随着资源勘探开发技术的飞速发展,极地和深海区域油气资源成为世界各国竞争与博弈的新领域[1]。在航线运输方面,航道沿线国家未来将会以北极航道作为国际贸易的主要运输干线,对运输极地资源有着至关重要的影响[2]。极地和深海资源开发与运输需要结合适宜低温地区的探测设备、低温海洋环境模拟取样、极地和深海环境监测等众多项目来获取极地环境的真实数据,这些数据的获得也离不开与极地运输船舶、深海开发装备相关的技术和设备[3,4]。为了保证资源开采装备的安全性,选用的材料逐渐由大壁厚、Ni-Cr-Mo-V高强钢所替代[5,6]。但是随着钢强度的提高,其组织也愈加复杂,塑性储备降低,如果受到外界或者自身应力情况下,其腐蚀敏感性将大幅增加。因此,研究Ni-Cr -Mo-V高强钢在低温海洋环境中的腐蚀对于装备安全具有重要意义。
极地和深海的海洋环境较为复杂,海水温度、海洋微生物以及外界环境气温等因素有较大差距[7]。极地海洋在较浅深度时其溶解氧含量与其他海洋环境差别较大,在南极海域深度为150~470 m范围其溶解氧含量大致在4.0~5.43 mL/L[8],而大部分合金钢在海水中多属于吸氧腐蚀,且一般是由阴极反应决定其反应速率,随着溶解氧浓度的增大,金属在海水中的阴极反应速率增加导致金属腐蚀速率增加[9~11]。Sun等[12]研究表明溶解氧含量的降低会降低X70钢的电化学反应速率,并且溶解氧含量减少,阴极反应由氧扩散过程变为氧去极化过程。Xue等[13]研究表明,碳钢的腐蚀质量损失随着溶解氧的含量升高而显著增加,在初始阶段的无氧溶液中,碳钢表现出阳极溶解的特征,而在有氧溶液中由于氯离子对腐蚀产物膜的破坏作用,钢的状态最初趋于伪钝化并迅速转变为活性溶解。因此,极地海洋环境中的溶解氧浓度对于低合金高强钢的腐蚀起着重要影响,而目前关于该领域的报道较少。
在船舶和油气装备工作环境中,高强钢通常会承受结构载荷、海流作用、加工或焊接残余应力等普遍存在的局部拉伸应力,与海洋腐蚀环境综合作用会导致严重的应力腐蚀[14~16]。陆辉等[17]利用慢应变速率拉伸实验,研究了06Cr17Ni12Mo2Ti不锈钢在不同温度和溶解氧浓度的超临界水中的应力腐蚀规律,不锈钢的应力腐蚀开裂程度随着溶解氧浓度的增加而增加,而升高温度则会降低应力腐蚀开裂程度。邱景等[18]通过四点弯曲实验结合宏微观分析,研究了TMCPX80管线钢焊接件在酸性海水中的应力腐蚀规律,在酸性海水中的S2-较少,因此反而焊接头处的应力腐蚀不明显。而目前在低温环境中的低合金高强钢应力腐蚀研究较少,因此,研究高强钢在模拟低温环境下的拉伸预应力与海洋中溶解氧因素协同作用下的腐蚀过程,揭示其腐蚀机理,对于极地船舶和探测设备的设计与安全运行具有重要参考意义。
