Ni-Cr-Mo-V钢是海工装备尤其是舰艇制造过程中广泛应用的一类合金钢,根据其合金元素含量和强度的不同,其应用部位及场景也存在一定差异。同时,随着合金元素含量、夹杂物等成分及微观组织结构的变化,Ni-Cr-Mo-V钢的腐蚀行为也显著不同。为提升舰艇用钢的使役性能,舰艇用合金钢向高强度和耐腐蚀方向发展。因此,研究合金元素及微观组织结构变化对Ni-Cr-Mo-V钢海水环境腐蚀行为的影响对于舰艇用钢选材及防护设计具有重要意义。
钢铁在冶炼过程中不可避免地会形成夹杂物。夹杂物作为合金钢中的结构缺陷,已被证明在腐蚀萌生及扩展过程中起重要作用。夹杂物的类型、尺寸、数量等是诱发局部腐蚀的关键因素[1]。目前,相关学者对夹杂物诱发局部腐蚀的机理进行了大量研究[2~10]。Wang等[2]研究了EH36钢中(Ca,Mg,Al)-O x -S y 夹杂物与局部腐蚀的关系,结果表明夹杂物的溶解顺序为:CaS优先溶解,完全溶解后xCaO·yAl2O3溶解,最后MgO·Al2O3脱落,形成稳定的凹坑。Nishimoto等[6]观察到304L不锈钢中CaS夹杂物在0.1 mol/L NaCl中浸泡1 h期间大部分溶解。CaS优先溶解并引发初期腐蚀。夹杂物与铁基体之间形成的酸性环境进一步促进了夹杂物的溶解[11]。Li等[10]认为夹杂物边界处的结构缺陷区作为优先发生腐蚀的阳极相,夹杂物中的CaS发生化学溶解。Liu等[5]研究了Al2O3夹杂物对Q460 NH钢在模拟海洋环境中局部腐蚀行为的影响,研究表明Al2O3夹杂物是局部腐蚀的主要原因。Zhang等[12]探讨了硫化物-氧化物复合夹杂物对点蚀萌生的影响,结果表明富含硫化铁的区域会优先诱发点蚀,大尺寸夹杂物引起腐蚀的速率比小尺寸夹杂物更快。
此外,由于晶界属于晶体缺陷,并且具有较高的电化学活性[13],晶粒尺寸也影响合金钢的腐蚀行为。目前,关于晶粒尺寸对耐蚀性能的影响存在争议。例如,由于晶界密度不同导致的钝化膜溶解和点蚀程度不同,随着晶粒尺寸的增加,304不锈钢由于钝化膜的稳定性降低而表现出较低的抗点蚀能力[14]。Zhao等[15]研究表明晶粒细化提高了304不锈钢在3.5%(质量分数)NaCl溶液中的耐腐蚀性。晶粒尺寸更细,有利于耐蚀性的提高[16]。而Wang等 [17]提出了相反的观点,他们得出晶粒细化降低了钢在NaCl溶液中的耐蚀性的结论。此外,合金钢中合金元素的添加,如Ni、Cr,会细化晶粒尺寸[18],这些合金元素在材料的腐蚀过程中会促进腐蚀产物层中的α-FeOOH的转化,提高锈层的致密性[19~21]。因此,合金元素和晶粒尺寸对合金高强钢腐蚀行为的影响也不容忽视。
基于上述背景以及舰艇开发对于高性能钢铁材料的需求,本文选择两种Ni-Cr-Mo-V钢为研究对象,探究了两种合金钢夹杂物诱发腐蚀萌生与扩展的差异。采用失重测试、电化学测试评估了两种合金钢的耐蚀性,通过扫描电镜 (SEM)、激光共聚焦显微镜 (CLSM) 对试样表面锈层进行分析,明确了合金元素、夹杂物、微观组织结构对Ni-Cr-Mo-V钢的影响机制,为舰艇用钢的开发与安全服役提供数据支持。
