在过去的几十年中,温度对压水堆一回路系统中常用低合金钢在硼酸溶液中腐蚀行为和机理的影响已被广泛报道[3,8,9]。Xiao等[10,11]认为温度对A508Ⅲ钢在硼酸溶液中的腐蚀速率与溶解氧浓度有关。在饱和溶解氧时,腐蚀速率随温度升高先增加后减小;在低溶解氧条件下,腐蚀速率单调增加。此外,通过改变温度调节低合金钢表面氧化层的成分和微观形貌可以降低应力腐蚀敏感性,从而降低镍基低合金钢的应力腐蚀开裂[8,12]。Lim等[13]研究表明SA508低合金钢在硼酸溶液中的腐蚀速率随硼酸浓度的增加而稳步增大;随温度的升高先逐渐增大后迅速下降,归因于腐蚀产物膜成分的变化。Fyfitch和Xu[14]研究表明低合金钢在浓硼酸溶液中的腐蚀速率随温度的升高急剧增加。虽然目前有大量低合金钢在硼酸溶液中的腐蚀数据,但对于螺栓钢在硼酸溶液中的腐蚀研究相对较少,国外仅有一些美标A193 B7钢的腐蚀数据[15],而对于国产42CrMoE螺栓用钢在压水堆一回路泄漏硼酸溶液中的腐蚀行为与机理尚未解释清楚。
本文通过模拟压水堆一回路硼酸溶液泄漏位点的工作环境,采用电化学阻抗谱(EIS)、动电位极化曲线和浸泡失重实验研究了25~97.5℃时42CrMoE钢在硼酸溶液中的腐蚀特性,有助于了解42CrMoE螺栓用钢在压水堆泄漏硼酸环境中腐蚀的机理。
1 实验方法
实验所用材料为压水堆一回路螺栓用42CrMoE低合金钢,其主要化学成分(质量分数,%)为:C 0.39、Si 0.34、Mn 0.60、Cr 0.98、Mo 0.17、P 0.0087、S 0.0037、Fe余量。将试样打磨至2000#后机械抛光,用4%硝酸酒精浸蚀,观察其金相显微组织。
根据调研分析压水堆核电站一回路工况,在硼酸溶液泄漏位点,硼酸溶液与LiOH存在不同程度的浓缩,因此使用等比例浓缩的8800 mg/L H3BO3 + 13.5 mg/L LiOH溶液模拟压水堆核电站一回路螺栓附近局部硼酸浓溶液。温度选用25、60和97.5℃,以模拟一回路系统中不同螺栓表面不同硼酸溶液的温度梯度。
电化学测试试样尺寸为 10 mm × 10 mm × 2 mm,用铜导线点焊连接,将其镶嵌于环氧树脂并保留1 cm2的工作面积。镶嵌后的试样表面用SiC砂纸打磨至2000#,清洗吹干待用。在25℃ (pH = 4.53)、60℃ (pH = 4.47)、97.5℃ (pH = 4.45)的温度下,采用三电极体系:Pt片为辅助电极,Ag/AgCl电极为参比电极,42CrMoE为工作电极进行电化学测试。在电化学测试之前,进行30 min的开路电位(OCP)使表面稳定,以10 min内上下波动10 mV范围内为稳定标准。EIS测试频率范围为105~10-2 Hz,幅值为10 mV;动电位极化曲线的电势扫描范围为-1.2~0.2 V,扫描速率为0.5 mV/s。所有测试至少重复3次以减小实验误差。
浸泡实验采用50 mm × 25 mm × 3 mm的长方体试样,挂孔直径为2 mm。浸泡前用碳化硅砂纸将样品表面研磨至800#,然后在无水乙醇和去离子水中超声清洗,吹干后称重。为了减小误差,每种条件设置两块平行试样。实验分为5、10、20和30 d 4个周期进行。浸泡结束后,利用数码相机对锈层的宏观形貌进行观察。刮取表面的腐蚀产物在研钵中研磨制成粉末,利用X射线衍射仪(XRD, Ultima IV)进行相组成分析。然后,用除锈液(500 mL HCl + 500 mL H2O + 3.5 g六次甲基四胺)去除腐蚀产物,除锈后的试样用去离子水和无水乙醇洗净。吹干后再次使用电子天平称重并计算其腐蚀速率。腐蚀速率计算公式如下。使用扫描电子显微镜(SEM, FEI Quanta 250)和3D激光共聚焦显微镜(CLSM, KEYENCE VK-X250)对除锈样品的微观腐蚀形貌进行观察和测量。