1 实验方法
材料选用船用Ni-Cr-Mo-V高强钢。运用Bruker荧光光谱仪测试试样化学成分,测得本次实验研究所采用的高强钢的化学成分(质量分数,%)为:Ni 4.738,Cr 0.674,Mo 0.496,V 0.106,Si 0.172,Mn 0.470,Fe 92.878。将试样切成120 mm × 15 mm × 3 mm,抛光备用,并利用硅橡胶涂覆露出15 mm × 15 mm试样表面,试验介质为青岛海域天然海水。采用四点弯曲试验装置对试样施加预应力。将试样放入青岛天然海水中浸泡5 d,试验温度5℃,溶解氧含量(DO)分别为3.5、4.5、5.5 和6.5 mg/L。船用Ni-Cr-Mo-V高强钢的屈服强度为820.7 MPa,施加拉应力分别为50%屈服强度(Rp0.2)和100%Rp0.2(挠度对应为1.78和3.56 mm)。
通过尼康数码相机观测记录宏观形貌。利用ULTRA55扫描电子显微镜(SEM)进行微观腐蚀形貌分析。利用HIROX KH-8700数字视频显微镜观察不同实验条件下腐蚀产物层下金属基体腐蚀形貌。
电化学测试采用PARATAT 2273电化学工作站,采用三电极体系,参比电极为218型Ag,AgCl/KCl (sat'd)电极;辅助电极为铂铌丝电极;工作电极为四点弯曲电极试样。电化学阻抗谱(EIS)测试交流激励信号幅值为10 mV,扫描范围为105~10-2 Hz。动电位极化扫描速率为0.333 mV/s,扫描范围为-300~300 mV(vs. OCP)。
2 结果与讨论
2.1 宏观腐蚀形貌分析
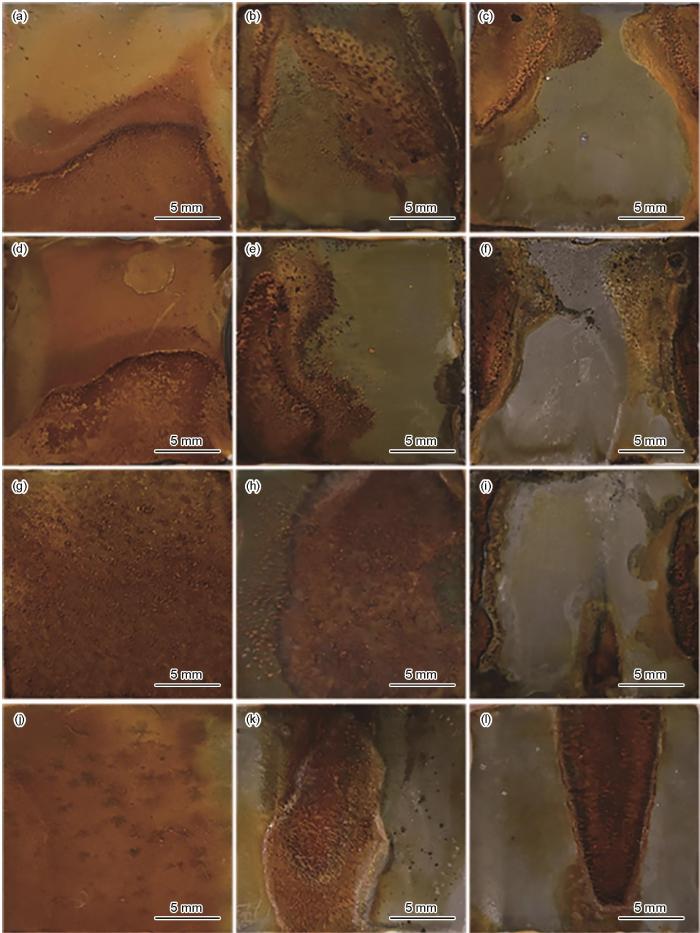
图1为施加不同应力的Ni-Cr-Mo-V高强钢在不同溶解氧含量下浸泡5 d的腐蚀形貌变化。未施加应力时,随着DO含量的增加,试样表面的腐蚀产物逐渐增加,腐蚀产物分布更为均匀;施加应力后,DO为3.5和4.5 mg/L时,均匀腐蚀随着施加应力的增大逐渐转变为局部腐蚀,腐蚀逐渐向试样中心靠近边缘的地方扩展。当DO达到5.5和6.5 mg/L,如图1g~i和图1j~l所示,随着应力增大,试样中心部位腐蚀逐渐变为严重。溶解氧含量的变化对于加载应力试样的影响与未加载应力试样的影响有较为明显的不同,在不施加应力的状态下,增加溶解氧会使O2去极化作用增强,使得腐蚀效率增加[19, 20]。对于施加应力的试样,溶氧量的增加会进一步增加带应力高强钢的应力集中,而引起局部腐蚀。对比施加不同应力相同氧含量的情况下,试样施加应力后,DO含量越高其试样表面的局部腐蚀越集中,试样中心靠近边缘处受应力最大部位腐蚀愈发严重。因此溶解氧含量越高,施加应力后,会造成高强钢应力集中部位腐蚀更为严重,由均匀腐蚀转为局部腐蚀。