1 实验方法
本文实验选用材料为两种Ni-Cr-Mo-V钢,两种钢的合金元素含量尤其是Ni、Mo含量存在显著差异。为区别两种钢,分别命名为A钢和B钢。表1是两种钢的合金元素含量,其中B钢的含C量略低于A钢,但Ni、Mo含量显著高于A钢。本文实验在人工海水中进行,人工海水组成包括(g/L):NaCl 24.53,Na2SO4 4.09,NaHCO3 0.201,KCl 0.695,MgCl6·H2O 11.1,CaCl2 1.16,KBr 0.101。
表 1 实验中所用两种Ni-Cr-Mo-V钢的化学成分
Table 1
| Material | C | S | P | Si | Mn | Ni | Cr | Mo | V | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| Steel A | 0.08 | 0.003 | 0.01 | 0.28 | 0.51 | 4.62 | 0.56 | 0.47 | 0.06 | Bal. |
| Steel B | 0.05 | 0.003 | 0.01 | 0.27 | 0.83 | 7.27 | 0.59 | 0.64 | 0.07 | Bal. |
电化学实验在Zahner Zennium-E电化学工作站上进行。将试样切割成10 mm × 10 mm × 3 mm,用环氧树脂密封并暴露出1 cm2的工作面积。将工作面打磨至1500#,依次用酒精、蒸馏水清洗,冷风吹干。电化学测试均采用三电极系统,其中,铂片为对电极,饱和甘汞电极(SCE)为参比电极,试样为工作电极。为减小欧姆降,参比电极与工作电极之间的距离保持在1 mm左右。
电化学测试包括开路电位(OCP)、电化学阻抗谱(EIS)以及动电位极化曲线测量。将试样放入人工海水溶液中,对工作电极施加-1 VSCE的电位5 min,以去除试样表面的氧化膜。随后测试OCP 1 h,以达到稳定状态。之后进行EIS测试,EIS扫描范围为105~10-2 Hz,并施加10 mV的正弦扰动电压。EIS实验数据用ZsimpWin软件拟合。动电位极化曲线阳极部分的扫描范围为(OCP-0.05 V)~0.2 V,阴极部分扫描范围为-1.2 V~(OCP+0.05 V),扫描速率均为0.333 mV/s。所有测试均在25℃下进行。每组实验均重复3次以保证重现性。将尺寸为10 mm × 10 mm × 3 mm的试样用SiC砂纸依次研磨至5000#,然后用1.5 μm的金刚石抛光膏抛光至表面无划痕,然后将试样在人工海水溶液中浸泡5 s、10 s、30 s、60 s、600 s、1200 s、1800 s、3600 s 、24 h,依次用蒸馏水、无水乙醇冲洗并冷风吹干。采用线切割在钢板上沿垂直于轧向方向切取10 mm × 10 mm × 3 mm的金相试样,用SiC砂纸逐级打磨至5000粒度,然后抛光至1.5 μm粗糙度,用酒精清洗并冷风吹干。