其中,v为腐蚀速率,mm/a;W为腐蚀失重,g;S为试样暴露面积,cm2;T为暴露时间,h;D为材料密度,g/cm3。
2 实验结果
2.1 42CrMoE钢显微组织
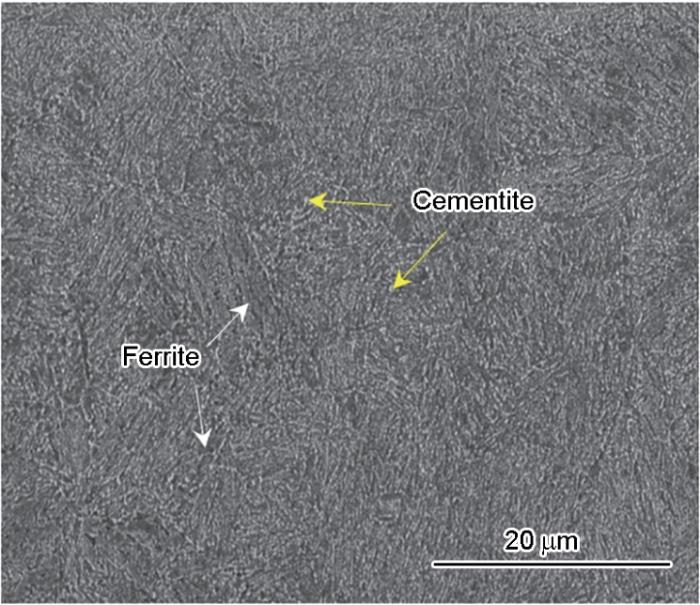
42CrMoE的金相组织如图1所示。从图中可以看出,42CrMoE为铁素体+渗碳体组织,组织相对比较均匀、晶粒尺寸适中。
图1
2.2 电化学测试结果
图2
图2
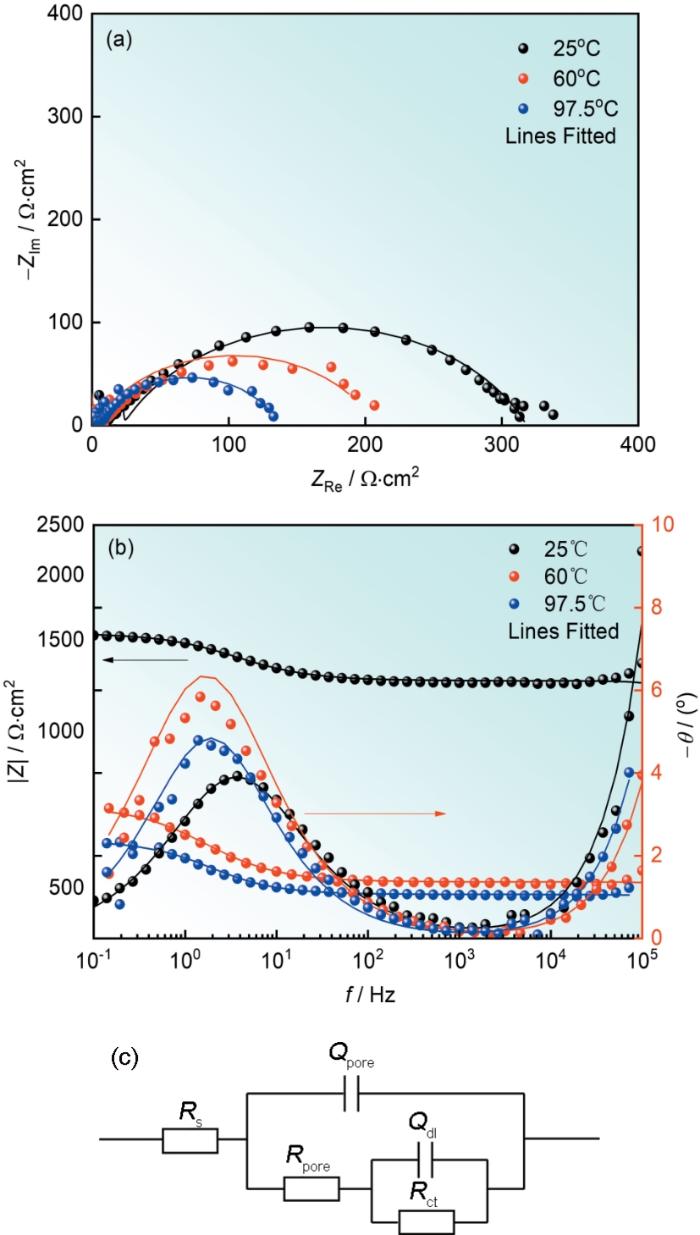
42CrMoE钢在不同温度下8800 mg/L硼酸溶液中的EIS和等效电路
Fig.2
Nyquist (a) and Bode (b) plots of 42CrMoE steel in 8800 mg/L boric acid solution at different temperatures and equivalent circuit diagram (c)
表1 42CrMoE 钢在不同温度的8800 mg/L硼酸溶液中阻抗谱拟合结果
Table 1
Temperature oC | Rs Ω·cm2 | Qpore × 10-4 Ω-1·cm-2·s n | npore | Rpore,sol Ω·cm2 | Qdl × 10-4 Ω-1·cm-2·s n | ndl | Rct Ω·cm2 | χ2 × 10-4 |
|---|---|---|---|---|---|---|---|---|
| 25 | 1.15 | 1.36 × 10-2 | 1.00 | 883.6 | 4.62 | 0.70 | 302.4 | 0.49 |
| 60 | 1.26 | 8.25 | 0.80 | 117.20 | 5.48 | 1.00 | 205.00 | 4.30 |
| 97.5 | 1.15 | 2.01 | 0.80 | 3.02 | 7.20 | 0.90 | 68.00 | 0.71 |
图3
图3
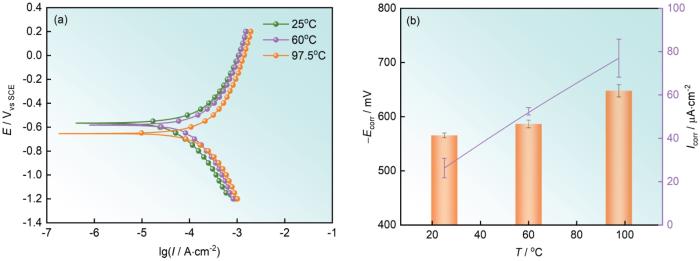
42CrMoE钢在不同温度下硼酸溶液中的动电位极化曲线和拟合获得的腐蚀电位和腐蚀电流密度
Fig.3
Potentiodynamic polarization curves (a) of 42CrMoE steel in boric acid solution at different temperatures, and fitting values of corrosion potential and corrosion current density (b)
2.3 浸泡实验分析
为了研究不同温度条件下42CrMoE钢在硼酸溶液中的腐蚀速率,开展了不同温度硼酸溶液中的浸泡实验。图4为不同温度下42CrMoE钢在硼酸浓度为8800 mg/L的溶液中不同浸泡周期(5、10、20和30 d)的腐蚀速率。由图可知,42CrMoE钢在近饱和硼酸溶液中的腐蚀速率明显随温度的升高而增大。在温度为25和60℃时,42CrMoE钢的腐蚀速率相对较小,平均腐蚀速率均小于1 mm/a。而当温度为97.5℃时,平均腐蚀速率均大于1 mm/a。此外,25和60℃时,42CrMoE钢在硼酸溶液中的腐蚀速率随浸泡周期的延长呈现逐渐减小的趋势,低温时在表面形成一层腐蚀产物膜,阻碍了离子的扩散,从而降低了腐蚀速率。97.5℃时,腐蚀速率呈现先减小后快速增大的趋势,这是因为在高温时浸泡前期生成的腐蚀产物膜有一定的阻碍作用,而长期浸泡时腐蚀速率增大的原因可能是钢表面腐蚀产物的脱落或溶解,导致硼酸溶液中的腐蚀性物质更容易扩散到钢表面促进钢的溶解[13]。
图4
图4
42CrMoE钢在不同温度下8800 mg/L硼酸溶液中的腐蚀速率
Fig.4
Corrosion rates of 42CrMoE steel in 8800 mg/L boric acid solution at 25oC (a), 60oC (b) and 97.5oC (c)
