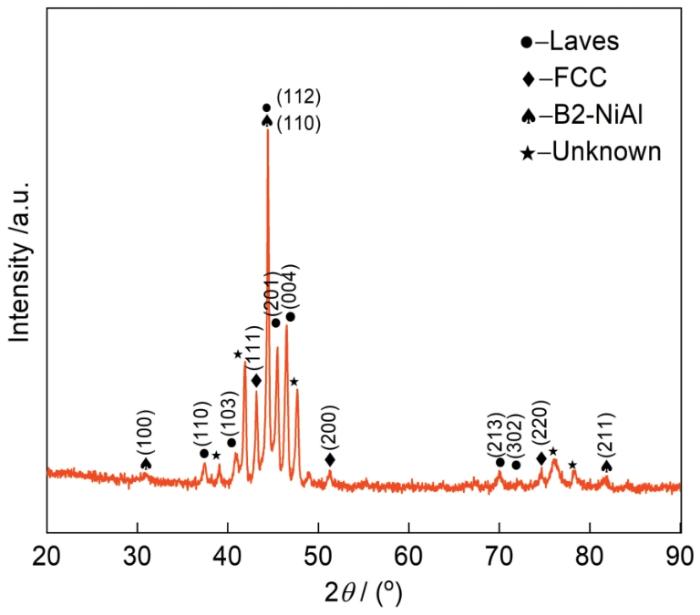
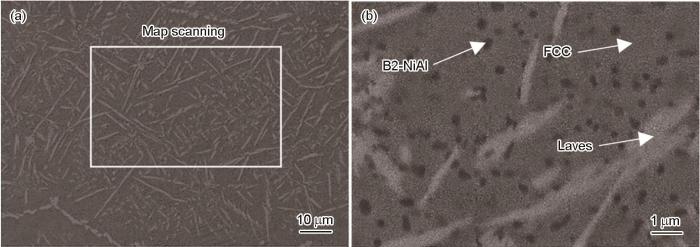
图1为HEA-15合金腐蚀前XRD谱,形成了FCC相、Laves相和B2-NiAl相,PDF卡片编号分别为PDF#33-0945、PDF#17-0908和PDF#44-1188,由于其它元素的固溶,FCC相晶格常数增大,Laves相晶格常数减小,导致FCC相衍射峰向左偏移,Laves相衍射峰向右偏移;同时由于FCC相晶格畸变较大,导致了FCC相衍射峰减弱[21]。图2为腐蚀前HEA-15合金的微观组织,表1为腐蚀前特征区域的能谱点扫描结果。可以看出,白色枝晶区域富含Mo和Nb,其它元素都比较贫乏,结合XRD结果可知其为Laves相。灰色枝晶间区域富集Fe和Cr,Ni和Al接近名义成分含量,贫Mo,不含Nb,结合XRD结果可知其为FCC相。灰色枝晶间区域内均匀的分布着黑色沉淀物(图2b),EDS测量显示黑色沉淀物中富含Ni和Al,不含Nb,结合XRD结果可知其为B2-NiAl相。同时用Image J对图2b进行处理,计算了各个相的占比,得出HEA-15合金的FCC相、Laves相和B2-NiAl相分别占比约75.95%、19.09%和4.96%。
图1
图2
图2
HEA-15合金腐蚀前的微观组织
Fig.2
Microstructure of HEA-15 alloy before corrosion (a) and enlarged region in Fig.2a (b)
表1 HEA-15合金中各元素质量分数
Table 1
| Region | Fe | Cr | Mo | Nb | Al | Ni |
|---|---|---|---|---|---|---|
| Map scanning | 34.5 | 29.8 | 14.9 | 2.9 | 2.9 | 15.0 |
| Laves phase (white) | 29.1 | 20.6 | 23.7 | 13.4 | 1.7 | 11.5 |
| FCC phase (gray) | 36.9 | 32.8 | 13.0 | - | 2.6 | 14.7 |
| B2-NiAl phase (black) | 30.4 | 28.0 | 11.0 | - | 6.9 | 23.7 |
2.2 腐蚀后表面组织分析
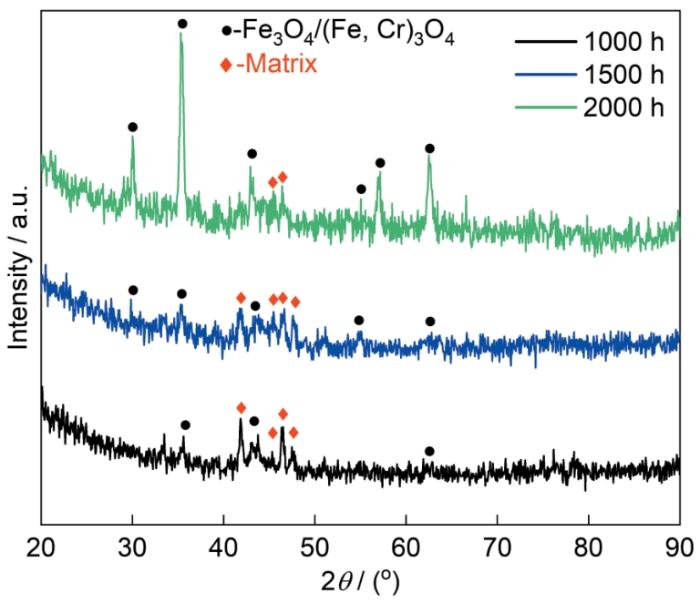
图3是HEA-15合金在液态LBE中腐蚀不同时间后表面的XRD谱。可以看出,HEA-15合金表面生成了Fe3O4和(Fe,Cr)3O4,未检测到其它的氧化物,均探测到了基体峰。
图3
图3
不同腐蚀时间后HEA-15合金表面的XRD谱
Fig.3
XRD patterns of HEA-15 alloy surface after different corrosion times
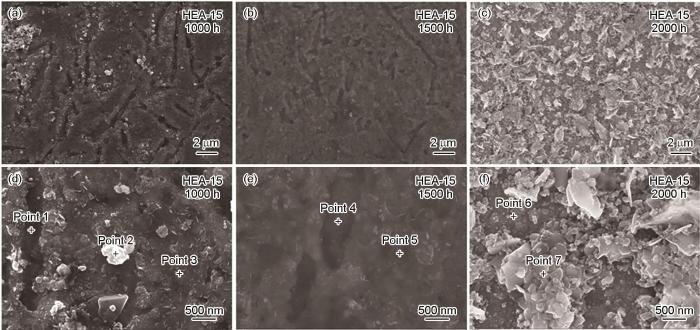