图1
图1
施加不同应力的Ni-Cr-Mo-V高强钢在不同溶解氧的天然海水中浸泡5 d后的腐蚀宏观形貌
Fig.1
Macro-morphologies of Ni-Cr-Mo-V steel immersed for 5 d in natural seawater containing 3.5 mg/L (a-c), 4.5 mg/L (d-f), 5.5 mg/L (g-i) and 6.5 mg/L (j-l) DO under applied tensile stresses of 0%Rp0.2 (a, d, g, j), 50%Rp0.2 (b, e, h, k) and 100%Rp0.2 (c, f, i, l)
2.2 微观腐蚀形貌及金属表面腐蚀分析
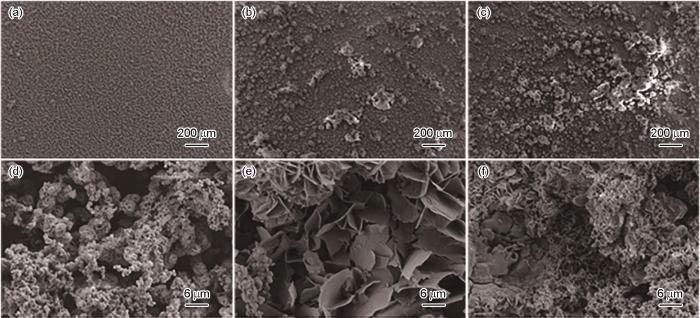
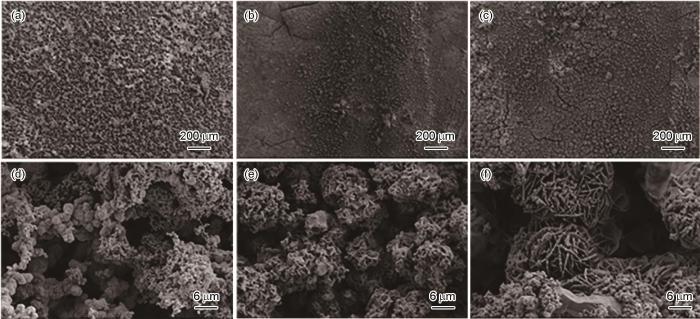
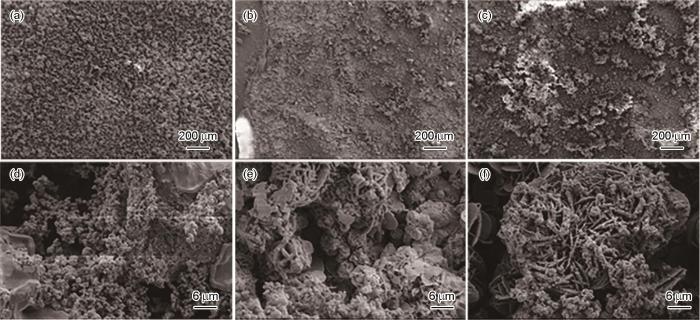
图2~5为施加不同应力的Ni-Cr-Mo-V高强钢在不同溶解氧含量的天然海水中浸泡5 d后得到的腐蚀产物高倍和低倍形貌。当DO为3.5和4.5 mg/L时,随着外加载荷的增加,高强钢腐蚀产物分布不均匀,腐蚀产物发生团聚;而且随着应力的增加腐蚀产物由尺寸较小的清晰可见的花瓣状腐蚀产物,逐渐变为直径较大,且表面附着有绒球状的腐蚀产物。当DO较高为5.5和6.5 mg/L时,相对于无应力试样,恒应变试样表面生成的腐蚀产物发生开裂,且随着外加载荷的增加,试样表面的产物增多发生聚集。而且高倍显微镜下观察,溶氧量较高情况下的腐蚀产物膜层明显比溶氧量较低的厚,而且随着应力增加,腐蚀产物之间的间隙变大,腐蚀产物较为疏松。因此,高强钢的腐蚀速率与溶解氧含量呈正相关。而且随着溶解氧含量的增加,施加应力的增大,高强钢腐蚀产物膜层更容易发生开裂,海水中的Cl-更容易到达高强钢基体[21],加速了该处的腐蚀速率,造成了金属表面应力集中的地方腐蚀更严重。
图2
图2
施加不同应力的高强钢在含3.5 mg/L DO的天然海水中浸泡5 d后的微观形貌
Fig.2
Microscopic morphologies of Ni-Cr-Mo-V steel after immersion for 5 d in 3.5 mg/L DO natural seawater under applied stresses of 0%Rp0.2 (a, d), 50%Rp0.2 (b, e) and 100%Rp0.2 (c, f)
图3
图3
