热障涂层主要应用在航空发动机热端部件上,可以将叶片与高温火焰隔离,降低叶片温度,提高发动机效率,延长热端部件的使用寿命[1]。镍铝金属间化合物具有高熔点,高比强度以及良好的耐腐蚀性和抗氧化性等性质,而受到航空工业的广泛关注[2]。镍铝金属间化合物被广泛应用于高温部件,其中,Ni3Al被应用于辐射燃烧器管、炉辊、热处理夹具和耐腐蚀夹具等,而NiAl则被用于叶片和燃烧室衬里。镍铝金属间化合物在室温下呈现低断裂韧性和低延展性,是限制其作为高温结构部件应用在涡轮发动机上的原因之一[3]。此外,镍铝金属间化合物另一应用出现在镍基高温合金的热障涂层中。热障涂层体系由粘结层和陶瓷顶层构成。NiCrAlY体系粘结层的作用之一就是防止高温气体直接接触基体引起的热侵蚀与氧化。NiCrAlY粘结层中主要的物相就是镍铝金属间化合物,包括β-NiAl和γ'-Ni3Al,其他物相为γ-Ni固溶体相和Ni17Y2[4~6]。据文献研究,热障涂层粘结层在室温下储存一段时间之后,表面会有腐蚀斑点出现。陶瓷顶层的喷涂粉末中存在杂质,其水解之后使腐蚀介质呈酸性。粘结层在酸性环境下腐蚀是其表面出现腐蚀斑点的重要因素[7]。
在世界范围内,中国的一次能源消耗中煤炭占比远超世界平均水平,约占70%。煤炭燃烧并进行脱硫处理之后,会向空气之中排放部分SO2和SO3。而SO2和氮氧化物(NO x )是形成酸雨的主要酸性污染物[8,9]。近年来,随着中国采取控制与减少SO2排放的相关措施,酸雨类型已经逐渐由硫酸型转变为硫酸硝酸混合型,降雨之中SO
1 实验方法
采用99.99%(质量分数)Ni粉和99.99%(质量分数)Al粉真空熔炼制备成4种Ni-Al合金,其中Ni∶Al摩尔比分别为75∶25、80∶20、85∶15和95∶5,将这4种Ni-Al合金分别命名为Ni75Al25,Ni80Al20,Ni85Al15,Ni95Al5。通过电火花线切割制备得到圆片状样品,尺寸为Φ10 mm × 3 mm。先使用SiC砂纸从240目逐级研磨至2000目,最后使用1 μm的金刚石颗粒进行抛光处理。采用SmartLab SE型X射线衍射仪(XRD)研究样品物相结构,采用Zeiss Ultra Plus型扫描电子显微镜(SEM)和X-Max 50型X射线能谱仪(EDS)研究样品表面形貌和元素分布,采用Escalab 250Xi型X射线光电子能谱(XPS)研究样品表面元素组成及化学状态。
电化学测试均使用CorrTest 2350型电化学工作站。腐蚀环境为室温下自然充气的1 mol/L H2SO4。所有电化学测试均采用标准的三电极系统。样品使用环氧树脂封装,仅暴露单面Φ 10 mm的圆片作为工作电极。参比电极、对电极分别为饱和甘汞电极和铂电极。极化曲线测试前,先将样品在溶液中浸泡20 min,待开路电位(OCP)稳定后,此时电位变化小于0.01 mV/s。极化电位以0.5 mV/s的扫描速率从-0.4 V(vs OCP)增至+0.6 V(vs OCP)。