图4和表2是不同腐蚀时间后HEA-15合金表面形貌和能谱点扫描结果。腐蚀1000 h后,HEA-15合金的表面形貌由平坦区域、凹陷区域以及平坦区域上方的白色结节状突起物(图4a和d)组成。与腐蚀1000 h后相比,腐蚀1500 h后表面的白色结节状突起物明显减少(图4b和e)。结合表2中的点1~5和腐蚀后XRD结果可以判断,腐蚀1000和1500 h后,平坦和凹陷区域均为Fe-Cr尖晶石,白色突起物是贫Cr的Fe-Cr尖晶石。腐蚀2000 h后,合金表面出现了大量的块状突起物 (图4c和f),结合表2中点6、7和腐蚀后XRD结果可以判断,在块状突起物附近的平坦区域为Fe-Cr尖晶石,外层的块状突起物为Fe3O4,块状Fe3O4尺寸约为1.5 µm。
图4
图4
不同腐蚀时间后HEA-15合金表面的微观形貌
Fig.4
Surface morphologies of HEA-15 alloy after corrosion for 1000 h (a, d), 1500 h (b, e) and 2000 h (c, f)
表2 图4的点扫描数据 (atomic fraction / %)
Table 2
| Region | O | Fe | Cr | Ni | Al | Mo | Nb |
|---|---|---|---|---|---|---|---|
| Point 1 | 35.6 | 22.2 | 23.2 | 7.5 | 3.3 | 6.2 | 2.0 |
| Point 2 | 54.0 | 19.5 | 17.1 | 3.5 | 2.8 | 3.1 | - |
| Point 3 | 37.8 | 21.2 | 23.4 | 9.4 | 3.9 | 4.2 | - |
| Point 4 | 41.9 | 18.0 | 20.0 | 7.0 | 3.6 | 7.0 | 2.6 |
| Point 5 | 36.6 | 24.4 | 23.4 | 8.3 | 3.1 | 4.2 | - |
| Point 6 | 29.6 | 26.4 | 23.9 | 8.9 | 3.4 | 6.5 | 1.3 |
| Point 7 | 60.0 | 26.3 | 8.1 | 1.9 | 1.7 | 2.0 | - |
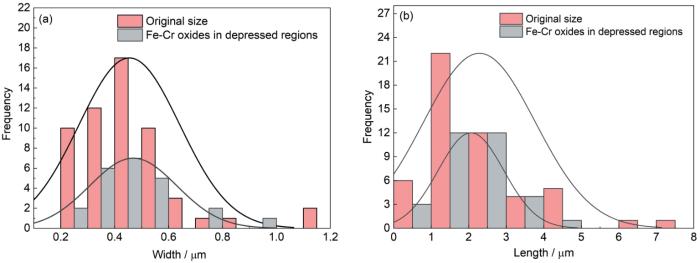
腐蚀1000和1500 h后,HEA-15合金在凹陷区域的Fe-Cr尖晶石可以检测到Nb,其它区域均检测不到Nb (图4和表2),可以推测凹陷区域下方基体为Laves相。为了进一步证实此猜测,对图2a中Laves相和图4a中氧化层凹陷区域宽度和长度进行了统计对比,如图5所示。可以看出,1000 h腐蚀后合金表面氧化层凹陷区域的宽度和长度都包含在其原始组织的Laves相尺寸范围内,因此可以判定氧化层凹陷区域下方基体是Laves相。而腐蚀2000 h后,合金表面没有发现明显的凹陷区域(图4c和f),但是在块状突起物附近的平坦区域(Fe-Cr尖晶石区域)可以检测到Nb,推测该区域下方基体也是Laves相。
图5
图5
图2a中Laves相和图4a中氧化层凹陷区域宽度和长度对比
Fig.5
Comparison of the widths (a) and lengths (b) between Laves phases in Fig.2a and the depression areas of the oxide layer after corrosion for 1000 h in Fig.4a
2.3 腐蚀后截面组织分析
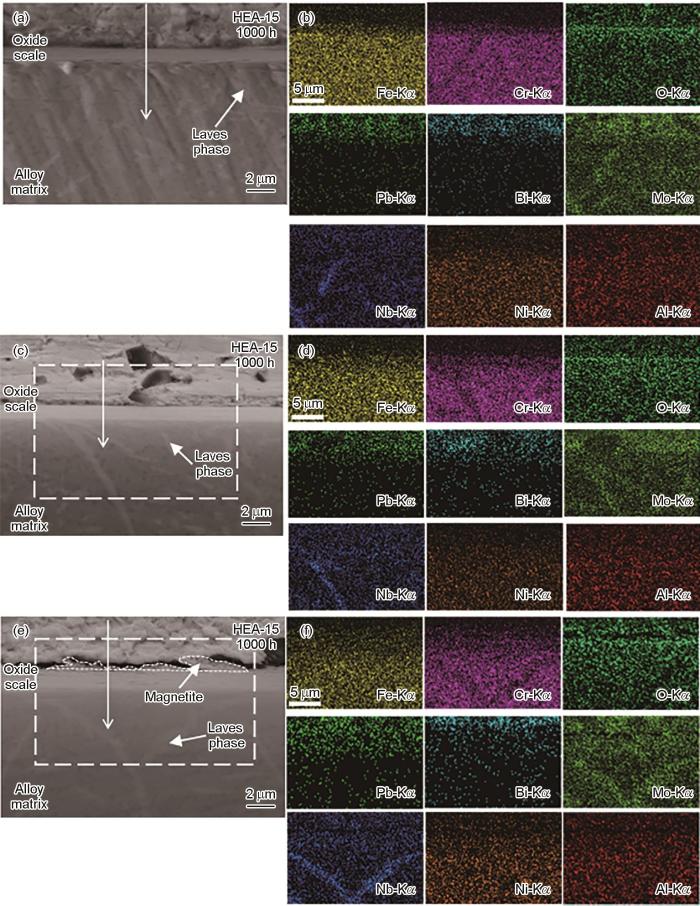