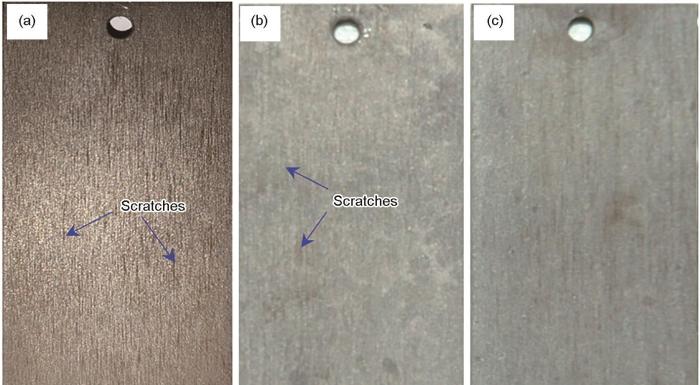
浸泡样品除锈后42CrMoE钢表面的宏观腐蚀形貌如图5所示。由图可见,在低温低浓度条件下,42CrMoE钢除锈后试样均存在明显的打磨痕迹,说明试样仅发生轻微的腐蚀。随着温度的升高,试样表面打磨痕迹逐渐消失,腐蚀愈发严重,出现了明显的局部点蚀坑。
图5
图5
42CrMoE钢在不同温度下8800 mg/L硼酸溶液中浸泡30 d后的宏观腐蚀形貌
Fig.5
Macroscopic corrosion morphologies of 42CrMoE steel after immersion in 8800 mg/L boric acid solution for 30 d at 25oC (a), 60oC (b) and 97.5oC (c)
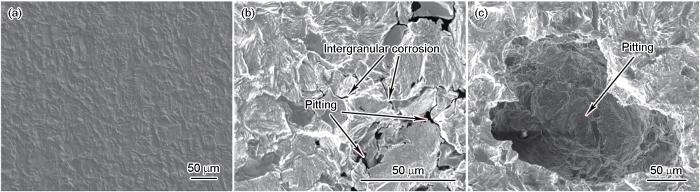
不同温度下42CrMoE钢在硼酸溶液中腐蚀后的微观形貌如图6所示。由图可见,在25℃下腐蚀程度轻微,呈现均匀腐蚀的特征。当温度升高至60℃时,试样表面存在明显的沿晶腐蚀现象,并且在晶界交界处存在少量的局部点蚀,点蚀坑尺寸较小。当温度升至97.5℃时,42CrMoE钢表面的点蚀坑快速生长,尺寸明显增大。这说明温度升高可以明显促进局部点蚀坑的生长,并且42CrMoE钢在硼酸溶液中的腐蚀类型从低温时的均匀腐蚀转变为高温时的局部腐蚀。
图6
图6
42CrMoE钢在不同温度下8800 mg/L硼酸溶液浸泡30 d后的微观腐蚀形貌
Fig.6
Micro-corrosion morphologies of 42CrMoE steel immersed in 8800 mg/L boric acid solution for 30 d at 25oC (a), 60oC (b) and 97.5oC (c)
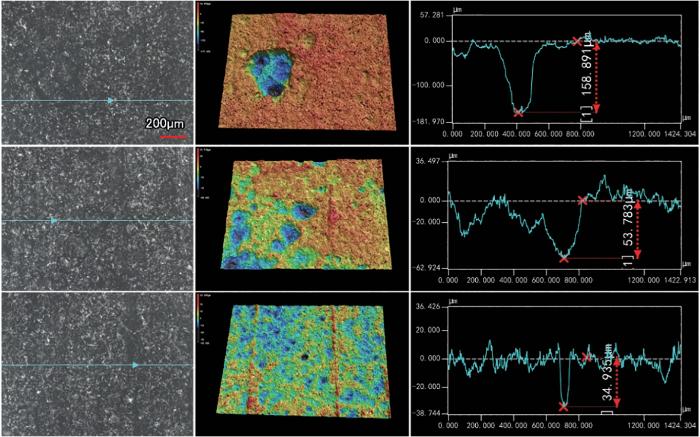
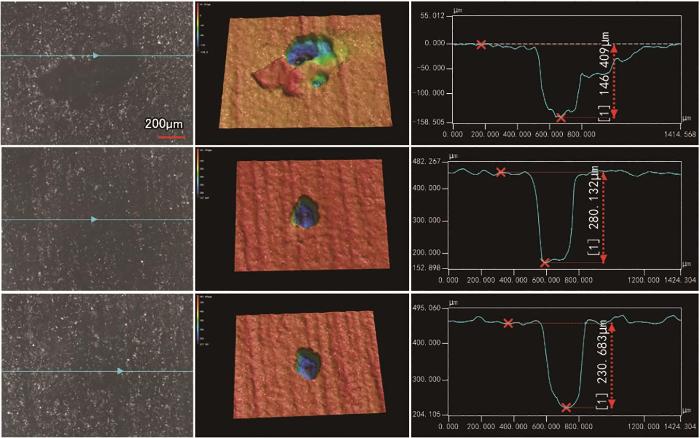
蚀坑深度是评价试验钢安全服役的一个重要指标,为了定量分析,采用3D激光共聚焦对浸泡不同周期的腐蚀形貌进行了观察。图7~9为采用3D CLSM观察不同温度下42CrMoE钢在硼酸溶液中浸泡30 d后的表面形貌。由图可见,25℃时,42CrMoE钢表面主要以均匀腐蚀为主,存在轻微的局部点蚀,点蚀坑的平均深度约为10.14 μm;温度为60℃时,42CrMoE钢在硼酸溶液中发生了较为严重的局部腐蚀,点蚀坑的生长方向主要以横向扩展为主,表面点蚀坑的平均深度约为82.54 μm,部分点蚀坑的深度高达158.89 μm;97.5℃时,42CrMoE钢表面发生了严重的点蚀,点蚀坑的平均深度约为219.07 μm,部分点蚀坑的深度高达280.13 μm。与25℃时的点蚀坑相比,97.5℃时点蚀坑的尺寸生长了约20倍。因此,42CrMoE钢在高浓度的硼酸溶液中随着温度的升高,表面局部点蚀坑尺寸显著增加。
图7
图7
42CrMoE钢在25℃下8800 mg/L硼酸溶液中浸泡30 d后LSCM表面形貌分析
Fig.7
LSCM surface analysis results of 42CrMoE steel after 30 d immersed in 8800 mg/L boric acid solution at 25oC
图8
图8
42CrMoE钢在60℃下8800 mg/L硼酸溶液中浸泡30 d后LSCM表面形貌分析
Fig.8
LSCM surface analysis results of 42CrMoE steel after 30 d immersed in 8800 mg/L boric acid solution at 60oC