EIS测试,直流电位采用OCP,交流幅值设置为10 mV,频率范围设置为105~10-2 Hz。线性极化电阻(LPR)测试,电位从-0.01 V(vs OCP)扫描至+0.01 V(vs OCP),扫描速率为0.1667 mV/s。每间隔0.5 h测试一次。其中,EIS和LPR的总测试时长均为48 h,且测试之前样品均在溶液中浸泡30 min,保证OCP稳定。为了保证实验的可靠性,动电位极化测试和EIS测试均重复了3次。
2 实验结果
2.1 真空熔炼样品物相结构
图1
图1
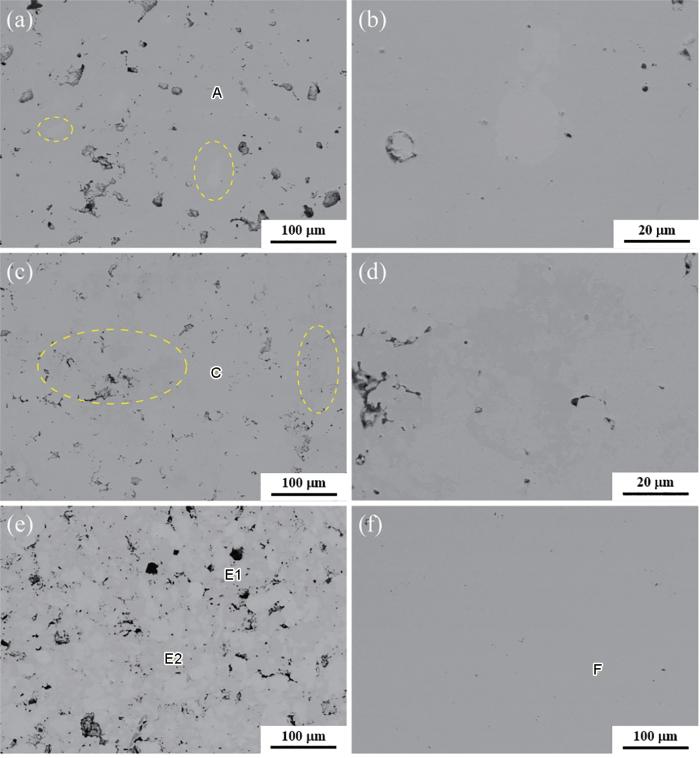
真空熔炼制备的4种Ni-Al合金SEM背散射电子像
Fig.1
SEM backscattered electron images of four alloys prepared by vacuum melting: (a, b) Ni75Al25, (c, d) Ni85Al15, (e) Ni80Al20, (f) Ni95Al5
表1 图1中不同区域的化学组成 (molar fraction / %)
Table 1
| Element | A | C | E1 | E2 | F |
|---|---|---|---|---|---|
| Al | 28.43 | 15.36 | 14.39 | 25.19 | 6.09 |
| Ni | 71.57 | 84.64 | 85.61 | 74.81 | 93.91 |
E1与E2两相共存于Ni80Al20之中,如图1e所示。图1中不同点区域A、C、F的元素组成见表1。结合表1和图1进行分析,A的Ni∶Al为71.57∶28.43对应Ni75Al25,C、F的Ni∶Al为84.64∶15.36、93.91∶6.09分别对应Ni85Al15、Ni95Al5。E1的Ni∶Al为85.61∶14.39,对应图1e的亮区;E2的Ni∶Al为74.81∶25.19,对应图1e的暗区。同时,两相的面积比接近1∶1,因此Ni80Al20实测的Ni∶Al接近80∶20。此外,Ni75Al25和Ni85Al15中存在部分标记区域为其他相,在图1a中标记区域元素组成对应于E1,在图1c中标记区域元素组成对应于E2,其标记区域放大图像分别见图1b和d。
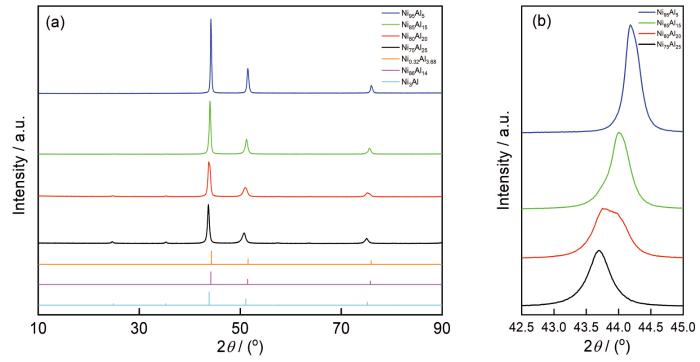
随着Ni:Al的增加,由于Ni的原子半径大于Al,导致衍射峰向更高角度偏移,如图2所示。Ni80Al20在2θ为43.8°左右的特征峰较宽,可能是不同特征峰叠加而成,由多相组成;而其他样品特征峰尖锐,主要由单相组成,如图2b所示。将样品的特征峰与ICSD卡片进行比较,并参考表1中的EDS数据,可以确定Ni75Al25对应Ni3Al(ICSD #58039),Ni85Al15与Ni86Al14(ICSD #107863)接近,Ni95Al5对应Ni3.68Al0.32(ICSD # 608801);E1与E2不同的Ni:Al与XRD分析中可能存在多相的假设是一致的。E1区对应Ni86Al14相,E2区对应γ'-Ni3Al相。