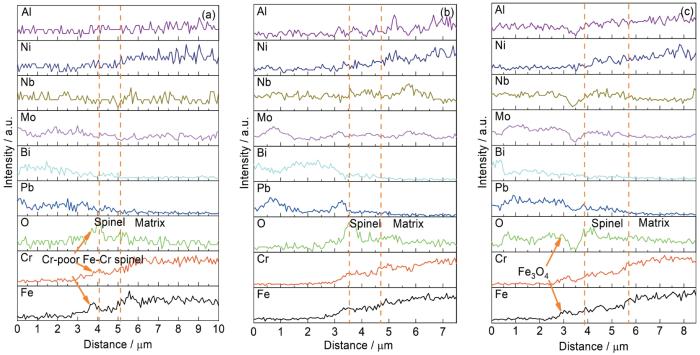
图6为不同腐蚀时间后HEA-15合金截面SEM和对应的EDS面扫描结果,图7是图6对应的线扫描结果。腐蚀1000和1500 h后,HEA-15合金表面均分布着厚度均匀的连续氧化层(图6a和c)。在氧化层区域观察到Fe和Cr的富集,没有Al的富集(图6b和d),结合图4可知,该氧化层为Fe-Cr尖晶石层,对应的是平坦和凹陷区域。在Fe-Cr尖晶石层外层出现明显的Fe和O信号峰和微弱的Cr信号峰 (图7a),再结合图4可知,该氧化物为贫Cr的Fe-Cr尖晶石,对应的是白色突起物。腐蚀2000 h后,合金表面的氧化层出现了明显的分离(图6e和f),结合图7c和图4可知,脱落的氧化物为Fe3O4。Fe3O4的脱落可能是因为其在向外生长过程中容易将LBE包裹住;同时其为疏松多孔结构,也容易导致LBE渗入,在LBE的作用下发生脱落[22]。未脱落的氧化层为Fe-Cr尖晶石。在基体和氧化层界面处未明显观察到因元素扩散而形成的过渡层[23]。结合图6a、c、e和图7可以看出,腐蚀1000和1500 h后的Fe-Cr尖晶石厚度分别约为1.12和1.24 µm,腐蚀2000 h后,Fe-Cr尖晶石的厚度约为1.93 µm,Fe3O4厚度约为0.70 µm。
图6
图6
HEA-15合金不同腐蚀时间后的截面形貌和EDS面扫描图
Fig.6
Cross-sectional morphologies (a, c, e) and EDS maps (b, d, f) of HEA-15 alloy after corrosion for 1000 h (a, b), 1500 h (c, d) and 2000 h (e, f)
图7
图7
HEA-15合金不同腐蚀时间后的线扫描结果
Fig.7
Line scan results of HEA-15 alloy after different corrosion times corresponding to the cross-section in Fig.6a (a), Fig.6c (b) and Fig.6e (c)
3 讨论
HEA-15合金在500℃、氧浓度为10-6%的液态LBE中腐蚀1000~2000 h后,Laves相区域表面仅生成了Fe-Cr尖晶石层,而FCC/B2-NiAl相区域生成了内层Fe-Cr尖晶石层,外层首先生成了贫Cr的Fe-Cr尖晶石,而后脱落,最终生成了Fe3O4。合金表面氧化层的生长特征与合金相组成引起的元素扩散差异有关。
HEA-15合金中的Fe和Cr在液态LBE中溶解度较高,导致其表面的Fe和Cr会向外扩散到液态LBE中[4,24],LBE中的O会向合金基体扩散。根据Tsai等[25]的研究,Cr和Fe在Co-Cr-Fe-Mn-Ni高熵合金中扩散速率较快。因此可推测,腐蚀1000 h后,向基体扩散的O与从基体向外扩散的Cr、Fe结合在表面形成Fe-Cr尖晶石。同时,Lobnig等[26]的研究表明,Fe-Cr-Ni-Mn合金表面生成的Cr2O3对Fe向外扩散速率影响较小,而对Cr的向外扩散速率降低影响较为明显。因此可推测,在本研究中Fe可以通过Fe-Cr尖晶石向外扩散,而Cr的向外扩散会受到抑制。另外,HEA-15合金中包含FCC相、Laves相和B2-NiAl相,元素在Laves相中扩散相对较为困难[27,28]。