图9
图9
42CrMoE钢在97.5℃下8800 mg/L硼酸溶液中浸泡30 d后LSCM表面形貌分析
Fig.9
LSCM surface analysis results of 42CrMoE steel after 30 d immersed in 8800 mg/L boric acid solution at 97.5oC
2.4 腐蚀产物分析
利用XRD对不同温度下42CrMoE钢浸泡周期为30 d的锈层进行物相分析,如图10所示。结果表明,不同温度下42CrMoE钢在模拟硼酸溶液中形成的腐蚀产物主要相由Fe3O4、α-FeOOH和γ-FeOOH组成。低温时,42CrMoE钢发生轻微腐蚀,少量的腐蚀产物吸附在钢表面。当温度升高时,腐蚀产物的相组成并没有太大的改变,且成分主要以Fe3O4和γ-FeOOH为主,具有保护性的α-FeOOH产物相对较少。
图10
图10
42CrMoE钢在不同温度下8800 mg/L硼酸溶液中浸泡30 d后表面XRD谱
Fig.10
XRD patterns of 42CrMoE steel after 30 d immersion in 8800 mg/L boric acid solution at different temperatures
3 分析讨论
电化学测试结果表明,温度升高降低了42CrMoE钢在硼酸溶液中耐蚀性。从电化学动力学角度看, 42CrMoE钢在硼酸溶液中的腐蚀电流密度随温度升高而增大,从而加速了其阳极溶解过程。此外,螺栓表面不同温度的硼酸溶液中会溶解不同浓度的O2,高溶解氧与低pH硼酸溶液中会生成Fe3+离子,Fe3+发生水解反应以及氧化还原反应促进基体的溶解(
浸泡实验结果(图4)表明,42CrMoE钢在硼酸溶液中的腐蚀速率随温度升高而急剧增大,这归因于硼酸溶液泄漏到螺栓表面发生腐蚀的过程中,低温时腐蚀产物吸附在基体表面形成一层腐蚀产物膜,这层膜抑制了金属表面与溶液的传质过程。众所周知,α-FeOOH是热力学稳定相,可有效提高腐蚀产物膜的稳定性[30,31]。XRD结果(图10)表明,随着温度逐渐升高,腐蚀产物膜中Fe3O4的含量有所增加,具有保护性的α-FeOOH含量相对减少,降低了腐蚀产物膜的保护性。从而导致42CrMoE钢在硼酸溶液的腐蚀行为从低温时的均匀腐蚀转变为严重的局部点蚀。由图7~9可知,42CrMoE钢在硼酸溶液中的局部点蚀坑深度从8.671 μm增加到280.132 μm,这归因于硼酸溶液的pH降低引起点蚀坑内形成较大的pH差,导致局部腐蚀加剧[32]。此外,在酸性环境中温度升高可以加速腐蚀产物膜的溶解。这种双重作用明显加速了42CrMoE钢在高温硼酸溶液中的腐蚀。
4 结论
(1) 随着温度的升高,42CrMoE钢在硼酸溶液中的腐蚀电流密度增大和硼酸溶液的pH降低,导致42CrMoE钢在硼酸溶液中的耐蚀性显著降低。
(2) 42CrMoE钢在硼酸溶液中的腐蚀行为从低温时的均匀腐蚀转变为高温时的局部点蚀为主。此外,点蚀坑的尺寸随温度升高显著增大,97.5℃时点蚀坑的尺寸约为25℃时的20倍。
(3) 42CrMoE钢在硼酸溶液中的腐蚀速率随温度升高逐渐增大,在97.5℃时腐蚀速率能够达到1.3 mm/a,约为25℃时的6倍。随着浸泡时间的延长,低温时的腐蚀速率逐渐减小;高温时呈现先减小后增加的趋势。
参考文献
Mechanisms and growth rate models for stress corrosion cracking in high temperature water
[J].
高温水中应力腐蚀开裂机理及扩展模型
[J].
Effects of tempering temperature on the impact toughness of steel 42CrMo