图2
图2
真空熔炼制备样品的XRD谱
Fig.2
XRD patterns of four Ni-Al alloys prepared by vacuum melting (a) and local enlargement patterns of Fig.2a (b)
2.2 电化学测试
2.2.1 动电位极化曲线分析
图3
图3
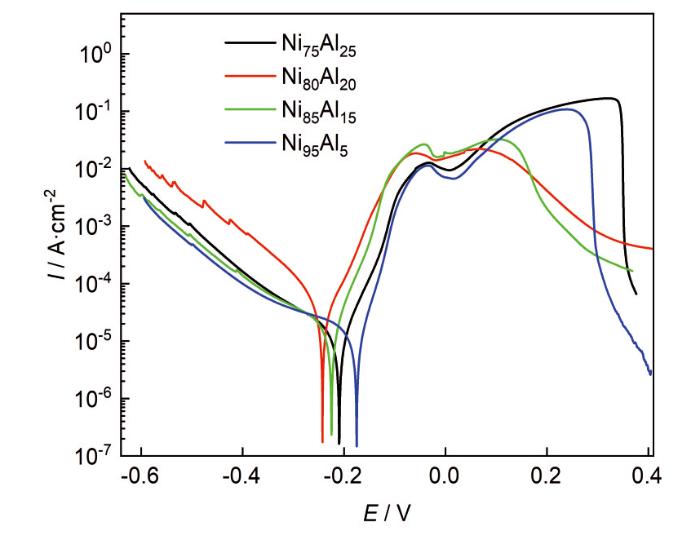
4种合金样品的动电位极化曲线
Fig.3
Potentiodynamic polarization curves of four Ni-Al alloys
样品的极化曲线测试拟合结果如表2所示。自腐蚀电位(Ecorr)值可以排序为:Ni95Al5>Ni75Al25>Ni80Al20≅Ni85Al15。对比不同样品的Ecorr,可以看出Ni95Al5合金最稳定,其次是Ni75Al25,最活跃的合金是Ni80Al20和Ni85Al15,Ni80Al20和Ni85Al15的腐蚀电位接近,相差1 mV。对比4种样品的自腐蚀电流密度(Icorr),Ni80Al20的Icorr最高,为37.3 µA·cm-2,约为其他样品的3~4倍;其余样品的Icorr接近,Ni95Al5的Icorr最低,为9.25 µA·cm-2。在1 mol/L H2SO4中,Ni95Al5性质最稳定,腐蚀速率最慢,Ni80Al20性质活跃,腐蚀速率最快。
表2 4种合金样品极化曲线拟合参数
Table 2
| Sample | Ecorr mV | Icorr µA·cm-2 | ba mV·dec-1 | bc mV·dec-1 |
|---|---|---|---|---|
| Ni75Al25 | -0.211 | 11.88 | 38.65 | 128.96 |
| Ni80Al20 | -0.223 | 37.3 | 47.79 | 126.09 |
| Ni85Al15 | -0.224 | 13.72 | 47.4 | 142.41 |
| Ni95Al5 | -0.172 | 9.25 | 35.57 | 139.12 |
2.2.2 腐蚀形貌分析
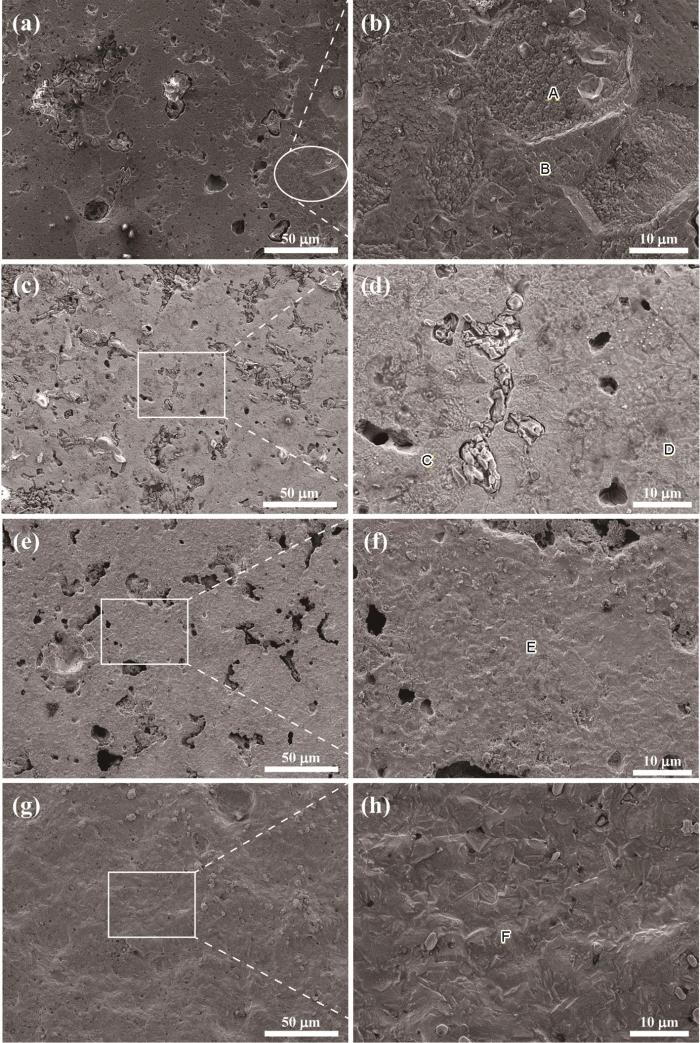
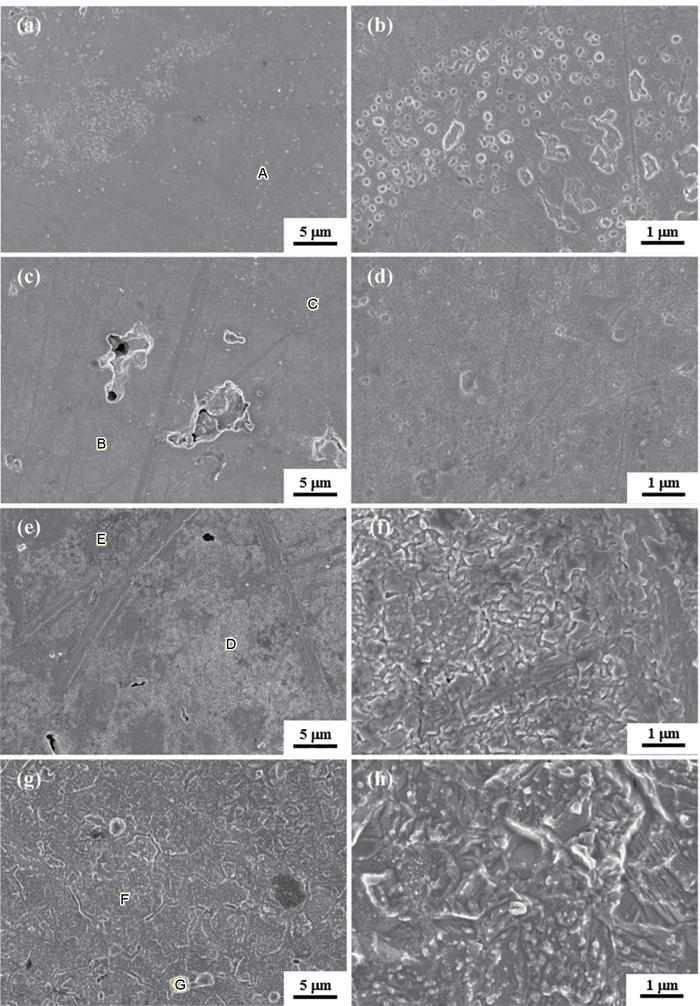
所有样品的表面都存在较多的孔缺陷,与图2中的原始形貌相对应。腐蚀产物填充了部分孔缺陷,特别是在Ni80Al20中,腐蚀产物在孔缺陷中析出更为明显,如图4所示。A点和B点的腐蚀表面均呈现鳞片状,其中,A点附近的鳞片更加致密。对图4中标记的点区域进行EDS扫描,其对应的元素组成如表3所示。其中,A点和B点的元素组成非常接近。因此,A点和B点的形貌差异对应着不同腐蚀程度的Ni75Al25单相,如图4b所示。图4d所示D点形貌与图4b所示A点形貌相似,Ni∶Al均接近75∶25;图4d所示C点形貌与图4f所示E点形貌相似,Ni∶Al均接近85∶15。Ni85Al15表面覆盖了一层均匀且致密的腐蚀产物,如图4f所示。Ni95Al5呈现均匀的腐蚀形貌,F点的Ni∶Al接近未发生腐蚀时样品的Ni∶Al,如图4h所示。