因此,在FCC/B2-NiAl相中的Fe和少量Cr通过Fe-Cr尖晶石向外扩散在其表面生成了贫Cr的Fe-Cr尖晶石,在Laves相表面只生成了薄薄的Fe-Cr尖晶石(图4a和d)。腐蚀1500 h后,Laves相区域上方仍然只生成了一层连续的Fe-Cr尖晶石层。相比于腐蚀1000 h后,发现FCC/B2-NiAl表面贫Cr的Fe-Cr尖晶石区域明显减少,一方面可能是由于Fe-Cr尖晶石层不断增厚,使得Fe和Cr向外扩散变得更加缓慢,导致FCC/B2-NiAl相区域外层贫Cr的Fe-Cr尖晶石难以继续生长,另一方面可能是由于腐蚀1000 h后生成的贫Cr的Fe-Cr尖晶石不连续(图4b和e),在腐蚀过程中发生脱落。腐蚀2000 h后,Laves相区域上方仍然为Fe-Cr尖晶石层。相比于腐蚀1000和1500 h后,由于Fe-Cr尖晶石层厚度继续增加,进一步抑制了Cr的向外扩散,而Fe继续向外扩散,在FCC/B2-NiAl相外层形成了Fe3O4层,内层为Fe-Cr尖晶石层(图4c和f)。在合金和氧化层的界面处也没有观察到明显的过渡层,可能是本研究中的高熵合金3种相分布较为均匀,同时Laves相对基体元素的亲和力都较高[27,28],因此很难发生元素大量向外扩散。
表3 不同腐蚀时间氧化层的厚度
Table 3
| Oxide type | 1000 h | 1500 h | 2000 h |
|---|---|---|---|
| Fe-Cr Spinel | 1.12 | 1.24 | 1.93 |
| Fe3O4 | - | - | 0.70 |
4 结论
(1) 制备了具有FCC相、Laves相和B2-NiAl相的多相高熵合金,其中,FCC相、Laves相和B2-NiAl相占比分别约为75.95%、19.09%和4.96%。
(2) HEA-15合金表现出优异的耐铅铋腐蚀性。在500℃、氧浓度为10-6%的液态LBE中生成了连续致密保护性的内层氧化物Fe-Cr尖晶石,外层氧化物只出现在FCC/B2-NiAl相区域,随着腐蚀时间的增加,外层氧化层发生了从贫Cr的Fe-Cr尖晶石到Fe3O4的演变。腐蚀1000、1500和2000 h,HEA-15合金表面的尖晶石厚度分别为1.12、1.24和1.93 µm。Fe3O4只出现在腐蚀2000 h样品中,厚度为0.70 µm。这与合金中Laves相有关,Laves相对基体元素的亲和力高,可以阻碍基体金属元素的向外扩散,有效的延迟氧化层的生长。
(3) XRD和微观分析表明,HEA-15合金在500℃、氧浓度为10-6%的液态LBE环境中腐蚀1000~2000 h后,均未发生明显的元素溶解和相变,保持稳定的相结构。
参考文献
Generation IV international forum: a decade of progress through international cooperation
[J].
A Technology Roadmap for Generation IV Nuclear Energy Systems
[R].
Lead-alloy coolant technology and materials-technology readiness level evaluation
[J].