[J].<p>42CrMo heat–resistant steel is a kind of structural steel, which is widely used in structural components such as crane weight–on–wheel, automobile crank shaft, locomotive gear hub and so on, for its good hardening ability, high temperature strength, good creep resistance, and little quenching deformation. However, in industry application, mismatching between the strength and the toughness always occurs for 42CrMo structure components. In order to solve the problem that the strength does not match the toughness in the manufacturing process for the polar crane for the nuclear power station, the effect of tempering temperature on the morphology and distribution of carbides and the impact toughness has been investigated for steel 42CrMo in this study. The experimental results indicated that the microstructure of the quenched steel 42CrMo after 500—650 ℃ tempering was characterized by tempering sorbite. As the tempering temperature increased, the Charpy absorbed energy at –12℃ initially increased and then decreased. The flake carbides after 500 and 530 ℃ tempering are not evenly distributed on the original martensite boundaries, the Charpy absorbed energy are 26 and 44 J, respectively. While the granular carbides are evenly distributed in the microstructure after 600℃ tempering, the Charpy absorbed energy reaches a maximum value of 104 J. When the tempering temperature is higher than 600 ℃, granular carbides coarsened obviously and the Charpy absorbed energy reduced notably. The morphology and distribution of carbides is the key factor that influences the impact toughness of steel 42CrMo. Morphology and structure analysis for the carbide