将抛光好的表面用4%硝酸酒精溶液蚀刻10 s,依次用蒸馏水、酒精清洗吹干,采用Lab. A1型金相显微镜观察其微观组织,随后用Quanta 250型SEM观察。将样品在10%高氯酸和90%乙醇的溶液中在25 V下电抛光约15 s,采用电子背散射衍射技术(EBSD,EDAX-TSL)分析其晶体学特征。使用OIM analysis 7.3软件获得反极图(IPF)、核平均取向差(KAM)、晶界角度和平均晶粒尺寸数据。采用SEM和XK-X260K型CLSM对去除表面腐蚀产物后试样表面形貌进行观察。
2 结果及讨论
2.1 微观结构表征
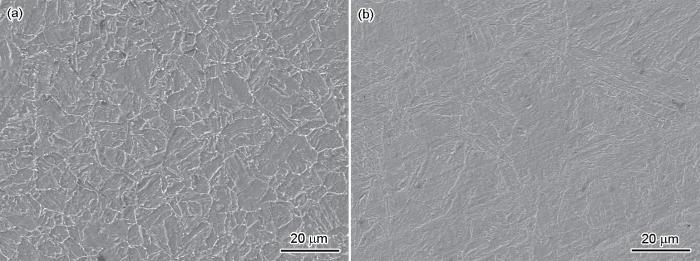
图1是两种Ni-Cr-Mo-V钢的SEM形貌。可以看出,两种合金钢均为贝氏体钢,其中A钢由粒状贝氏体和少量板条贝氏体组成,B钢主要由较细的板条状贝氏体组成。
图1
图1
两种钢的微观组织形态
Fig.1
Microstructure morphologies of steel A (a) and steel B (b)
为了进一步探究两种钢的微观组织差异,采用EBSD对两种钢进行了晶体学信息分析,其结果如图2所示。两种钢均具有均匀的晶体特性,没有明显的择优取向。KAM通常用于表示金属局部晶格的旋转程度,可以定性评估金属内部应变或局部残余应力的分布[22, 23]。晶界和相界的颜色越深,意味着内应力和局部应变积累越大,因此腐蚀萌生的概率越大。与B钢相比,A钢具有较高的KAM值。由图2d可以看出,A钢的晶粒尺寸在2~25 μm范围内,3~6 μm占大部分。B钢则是5~14 μm晶粒为主。与B钢相比,A钢具有更小的晶粒尺寸,这对应于晶界的增加。通常将晶界旋转角(RA)分为两类:取向差角为3°~15°称为低角度晶界(LAGB),取向差角大于15°为高角度晶界(HAGB)。如图2所示,A钢比B钢具有更高比例的HAGB。研究表明,无Nb钢中大多数不可逆陷阱是夹杂物、微孔和HAGB,并且大多数可逆陷阱是位错、小角度晶界和空位[24]。此外,由于HAGB具有较高的能量,其耐蚀性更差。
图2
图2
两种钢基于EBSD测量的IPF取向图,KAM图,晶界分布图和晶粒尺寸统计图
Fig.2
EBSD measurements for steel A (a1-d1) and steel B (a2-d2): (a1, a2) IPF orientation maps, (b1, b2) KAM maps, (c1, c2) grain boundary distribution maps, (d1, d2) grain size statistics
2.2 电化学行为分析
图3
图3
两种合金钢在人工海水溶液中的Nyquist图,Bode图,动电位极化曲线阳极图和极化曲线阴极图
Fig.3