施加不同应力的高强钢在含4.5 mg/L DO天然海水中浸泡5 d后的微观形貌
Fig.3
Microscopic morphologies of Ni-Cr-Mo-V steel immersed for 5 d in 4.5 mg/L DO natural seawater under applied stresses of 0%Rp0.2 (a, d), 50%Rp0.2 (b, e) and 100%Rp0.2 (c, f)
图4
图4
施加不同应力的高强钢在含5.5 mg/L DO天然海水中浸泡5 d后的微观形貌
Fig.4
Microscopic morphologies of Ni-Cr-Mo-V steel immersed for 5 d in 5.5 mg/L DO natural seawater at applied stress of 0%Rp0.2 (a, d), 50%Rp0.2 (b, e) and 100%Rp0.2 (c, f)
图5
图5
施加不同应力的高强钢在含6.5 mg/L DO天然海水中浸泡5 d的微观形貌
Fig.5
Microscopic morphologies of Ni-Cr-Mo-V steel immersed for 5 d in 6.5 mg/L DO natural seawater under applied stresses of 0%Rp0.2 (a, d), 50%Rp0.2 (b, e) and 100%Rp0.2 (c, f)
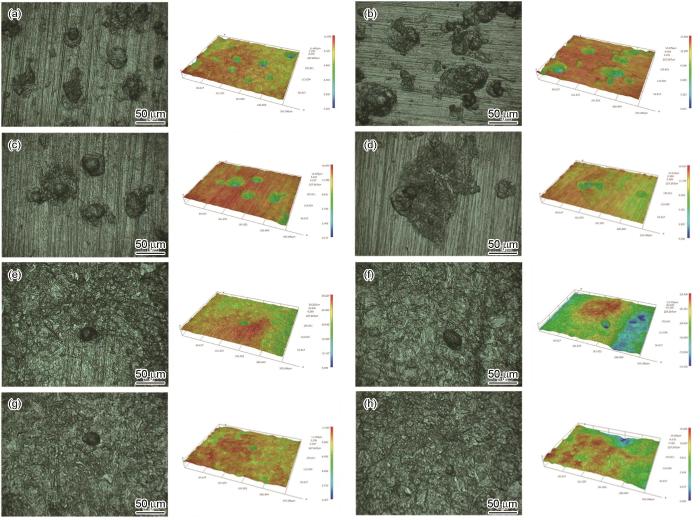
图6为Ni-Cr-Mo-V高强钢分别施加0%Rp0.2和100%Rp0.2应力后在不同溶氧量的自然海水中浸泡5 d的腐蚀微观形貌和三维形貌。DO为3.5和4.5 mg/L时,试样表面有清晰的腐蚀坑,制备试样的磨痕清晰可见。施加应力后的试样蚀孔的宽度明显增大,蚀孔间相互融合。当DO为5.5和6.5 mg/L时,高强钢表面由点蚀转向均匀腐蚀,尤其是当DO为6.5 mg/L时,试样表面蚀孔减少,且难以看见制备试样的磨痕,从三维图中可以看出,施加应力后的试样表面起伏较大,且沿受力垂直方向扩展。因此,溶解氧的升高会导致高强钢基体表面腐蚀加快,加快点蚀向均匀腐蚀转变的速率。而施加应力后,会使高强钢在低含氧量下点蚀扩展,相融合;而在高含氧量下,垂直受力方向的腐蚀速率明显加快,因为施加应力会在试样缺陷处或受力最大处产生应力集中,应力集中会加速金属基体的腐蚀。
图6
图6
高强钢在含不同浓度溶解氧的天然海水中浸泡5 d后三维点蚀形貌
Fig.6