图4
图4
4种Ni-Al合金样品极化测试后的表面形貌
Fig.4
Surface morphologies of four Ni-Al alloys after polarization test: (a, b) Ni75Al25, (c, d) Ni80Al20, (e, f) Ni85Al15, (g, h) Ni95Al5
表3 图4中不同点区域的化学成分 (molar fraction / %)
Table 3
| Element | A | B | C | D | E | F |
|---|---|---|---|---|---|---|
| O | 7.96 | 7.34 | 8.25 | 14.81 | 12.52 | 13.28 |
| Al | 23.33 | 24.27 | 13.80 | 20.00 | 10.26 | 5.56 |
| S | 0.68 | 0.55 | 0.36 | 0.82 | 1.08 | 0.86 |
| Ni | 68.03 | 67.84 | 77.59 | 64.36 | 76.14 | 80.30 |
进行48 h LPR测试后,样品的表面腐蚀形貌如图5所示。Ni75Al25表面均有腐蚀坑分布,局部相对密集,较小的腐蚀坑均呈圆形,如图5a所示。大型腐蚀坑的尺寸在0.1~1 µm之间,小型腐蚀坑的尺寸约为0.1 μm。随着腐蚀过程进行,小型腐蚀坑边缘接触后逐渐发展成不规则的大型腐蚀坑,如图5b所示。Ni80Al20表面的腐蚀形貌一致,在原始孔缺陷中观察到腐蚀产物。Ni80Al20表面除了出现与Ni75Al25相似的腐蚀坑外,密集的晶粒裸露在表面,如图5d所示。此外,Ni75Al25同样可见类似于Ni80Al20表面晶粒裸露现象,如图5b所示。Ni75Al25和Ni80Al20在H2SO4中的腐蚀孔的形貌与纯Al在酸中的腐蚀孔的形貌相似[25,26]。Ni85Al15表面部分区域呈现不规则晶粒,其他区域腐蚀产物呈片状堆叠,如图5e所示。Ni95Al5表面粘附有球形颗粒,其直径约为1~2 μm,并且表面形成了长度约为3 μm的棒状空隙,如图5g所示。Ni95Al5表面呈现不同取向的条纹状块体,许多尺寸小于或接近0.1 μm的小颗粒附着在表面,如图5h所示。
图5
图5
4种Ni-Al合金样品48 h线性极化电阻测试后的表面形貌
Fig.5
Surface morphologies of four Ni-Al alloys after linear polarization resistance test for 48 h: (a, b) Ni75Al25, (c, d) Ni80Al20, (e, f) Ni85Al15, (g, h) Ni95Al5