Nyquist diagram (a), Bode diagram (b), anodic (c) and cathodic (d) kinetic potential polarization curves of two steels in artificial seawater solution
由动电位极化曲线结果可以看出,A钢和B钢阳极分支及阴极分支相似,表明了这两种钢具有相同的腐蚀动力学过程。阳极分支表现出钢的活性溶解过程,从开路电位至-875 mV左右为氧还原极限扩散控制区,低于-875 mV为析氢(电荷转移控制)与吸氧极限扩散控制混合区。与A钢相比,B钢具有更高的腐蚀电位,以及较低的电流密度,这表明B钢具有较高的热力学稳定性,其耐蚀性也较好。
2.3 夹杂物初期腐蚀萌生行为
图4
图5
图5
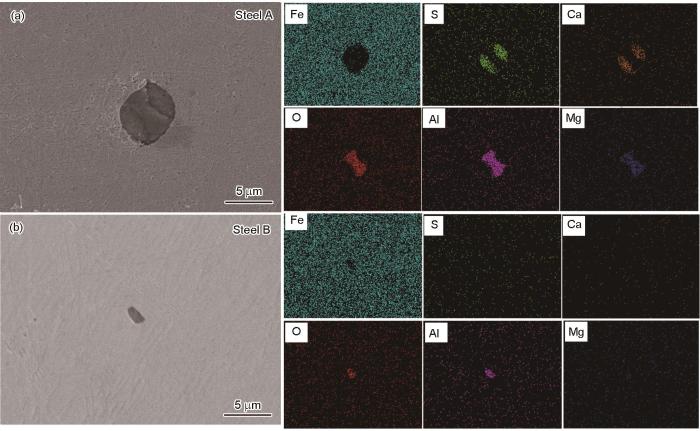
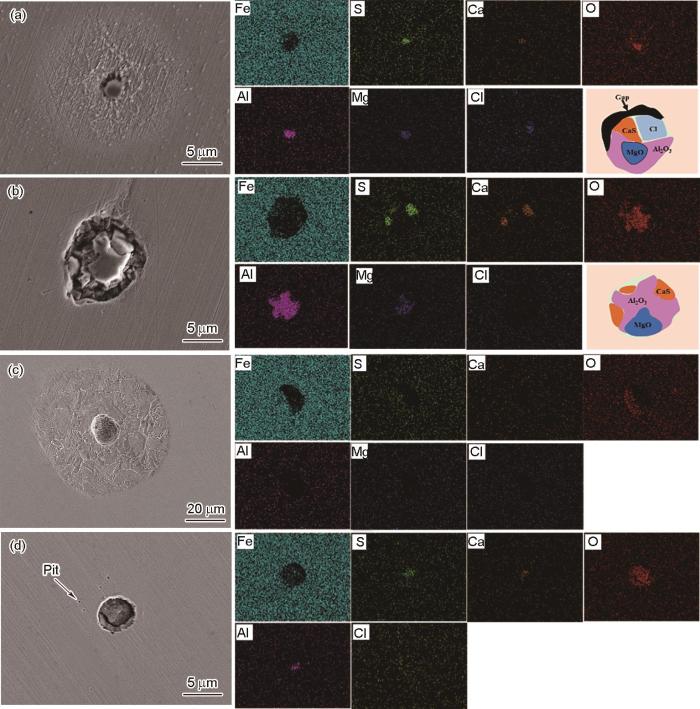
两种钢中夹杂物的SEM形貌和EDS结果
Fig.5
SEM images and EDS results of inclusions in steel A (a) and steel B (b)
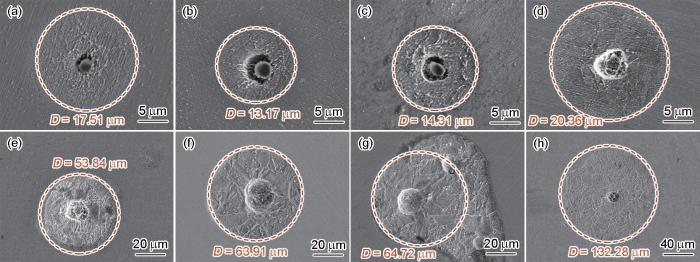
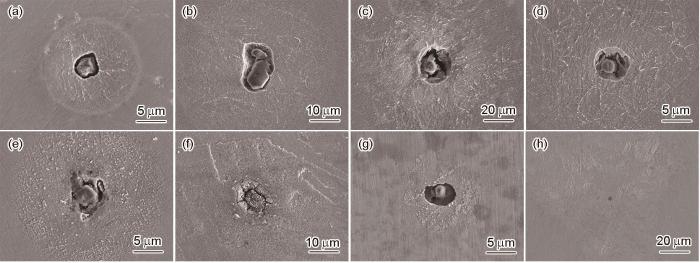
图6为A钢浸泡后的表面形貌。结果显示,浸泡5 s后夹杂物与基体之间产生空隙,直至浸泡60 s后夹杂物完全溶解。并且,夹杂物周围基体呈现出晶内优先溶解,晶界被保留,腐蚀范围近似圆形状。浸泡5 s后的腐蚀区域直径为15.36 μm,浸泡时间延长至3600 s时,腐蚀范围直径扩大至126.49 μm,比5 s时的直径高一个数量级。
图6
图6
A钢在人工海水溶液中浸泡不同时间后夹杂物诱发局部腐蚀的SEM形貌
Fig.6
SEM morphologies of inclusions induced localized corrosion of steel A after immersion in artificial seawater solution for 5 s (a), 10 s (b), 30 s (c), 60 s (d), 600 s (e), 1200 s (f), 1800 s (g) and 3600 s (h)
图7
图7
A钢浸泡3600 s后的活性夹杂物和非活性夹杂物SEM形貌及EDS结果
Fig.7
SEM morphologies of active and inactive inclusions of steel A after 3600 s immersion: (a) macroscopic corrosion morphology, (b) inactive inclusions, (c) active inclusions
图8
图8
A钢浸泡5 s和600 s后活性夹杂物和非活性夹杂物的SEM形貌
Fig.8