was carried out by TEM together with EDS analysis, the results showed that the carbide after tempering treatment is (Fe, M)3C and Fe, Cr, Mo are the main alloy element in the carbide. When the tempering temperature is in the range of 560—600 ℃, the uniformly–distributed granular carides forms on the matrix and the impact toughness is over 60 J. As the tempering temperature continues increasing, the carbides will coarsen and the impact toughness will decrease. In order to obtain the good strength and toughness matching, for 42CrMo structure, it is recommended that the tempering temperature should be in the range of 550—590 ℃.</p>
回火温度对42CrMo钢冲击韧性的影响
[J].以核电站环形起重机用42CrMo耐热钢为研究对象, 分析了显微组织中碳化物形貌和分布随回火温度的变化及其对冲击韧性的影响.结果表明, 42CrMo钢经水淬后在500-650 ℃区间回火, 显微组织均为回火索氏体.随回火温度上升, -12 ℃冲击功先增加后减小; 经500和530 ℃回火后, 片状碳化物不均匀分布于原马氏体板条界上, 冲击功分别为26和44 J; 600 ℃回火后碳化物呈颗粒状弥散分布, 冲击功达到峰值104 J; 600 ℃以上回火, 颗粒状碳化物明显粗化,冲击功下降.碳化物的形貌和分布是影响42CrMo钢冲击性能的关键因素.
Effects of boric acid and lithium hydroxide on the corrosion behaviors of 316LN stainless steel in simulating hot functional test high-temperature pressurized water
[J].
Determining SCC resistance of stainless steel claddings in high- temperature water by constant load crack growth tests and slow strain rate tests
[J].
Research on ultra-high cycle fatigue behavior of 42CrMoE bolt material for nuclear power station
[J].
核电站用42CrMoE螺栓材料超高周疲劳性能研究
[J].
Dynamic recrystallization kinetics of 42CrMo steel during compression at different temperatures and strain rates
[J].
Review of stress corrosion crack initiation of nuclear structural materials in high temperature and high pressure water
[J].
核用结构材料在高温高压水中应力腐蚀裂纹萌生研究进展