表4 图5中不同区域的平均化学成分 (molar fraction / %)
Table 4
| Element | A | B | C | D | E | F | G |
|---|---|---|---|---|---|---|---|
| Ni | 70.17 | 79.48 | 69.35 | 78.10 | 66.71 | 91.43 | 0.98 |
| Al | 25.15 | 13.86 | 25.39 | 13.51 | 11.75 | 5.41 | 31.12 |
| O | 4.68 | 6.66 | 5.27 | 8.39 | 21.55 | 3.16 | 67.90 |
2.2.3 XPS测试
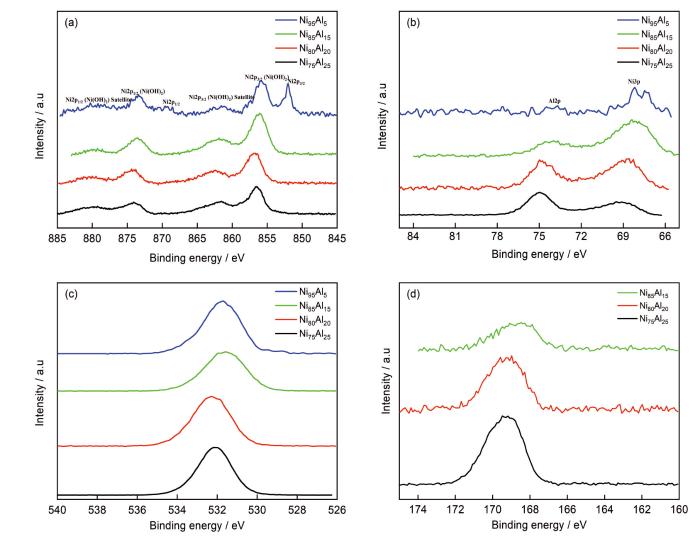
图6
图6
4种Ni-Al合金样品在1 mol/L H2SO4溶液中浸泡48 h之后的XPS谱
Fig.6
XPS spectra of four Ni-Al alloys after immersion in 1 mol/L H2SO4 solution for 48 h: (a) Ni 2p, (b) Al 2p, (c) O 1s, (d) S 2p
2.2.4 电化学阻抗谱分析
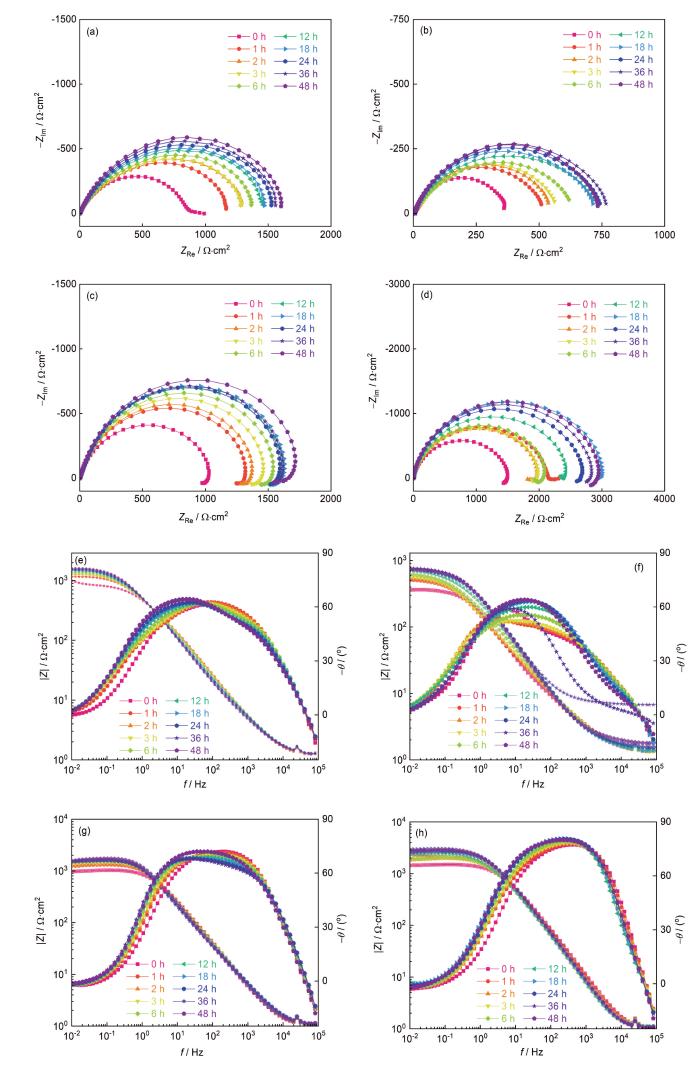
图7
图7
4种Ni-Al合金样品腐蚀不同时间后的电化学阻抗谱
Fig.7
Nyquist (a-d) and Bode (e-h) plots of Ni75Al25 (a, e), Ni80Al20 (b, f), Ni85Al15 (c, g) and Ni95Al5 (d, h) after corrosion for different time
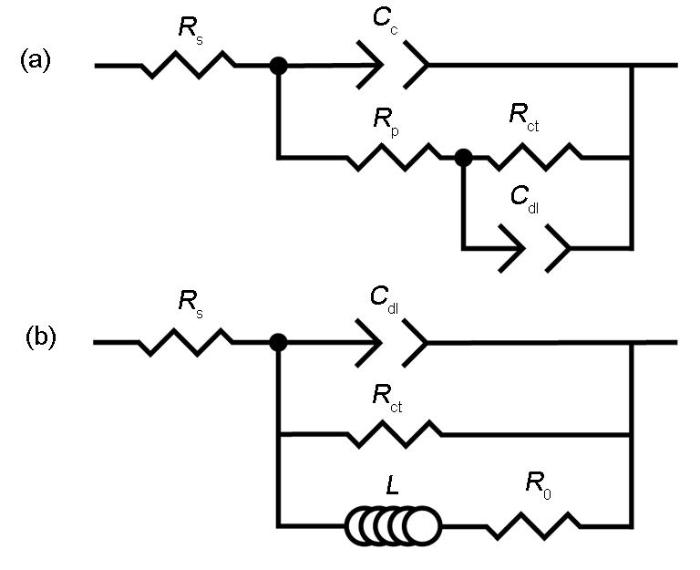
图8
图8
EIS拟合中采用的等效电路模型
Fig.8
Equivalent circuit model used for fitting EIS of Ni75Al25 and Ni80Al20 (a), and Ni85Al15 and Ni95Al5 (b)
随着腐蚀时间的延长,样品的阻抗半圆环的半径在增加,如图7a~d所示。Ni75Al25的Rct值从914 Ω(0 h)不断增大到1705 Ω(48 h),Ni80Al20的Rct值从364 Ω(0 h)不断增大到764 Ω(48 h)。Ni80Al20相位角最大值从40°(0~6 h)变为60°(12~48 h),lgf-θ曲线的峰型由双峰变为单峰,如图7f所示。其中,f表示频率,θ表示相位角。Ni85Al15和Ni95Al5的Bode图形状相似,相位角的最大值接近70°,如图7g和h所示。Ni85Al15的Rct值从1099 Ω(0 h)增加到1962 Ω(48 h),Ni95Al5的Rct值从1653 Ω(0 h)增加到3083 Ω(48 h)。随着腐蚀时间的延长,样品Rct值的增大表明样品的腐蚀速率减慢。