SEM morphologies and EDS results of active (a, c) and inactive (b, d) inclusions of steel A after immersion for 5 s (a, b) and 600 s (c, d)
图9
图9
B钢在人工海水溶液中浸泡不同时间后夹杂物诱发局部腐蚀的SEM形貌
Fig.9
SEM morphologies of inclusions induced localized corrosion of steel B after immersion in artificial seawater solution for 5 s (a), 10 s (b), 30 s (c), 60 s (d), 600 s (e), 1200 s (f), 1800 s (g) and 3600 s (h)
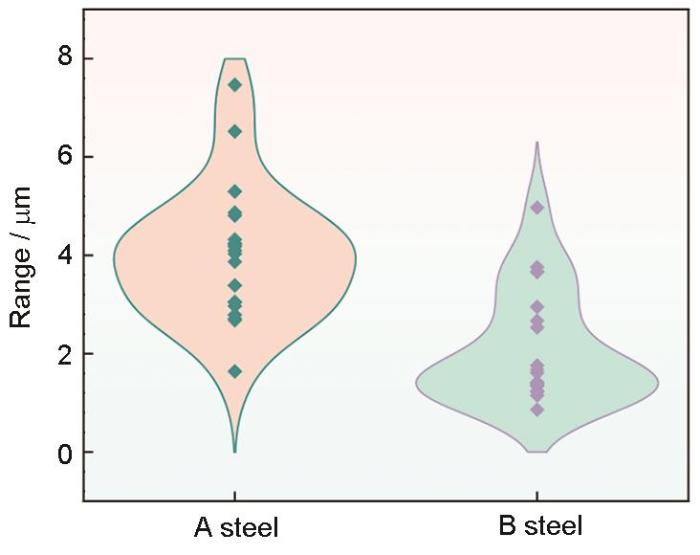
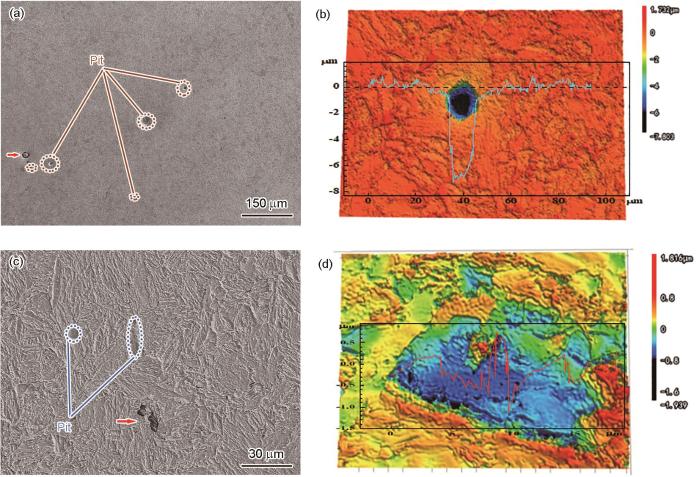
为进一步探究两种合金钢腐蚀萌生差异,对试样浸泡24 h后,采用SEM和CLSM观察其形貌。浸泡24 h后,表面形貌呈现出均匀腐蚀行为,两种钢中均观察到未溶解的夹杂物,如图10中红色箭头所示。与B钢相比,A钢中夹杂物引发的点蚀坑形状规则且深度更深,达到了7.8 μm。B钢中的点蚀坑较浅,最大深度仅为1.94 μm,并且最大深度不在夹杂物位置。
图10
图10
两种钢浸泡24 h后的SEM形貌和CLSM结果
Fig.10
SEM morphologies (a, c) and CLSM results (b, d) of steel A (a, b) and steel B (c, d) after 24 h immersion
由于夹杂物的存在,基体表面钝化膜不连续,导致腐蚀更容易在夹杂物与基体的界面处发生。根据以上结果,造成两种合金钢初期腐蚀萌生差异的主要原因在于夹杂物的类型和尺寸不同。结合A钢中CaS-MgO-Al2O3类型夹杂物初期腐蚀萌生演化规律,由于CaS在腐蚀过程中优先溶解,导致夹杂物与基体之间产生空隙,为Cl-提供了传输通道,如图8所示。CaS在溶解过程中将发生如下反应:
CaS溶解产生的空腔导致局部酸化,将进一步促进基体溶解:
2.4 均匀腐蚀分析
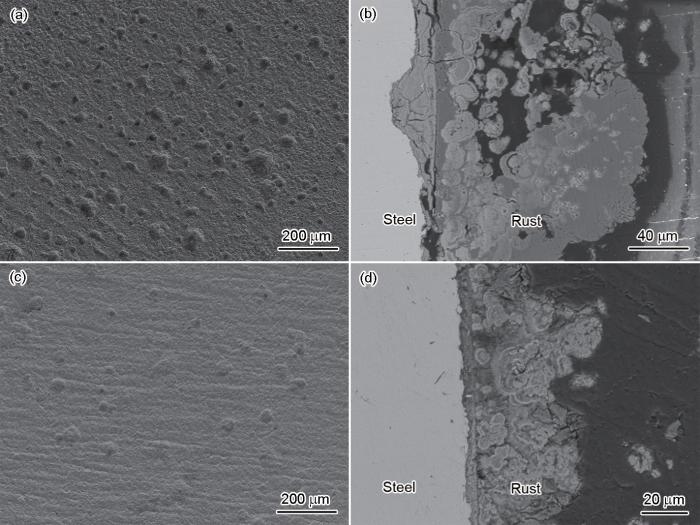
为进一步探究两种合金钢腐蚀差异,对腐蚀42 d后的试样表面和截面形貌进行了表征,如图11所示。两种钢均出现均匀腐蚀,A钢试样表面点蚀坑数量明显多于B钢。A钢和B钢的腐蚀产物层均呈现双层结构,即相对致密的内层结构和疏松的外层结构。A钢的内锈层厚度明显大于B钢,B钢的内锈层较为平整,这归因于B钢中的夹杂物尺寸较小,其引发局部腐蚀的程度较轻,在样品表面没有较深的腐蚀坑。A钢的内锈层分布着横向和纵向裂纹,这些裂纹可作为腐蚀性离子的传输通道,从而导致腐蚀速率增加。