Three dimensional morphologies of pitting pits of Ni-Cr-Mo-V steel after immersion for 5 d in natural seawater containing 3.5 mg/L (a, b), 4.5 mg/L (c, d), 5.5 mg/L (e, f) and 6.5 mg/L (g, h) DO under applied tensile stresses of 0%Rp0.2 (a, c, e, g) and 100%Rp0.2 (b,d,f,h)
2.3 腐蚀产物成分分析
表1为Ni-Cr-Mo-V高强钢施加0%Rp0.2和100%Rp0.2应力在不同溶解氧含量的天然海水中浸泡5 d后腐蚀产物的成分分析。表中各条件下主要元素都为Fe和O,证明该钢材腐蚀产物中主要的成分为Fe的氧化物组成。高溶解氧的环境下,腐蚀产物中其他元素成分明显增多。表中数据表明,腐蚀产物中Cr和Ni随着DO的增加而减小,尤其是在6.5 mg/L,100%Rp0.2时,腐蚀产物中基本没有Cr和Ni,推断在这些工况下腐蚀产物中缺少具有保护性的Cr和Ni的氧化物。由于Cr和Ni是增加低合金钢耐蚀性能的主要元素,这两种元素可以促进低合金钢产生致密的锈层,高拉应力和高溶解氧含量会导致高强钢的腐蚀产物层的致密度大大降低[22,23]。而且随着DO的增加,腐蚀产物中的Cl随之大幅度增加而富集在腐蚀产物中,Cl-会吸附在缺陷处和裂纹尖端的局部腐蚀处,进一步诱导腐蚀的发生[24]。
表1 在不同应力作用下高强钢在含不同浓度溶解氧的天然海水中腐蚀产物的化学成分 (mass fraction / %)
Table 1
| Condition | O | Na | Mg | S | Cl | K | Ca | Cr | Fe | Ni |
|---|---|---|---|---|---|---|---|---|---|---|
| 3.5 mg/L-0% Rp0.2 | 36.19 | / | / | 0.99 | 3.04 | / | / | 0.49 | 55.48 | 2.72 |
| 3.5 mg/L-100% Rp0.2 | 33.46 | / | / | 2.03 | 0.48 | / | / | 0.69 | 60.51 | 1.56 |
| 4.5 mg/L-0% Rp0.2 | 34.84 | / | / | 0.33 | 1.27 | / | / | 0.55 | 58.56 | 2.74 |
| 4.5 mg/L-100% Rp0.2 | 31.64 | / | / | 2.22 | 9.04 | / | / | 0.34 | 54.26 | 1.14 |