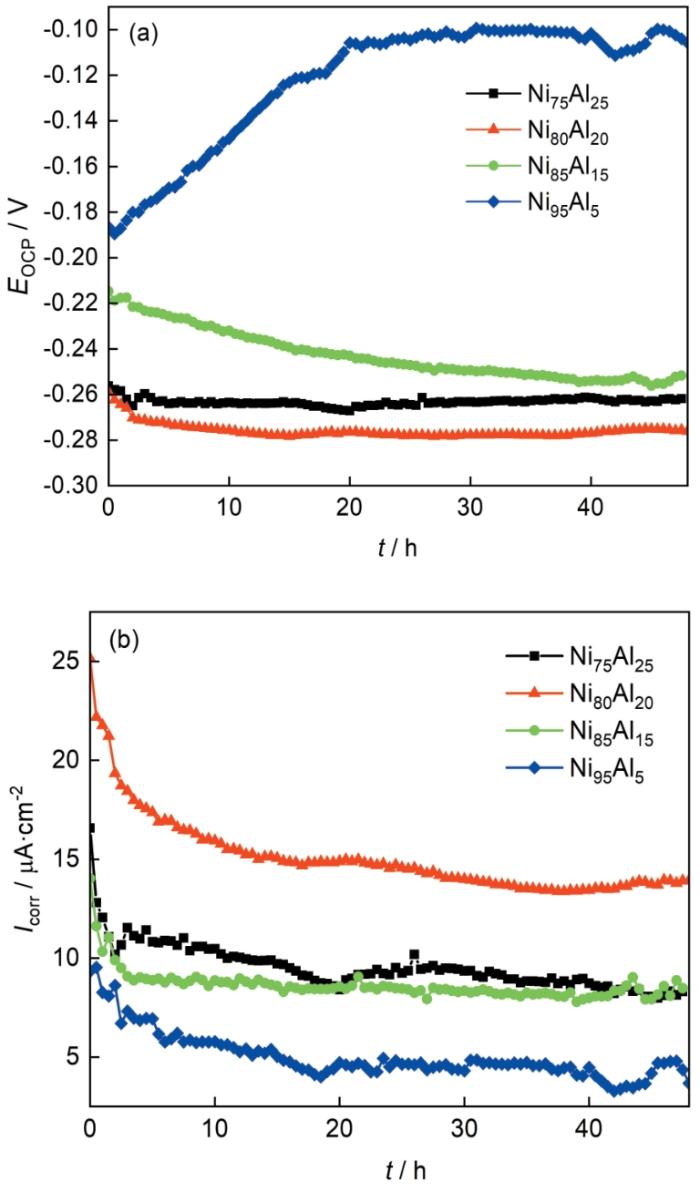
2.2.5 线性极化电阻测试分析
LPR测试中腐蚀电流密度(Icorr)采用Stern-Geary公式计算[33]。计算过程采用下列公式:
图9
图9
4种Ni-Al合金的Eocp和Icorr随浸泡时间的变化曲线
Fig.9
Eocp (a) and Icorr (b) values of four Ni-Al alloys as a function of immersed time
3 分析与讨论
Ni(OH)ads的存在是Ni85Al15和Ni95Al5的Nyquist阻抗谱中产生感抗弧的主要原因。本实验采取两种等效电路模型进行拟合,主要与Ni(OH)ads在表面吸附覆盖率有关,符合金属阳极溶解过程之中的复杂过程[39]。
Ni(OH)+形成后,它可以与H+结合生成Ni2+,也可以与H2O反应生成Ni(OH)2,这与H+和H2O的相对含量有关。XPS测试结果反应所有样品表面都存在Ni(OH)2,而Ni(OH)2在腐蚀溶液中不稳定,酸中Ni(OH)2的生成与溶解处于平衡状态。
对比其他Al的XPS谱,参考
根据极化曲线强极化区域拟合获得的样品拟合参数(表2)可知,Ecorr与样品的物相组成有关。其中,含有γ相的Ni95Al5的Ecorr最高,含有γ'相的Ni75Al25次之,而含有Ni86Al14相的Ni80Al20与Ni85Al15更低。EIS测试中阻抗整体呈现增加趋势,腐蚀初期增幅较大,腐蚀后期增幅较小且趋于稳定;同时LPR测试中Icorr变化也呈现出相似趋势,如图7和9所示。无论是EIS测试还是LPR测试,阻抗或者Icorr变化程度均未达到指数级。样品LPR测试48 h的表面形貌反应出晶粒裸露的特点,如图5所示。结合样品表面腐蚀形貌和电流密度进行分析,判断样品表面在未施加阳极极化腐蚀时,表面并未出现钝化。
4 结论
(1) 极化曲线测试中Ecorr可以排序为:Ni95Al5>Ni75Al25>Ni80Al20≅Ni85Al15,Icorr可以排序为:Ni80Al20>Ni85Al15>Ni75Al25>Ni95Al5。可以看出,Ni80Al20腐蚀速率较快,热力学性质不稳定,容易发生腐蚀;Ni95Al5腐蚀速率最慢,热力学性质最稳定,不易发生腐蚀。Ni75Al25腐蚀电位低于Ni95Al5,说明γ'-Ni3Al相较γ-Ni相更易发生腐蚀。
(2) 从LPR测试中可以观察到,24 h前样品的Icorr逐渐下降,且在24 h后逐渐趋于稳定,这与EIS测试中阻抗变化相符合。随着腐蚀时间的延长,表面腐蚀产物层的生成导致Icorr一定程度下降,随后表面腐蚀产物的生成与溶解趋于稳定,之后Icorr几乎不变。