图11
图11
两种钢浸泡42 d后的表面和截面形貌
Fig.11
Surface (a, c) and cross-sectional (b, d) morphologies of steel A (a, b) and steel B (c, d) after 42 d immersion
合金钢显微组织结构的差异将影响腐蚀初期点蚀坑的萌生和扩展、锈层的稳定性以及腐蚀产物的转换。具有单一均匀贝氏体结构的合金钢在腐蚀过程中的微电池反应不明显,并且增加了电荷转移电阻[27],与其他珠光体等双相结构钢相比具有良好的耐蚀性。腐蚀初期,腐蚀产物没有完全覆盖试样表面,发生强烈的电偶腐蚀,由于锈层形态较为松散,不具有保护作用,因此该阶段的腐蚀行为主要与钢的组织结构有关。A钢和B钢的显微结构均是贝氏体。基于EBSD表征,A钢较多的高角度晶界导致其具有更高的电化学活性,较高的KAM值表明其内应力和局部应变积累越大,更容易遭受腐蚀。在腐蚀的过程中,晶界作为晶体学缺陷将改变腐蚀的热力学过程以及动力学过程。随着腐蚀过程的进行,大量晶界被致密的腐蚀产物覆盖[28], 阻碍了基体与溶液之间的腐蚀反应,起到了一定的保护作用,腐蚀扩展到晶粒内部,导致晶粒出现优先溶解。由于B钢的低角度晶界含量高,提高了其抗点蚀性能[29]。并且KAM值也较低,与A钢相比具有较低的电化学活性。
3 结论
(1) B钢晶界和相界的内应力和局部应变积累低于A钢,且B钢中低角度晶界比例高,其耐蚀性相对较好。
(2) A钢的局部腐蚀萌生源于夹杂物,CaS的优先溶解促进了腐蚀的扩展,夹杂物整体溶解,最终导致点蚀坑向深处扩展。B钢中夹杂物周围腐蚀程度较小,由于基体溶解,引发了较浅的点蚀坑。
(3) A钢和B钢的腐蚀均由局部腐蚀扩展为均匀腐蚀。在腐蚀过程中夹杂物溶解形成的点蚀坑被腐蚀产物覆盖。A钢点蚀坑中形成的锈层由于应力集中而产生裂纹,恶化了其耐蚀性。
参考文献
Research progress on initiation mechanism of local corrosion induced by inclusions in low alloy steel
[J].
低合金钢中夹杂物诱发局部腐蚀萌生机制的研究进展
[J].夹杂物作为钢中不可避免的冶金缺陷,常会诱发局部腐蚀的萌生,进而对材料的耐蚀性能产生较大的影响。针对近年来各类夹杂物诱发局部腐蚀萌生的机理争议,本文总结了夹杂物诱发局部腐蚀萌生和发展的作用机制,包括电偶腐蚀机制、化学溶解机制和电偶腐蚀-化学溶解机制。夹杂物的化学成分、尺寸以及形状等是诱发局部腐蚀的关键影响因素。最后,对夹杂物诱发局部腐蚀机理研究和耐蚀钢调控的未来研究方向提出了展望。
Correlation between active/inactive (Ca, Mg, Al)-O x -S y inclusions and localised marine corrosion of EH36 steels
[J].