[J].对核电站常用不锈钢和镍基合金等结构材料在服役的高温高压水中的应力腐蚀裂纹萌生测试的实验方法、评价指标、影响因素和萌生机理等几个方面进行论述,并指出目前研究的不足和未来研究趋势。
Effect of temperature and dissolved hydrogen on oxide films formed on Ni and Alloy 182 in simulated PWR water
[J].
Effects of rare earth elements addition on mechanical properties and corrosion behavior of GCr15 bearing steel under different heat treatment conditions
[J].
The effects of temperature and aeration on the corrosion of A508III low alloy steel in boric acid solutions at 25–95°C
[J].
Corrosion and electrochemical behavior of A508Ⅲ low alloy steel in boric acid solutions with different concentrations
[J].
A508Ⅲ低合金钢在不同浓度硼酸溶液中的腐蚀与电化学行为
[J].
Effects of iron content in Ni-Cr-Fe alloys on the oxide films formed in an oxygenated simulated PWR water environment
[J].
Corrosion behavior of SA508 low alloy steels exposed to aerated boric acid solutions
[J].
Boric acid corrosion laboratory investigation of carbon and low-alloy steels in PWR systems
[A].
Boric acid corrosion of light water reactor pressure vessel head materials
[R].
Mechanistic aspect of near-neutral pH stress corrosion cracking of pipelines under cathodic polarization
[J].
Mechanistic aspect of stress corrosion cracking of X80 pipeline steel under non-stable cathodic polarization
[J].
Effect of secondary passivation on corrosion behavior and semiconducting properties of passive film of 2205 duplex stainless steel
[J].
Microstructure evolution and SSCC behavior of strain-strengthened 304 SS pre-strained at room temperature and cryogenic temperature
[J].
Effect of grain size on corrosion behavior of 304 stainless steel in coal chemical high salty wastewater
[J].