(3) EIS测试之中,感抗弧的出现对应于中间产物Ni(OH)ads的形成。XPS测试表明,样品中Ni的腐蚀产物以Ni(OH)2形式存在,Al的腐蚀产物以Al2(SO4)3或Al(OH)3的形式存在。Ni80Al20样品中两相腐蚀电位相差较小,约为10 mV,未观察到两相腐蚀形貌的差异,即本研究中两相之间不存在某一相的优先腐蚀。
参考文献
Research progress of thermal barrier coatings for aeroengine turbine blades
[J].
航空发动机涡轮叶片热障涂层研究进展
[J].
NiAl alloys for high-temperature structural applications
[J].
Emerging applications of intermetallics
[J].
Research progress on microstructure and preparation methods for MCrAlY bond coats
[J].
MCrAlY粘结层的微观组织及制备方法研究进展
[J].
Phase structure and properties of a NiCrAlYSi bond coating alloy
[J].
NiCrAlYSi粘结层合金相结构与性能研究
[J].
Formation mechanism of corrosion spots on thermal barrier coatings
[J].
热障涂层表面腐蚀斑点的形成机理研究
[J].
Development path choice and strategy framework of China's energy security
[J].
中国能源安全的路径选择与战略框架
[J].
Analysis of harmful elements causing air pollution in coal
[J].
煤炭中造成大气污染有害元素的分析
[J].
Analysis of the spatio-temporal changes in acid rain and their causes in China (1998–2018)
[J].
Interannual variation characteristics of acid rain from 2006-2021 in China
[J].
2006-2021年中国酸雨年际变化特征分析
[J].
Effect of phase composition and microstructure on the corrosion resistance of Ni-Al intermetallic compounds
[J].
Electrochemical and corrosion study of as-cast Ni x Aly intermetallic alloys: Influence of alloy composition and electrolyte pH
[J].