Handbook on lead-bismuth eutectic alloy and lead properties, materials compatibility, thermalhydraulics and technologies
[R].
Behavior of steels in flowing liquid PbBi eutectic alloy at 420-600 ℃ after 4000-7200 h
[J].
A review of steel corrosion by liquid lead and lead–bismuth
[J].
Opportunities for the LWR ATF materials development program to contribute to the LBE-cooled ADS materials qualification program
[J].
Influence of liquid lead and lead-bismuth eutectic on tensile, fatigue and creep properties of ferritic/martensitic and austenitic steels for transmutation systems
[J].
Study on corrosion products of T91 and 316L steels in oxygen controlled LBE for 600 hrs
[J].
T91和316L钢在氧控铅铋中600小时后腐蚀产物分析
[J].
Corrosion behavior of 316L and T91 steels in stagnant lead-bismuth eutectic at 550 °C
[J].
316L和T91不锈钢在550℃静态铅铋合金中的腐蚀行为
[J].
Al-containing ODS steels with improved corrosion resistance to liquid lead–bismuth
[J].
High entropy alloys: a focused review of mechanical properties and deformation mechanisms
[J].
Mechanical properties of high-entropy alloys with emphasis on face-centered cubic alloys
[J].
High-entropy alloys with heterogeneous microstructure: processing and mechanical properties
[J].
Research progress in refractory high entropy alloys for nuclear applications
[J].
核用难熔高熵合金的研究进展
[J].
Microstructures and properties of high-entropy alloys
[J].
Corrosion behavior of high strength AlCrFeNi multi-principal-component alloy in lead-bismuth alloy
[J].
铅铋合金环境中高强AlCrFeNi多主元合金的腐蚀行为
[J].