Stress Corrosion Cracking Behavior of 35CrMo Steel in Acidic Hydrogen Sulfide Solutions
[J].The combination of electrochemical corrosion technologies were performed to study corrosion of 35CrMo steel in H2S solution with various pH values by simulate the low concentrations of hydrogen sulfide environment of oilfield in the pit. Stress corrosion cracking(SCC) of 35CrMo steel in acidic hydrogen sulfide solutions with various pH values and temperature was investigated by U-bent specimen immersing test and slow strain rate tensile test. It is shown that 35CrMo steel exhibits a high susceptibility to SCC in acidic hydrogen sulfide solutions. The susceptibility and corrosion increases by decreasing the pH value of the solution. The susceptibility varied seriously with the change of pH value. The decrease of pH would accelerate the cathodic process greatly, leading to the increase of corrosion rate and hydrogen charging current density, resulting the local anodic dissolution and hydrogen embrittlement effect more significant. The different susceptibility to SCC of 35CrMo steel in 15-90 ℃, Maximum susceptibility in 35 ℃ and 90 ℃. The SCC mechanism of 35CrMo steel is hydrogen embrittlement(HE) mainly and combination of anodic dissolution(AD) in acidic hydrogen sulfide solutions.
35CrMo钢在酸性H2S环境中的应力腐蚀行为与机理
[J].通过模拟油田井下低浓度H2S的工作环境,采用电化学技术研究35CrMo钢在不同pH的H2S溶液中的腐蚀行为,通过慢应变速率拉伸试验和U形弯试样浸泡试验研究35CrMo在不同pH和温度条件下的H2S介质中的应力腐蚀开裂行为与机理。结果表明,35CrMo钢在酸性H2S环境下SCC敏感性较高,随着介质的pH降低,35CrMo钢的应力腐蚀敏感性增加,腐蚀速率加快。pH降低能够较大地促进35CrMo钢阴极过程的进行,导致腐蚀速率和充氢电流密度均相应增大,从而导致局部阳极溶解作用和氢脆作用增大。在温度为15~90 ℃,35CrMo钢应力腐蚀敏感性不同,35 ℃和90 ℃条件下的应力腐蚀敏感性大。在酸性H2S环境下35CrMo钢的应力腐蚀机制是以氢脆为主,阳极溶解为辅的协同机制。
Influence of ferric ions on corrosion behavior of A508Ⅲ low alloy steel in boric acid solutions
[J].
三价铁离子对低合金钢在硼酸溶液中腐蚀的影响
[J].
Effect of inclusions on initiation of stress corrosion cracks in X70 pipeline steel in an acidic soil environment
[J].
Effect of hydrogen-induced plasticity on the stress corrosion cracking of X70 pipeline steel in simulated soil environments
[J].
Inhibition effect of tannic acid and sodium molybdate for the flow corrosion of 304 stainless steel on 90° elbow
[J].
Microstructure and corrosion behavior of welded joint between 2507 super duplex stainless steel and E690 low alloy steel
[J].
Local additional potential model for effect of strain rate on SCC of pipeline steel in an acidic soil solution
[J].
Effect of cathodic protection on corrosion of pipeline steel under disbonded coating
[J].
Effect of dissolved oxygen on electrochemical corrosion behavior of 2205 duplex stainless steel in hot concentrated seawater
[J].The corrosion behavior of 2205 duplex stainless steel was investigated in hot concentrated seawater with different dissolved oxygen (DO) concentration by electrochemical measurement techniques and surface analysis methods. DO obviously enhances the cathodic reaction process, the formation of passive film and polarization resistance. With increasing the DO concentration from 0.34 to 3.06 mg L-1, the relative contents of Fe2O3 and Cr2O3 and the Cr-enrichment gradually enlarge in the passive film. The higher DO concentrations result in lower defect densities and thicker of space charge layers in the passive films, which may effectively inhibit the intrusion of aggressive chloride ions. The increment in DO concentration clearly increases the pitting potential, but decreases the repassivation potential. It may weaken both the occurrence and repassviation tendencies of stable pitting corrosion.
Fundamental understanding on the effect of Cr on corrosion resistance of weathering steel in simulated tropical marine atmosphere
[J].
Distinct beneficial effect of Sn on the corrosion resistance of Cr–Mo low alloy steel
[J].