Effect of Cu addition on the electrochemical corrosion performance of Ni3Al in 1.0 M H2SO4
[J].
Electrochemical corrosion characterization of nickel aluminides in acid rain
[J].
A thermodynamic analysis of the Ni-Al system
[J].
Nanocrystalline Ni3Al alloy produced by mechanical alloying of nickel aluminides and hot-pressing consolidation
[J].
Microstructure and mechanical properties of NiAl intermetallic compound synthesized by reactive sintering under pressure
[J].
A study of self-propagating high-temperature synthesis of NiAl in thermal explosion mode
[J].
Solidification microstructure maps in Ni-Al alloys
[J].
Electron and laser-based additive manufacturing of Ni-based superalloys: a review of heterogeneities in microstructure and mechanical properties
[J].
A brief review of processing techniques for NiAl intermetallic composites
[J].
Investigations on the microstructure and room temperature fracture toughness of directionally solidified NiAl-Cr(Mo) eutectic alloy
[J].
Transpassive dissolution of Ni in acidic sulfate media: a kinetic model
[J].
A study of aluminium corrosion inhibition in acid medium by an antiemitic drug
[J].
Garcinia indica as an environmentally safe corrosion inhibitor for aluminium in 0.5 M phosphoric acid
[J].
A review on C1s XPS-spectra for some kinds of carbon materials
[J].
Calibration of binding energy positions with C1s for XPS results
[J].
Characterization of α-Ni(OH)2 by XPS
[J].
Main and satellite features in the Ni 2p XPS of NiO
[J].The origin and assignment of the complex main and satellite X-ray photoelectron spectroscopy (XPS) features of the cations in ionic compounds have been the subject of extensive theoretical studies using different methods. There is agreement that within a molecular orbital model, one needs to take into account different types of configurations. Specifically, those where a core electron is removed, but no other configuration changes are made and those where in addition to ionization, there are also shake or charge-transfer changes to the ionic configuration. However, there are strong disagreements about the assignment of XPS features to these configurations. The present work is directed toward resolving the origin of main and satellite features for the Ni 2p XPS of NiO based on ab initio molecular orbital wave functions (WFs) for a cluster model of NiO. A major problem in earlier ab initio XPS studies of ionic compounds has been the use of a common set of orbitals that was not able to properly describe all the ionic configurations that contribute to the full XPS spectra. This is resolved in the present work by using orbitals that are optimized for averages of the occupations of the different configurations that contribute to the XPS. The approach of using state-averaged (SA) orbitals is validated through comparisons between different averages and through use of higher order excitations in the WFs for the ionic states. It represents a major extension of our earlier work on the main and satellite features of the Fe 2p XPS of FeO and proves the reliability and the generality of the assignments of the character and origin of the different features of the XPS obtained with orbitals optimized for SAs. These molecular orbital methods permit the characterization of the ionic states in terms of the importance of shake excitations and of the coupling of ionization of 2p and 2p spin-orbit split sub shells. The work lays the foundation for definitive assignments of the character of main and satellite XPS features and points to their origin in the electronic structure of the material.
Use of Al2(SO4)3 and acidified water glass as mixture depressants in flotation separation of fluorite from calcite and celestite
[J].To cope with the increase of sulfate gangue minerals in fluorite ores, flotation separation of fluorite from calcite and celestite using Al-2(SO4)(3) and acidified water glass (AWG) as mixture depressants was studied in this paper. The flotation separation performance and underlying mechanism were studied by flotation experiments, zeta potential measurements, microcalorimetric analysis, adsorption measurements, and X-ray photoelectron spectroscopy (XPS). The flotation results show that under optimum conditions, the inhibition effect of AWG on celestite is more remarkable with the assistance of Al-2(SO4)(3), but has no obvious effect on the flotation of fluorite. Zeta potential measurements show that both positively charged Al species and negatively charged Si species are adsorbed on the surface of the minerals. Microcalorimetric analysis confirms that the interaction between Al-2(SO4)(3) and celestite is more intense than that of calcite and fluorite, while AWG is more likely to be adsorbed on the calcite surface. Adsorption measurements reVeal that there is competitive adsorption between the depressant and collector and that the adsorption of the depressant AWG prevents the collector NaOL from adsorbing on the surface of the minerals. The XPS results show that Al species and Si species are chemically adsorbed on the surface of the minerals and change the chemical surroundings of the elements on the mineral surface. Moreover, compared with fluorite, Al species can more clearly enhance the adsorption of Si species on the celestite surface.
Nordstrandite (Al(OH)3) by XPS
[J].
The stern-geary and related methods for determining corrosion rates
[J].
The study of cathodic reactions in metallic corrosion
[J].
The mechanism of hydrogen evolution at nickel cathodes in aqueous solutions
[J].
Kinetics of the anodic dissolution of nickel in sulfuric acid solutions
[J].
Comparative potentiodynamic study of nickel in still and stirred sulfuric acid-potassium sulfate solutions in the 0.4-5.7 pH range
[J].
Corrundum (α-Al2O3) by XPS
[J].