A promising new class of high-temperature alloys: eutectic high-entropy alloys
[J].High-entropy alloys (HEAs) can have either high strength or high ductility, and a simultaneous achievement of both still constitutes a tough challenge. The inferior castability and compositional segregation of HEAs are also obstacles for their technological applications. To tackle these problems, here we proposed a novel strategy to design HEAs using the eutectic alloy concept, i.e. to achieve a microstructure composed of alternating soft fcc and hard bcc phases. As a manifestation of this concept, an AlCoCrFeNi2.1 (atomic portion) eutectic high-entropy alloy (EHEA) was designed. The as-cast EHEA possessed a fine lamellar fcc/B2 microstructure, and showed an unprecedented combination of high tensile ductility and high fracture strength at room temperature. The excellent mechanical properties could be kept up to 700 degrees C. This new alloy design strategy can be readily adapted to large-scale industrial production of HEAs with simultaneous high fracture strength and high ductility.
Influence of alloying elements (Cu, Ti, Nb) on the microstructure and corrosion behaviour of AlCrFeNi-based high entropy alloys exposed to oxygen-containing molten Pb
[J].
An energetic evaluation of dissolution corrosion capabilities of liquid metals on iron surface
[J].Using first principles calculations, dissolution corrosion of liquid metals on iron surfaces has been investigated by calculating adsorption energies of metal atoms in the liquid phase on the surface and escape energies of surface Fe atoms. The adsorption energies, characterizing the stability of the adsorbed atoms on the investigated surfaces, show that Bi is more stable than Pb and Au. The escape energies, representing the energy required for an Fe atom to escape from the surface, show that adsorbed Pb makes surface Fe atoms escape more easily than Bi and Au. The combination of adsorption energy and escape energy indicates that the corrosion capabilities of liquid metals decrease in the order Bi > Pb > Au. This is further proved by the investigation of surface properties, such as inter-layer distance, magnetic momentum and charge density difference. The results are consistent with experimental results that Fe can be corroded more severely in Bi than in Pb. In the case of liquid alloys, chemical proportions of compositions are incorporated to evaluate the corrosion capabilities of Pb-Bi eutectic (LBE) and Pb-Au eutectic (LGE). It is found that LBE has more severe corrosion capability than LGE. The energetic calculation is further developed in evaluating the effect of alloying elements in popular steels on the dissolution corrosion. The results indicate that Si, V, Nb and Mo may mitigate the dissolution corrosion of martensite steels in liquid Pb, Bi and Au.
Anomalous decrease in X-ray diffraction intensities of Cu-Ni-Al-Co-Cr-Fe-Si alloy systems with multi-principal elements
[J].
An atomistic insight into the corrosion of the oxide film in liquid lead-bismuth eutectic
[J].When used as a protective scale, the Fe3O4 layer covering the stainless steel surface in accelerator driven subcritical systems (ADS) is corroded by liquid lead-bismuth eutectics (LBE). By performing theoretical calculations, we reveal that both Pb and Bi at the interface between the LBE and the Fe3O4 scale, favorably adsorb onto the Fe3O4 surfaces, weakening the strength of Fe-O bonds nearby significantly. This facilitates the movement of iron atoms toward the deposited Pb(Bi) and away from the Fe3O4 surface, thus causing corrosion. Such corrosion behavior becomes severe if oxygen vacancies exist in the surface region.
Corrosion resistance and microstructural stability of austenitic Fe-Cr-Al-Ni model alloys exposed to oxygen-containing molten lead
[J].
Thermodynamic assessment of solubility and activity of iron, chromium, and nickel in lead bismuth eutectic
[J].
Sluggish diffusion in Co-Cr-Fe-Mn-Ni high-entropy alloys
[J].
Diffusion of cations in chromia layers grown on iron-base alloys
[J].
The role of Nb on the high temperature oxidation behavior of CoCrFeMnNbxNi high-entropy alloys
[J].
Retardation of 20%Cr steel oxidation with Laves phase precipitation
[J].
Corrosion behavior and mechanisms of ferritic/martensitic steels and austenitic stainless steels in liquid lead-bismuth eutectic
[J].
铁素体/马氏体钢和奥氏体不锈钢的液态铅铋腐蚀行为与机理
[J].
Effect of Ce on microstructure evolution of oxide scale for CLAM steel exposed to LBE containing 10-6 wt% oxygen at 500oC
[J].
Ellingham diagram
[A].
The influence of alloying elements on the development and maintenance of protective scales
[J].
Corrosion behavior and surface treatment of cladding materials used in high-temperature lead-bismuth eutectic alloy: a review
[J].