Quantitative analysis of initiation site of pitting corrosion on type 304 austenitic stainless steel
[J].
Towards a better understanding of localised corrosion induced by typical non-metallic inclusions in low-alloy steels
[J].
Role of Al2O3 inclusions on the localized corrosion of Q460NH weathering steel in marine environment
[J].
Cerium addition to CaS inclusions in stainless steel: Insolubilizing water-soluble inclusions and improving pitting corrosion resistance
[J].
Pit initiation on sensitized Type 304 stainless steel under applied stress: Correlation of stress, Cr-depletion, and inclusion dissolution
[J].
Unraveling the effect of sulfide-oxide complex inclusions on the localized corrosion mechanism for carbon steel
[J].
Role of complex nonmetallic inclusions on the localized corrosion resistance of wire arc additively manufactured super duplex stainless steel
[J].
Local corrosion characteristics of CaS/CaO-MgO-Al2O3 inclusions in low-alloy steel under multi-factor mechanisms
[J].
Role of inclusion and microstructure on corrosion initiation and propagation of weathering steels in marine environment
[J].
The pitting to uniform corrosion evolution process promoted by large inclusions in mooring chain steels
[J].
Anomalous evolution of corrosion behaviour of warm-rolled type 304 austenitic stainless steel
[J].
Effect of grain size on corrosion behavior of 304 stainless steel in coal chemical high salty wastewater
[J].
Effect of grain size on mechanical property and corrosion behavior of a metastable austenitic stainless steel
[J].
Effect of grain size and distribution on the corrosion behavior of Y2O3 dispersion-strengthened 304 stainless steel
[J].
Effect of grain size and crystallographic orientation on the corrosion behaviors of low alloy steel
[J].
Influence of Cr and Ni elements on the electrochemical and early corrosion behavior of FeMnAlC low-density steel
[J].
Fundamental understanding on the effect of Cr on corrosion resistance of weathering steel in simulated tropical marine atmosphere
[J].
Clarifying the effect of a small amount of Cr content on the corrosion of Ni-Mo steel in tropical marine atmospheric environment
[J].
Insight into the product film formed on Ni-advanced weathering steel in a tropical marine atmosphere
[J].
Improving the resistance of high-strength steel to SCC in a SO2-polluted marine atmosphere through Nb and Sb microalloying
[J].
A high-entropy alloy with dislocation-precipitate skeleton for ultrastrength and ductility
[J].
Effect of Nb on the hydrogen-induced cracking of high-strength low-alloy steel
[J].
Corrosion behaviour of a quenched and partitioned medium carbon steel in 3.5 wt.% NaCl solution
[J].
Mechanism of the influence of alloying elements in low-alloy steel on corrosion initiation and extension
[D].
低合金钢中合金元素对腐蚀萌生与扩展影响机制
[D].
Effects of Cr and Ni on the microstructure and corrosion resistance of high-strength low alloy steel
[J].
Dependence of corrosion resistance on grain boundary characteristics in a high nitrogen CrMn austenitic stainless steel
[J].Processing schedules for grain boundary engineering involving different types of cold deformation (tension, compression, and rolling) and annealing were designed and carried out for 18Mn18Cr0.6N high nitrogen austenitic stainless steel. The grain boundary characteristic distribution was obtained and characterized by electron backscatter diffraction (EBSD) analysis. The corrosion resistance of the specimens with different grain boundary characteristic distribution was examined by using potentiodynamic polarization test. The corrosion behavior of different types of boundaries after sensitization was also studied. The fraction of low-Σ boundaries decreased with increasing strain, and it was insensitive to the type of cold deformation when the engineering strain was lower than 20%. At the strain of 30%, the largest and smallest fractions of low-Σ boundaries were achieved in cold-tensioned and rolled specimens, respectively. The fraction of low-Σ boundaries increased exponentially with the increase of grain size. The proportion of low-angle grain boundaries increased with decreasing grain size. Increasing the fraction of low-Σ boundaries could improve the pitting corrosion resistance for the steels with the same grain size. After sensitization, the relative corrosion resistances of low-angle grain boundaries, Σ3 boundaries, and Σ9 boundaries were 100%, 95%, and 25%, respectively, while Σ27 boundaries, other low-Σ boundaries and random high-angle grain boundaries had no resistance to corrosion.