| 5.5 mg/L-0% Rp0.2 | 31.50 | / | / | 1.44 | 9.93 | / | / | 0.30 | 54.07 | 1.23 |
| 5.5 mg/L-100% Rp0.2 | 33.78 | 5.98 | / | 2.73 | 8.17 | 0.23 | 0.37 | / | 47.10 | 0.61 |
| 6.5 mg/L-0% Rp0.2 | 26.66 | 19.38 | 1.25 | 1.08 | 22.58 | 0.45 | 0.69 | / | 26.15 | 0.76 |
| 6.5 mg/L-100% Rp0.2 | 26.05 | 18.13 | 0.63 | 1.31 | 20.43 | 0.39 | 0.38 | / | 31.22 | / |
2.4 电化学研究与分析
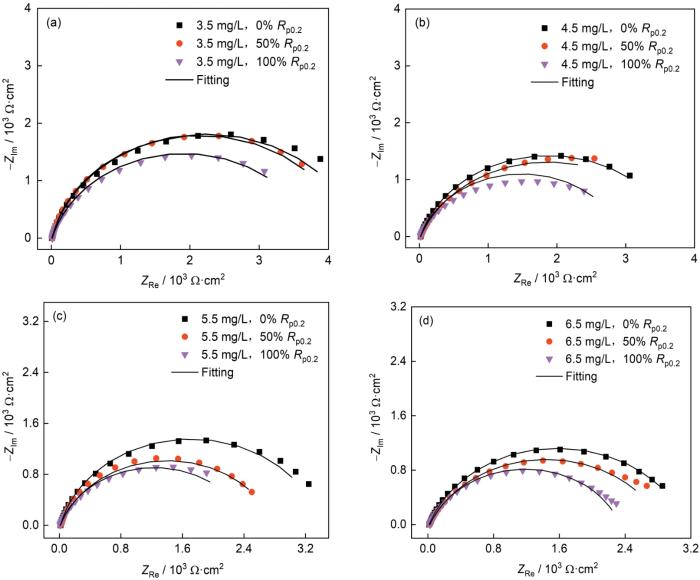
2.4.1 电化学阻抗谱结果与分析
图7
图7
高强钢在含不同浓度DO的天然海水中浸泡5 d后的Nyquist图
Fig.7
Nyquist plots of Ni-Cr-Mo-V steel immersed for 5 d in natural seawater containing 3.5 mg/L (a), 4.5 mg/L (b), 5.5 mg/L (c) and 6.5 mg/L (d) DO
图8
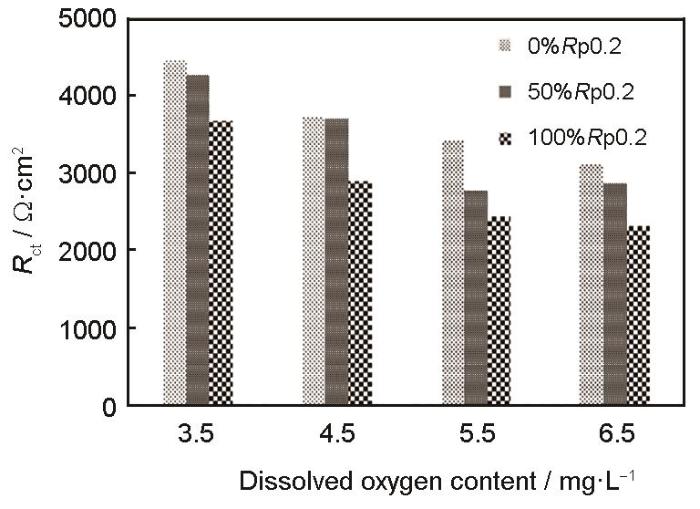
图8
高强钢在含不同浓度DO的天然海水中浸泡5 d后的Rct值
Fig.8
Rct values of Ni-Cr-Mo-V steel after 5 d immersion in natural seawater containing different concentrations of DO
2.4.2 极化曲线结果与分析
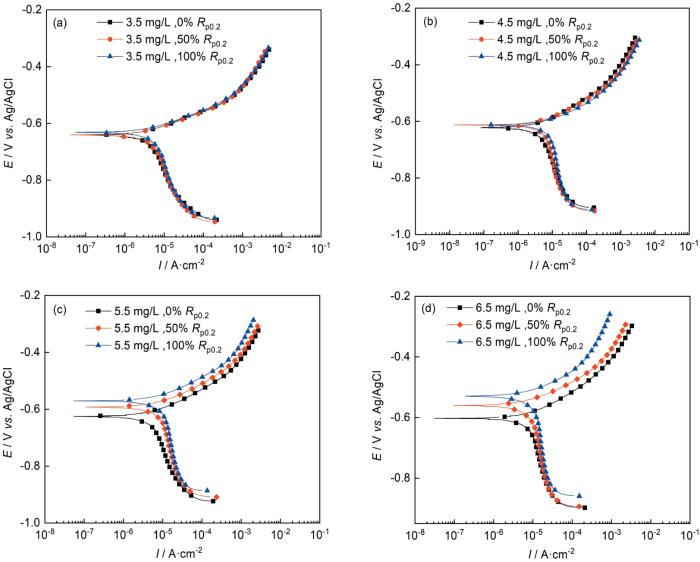
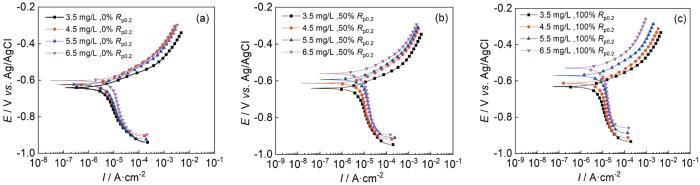
图9是施加不同应力后在3.5、4.5、5.5和6.5 mg/L DO的天然海水中浸泡5 d后,所得的动电位极化曲线,拟合参数见表2。图9表明,Ni-Cr-Mo-V高强钢在所有条件下都呈现活性溶解,随着溶解氧含量的增加,自腐蚀电流密度增加。溶解氧含量较高时,在Ni-Cr-Mo-V高强钢上施加应力越大对试样的腐蚀反应影响越大。当DO为3.5和4.5 mg/L时,随着试样上的施加应力增大,自腐蚀电位和阳极斜率未发生明显改变,但自腐蚀电流密度增大,证明在低溶解氧条件下,轻微加速了阴极反应的反应速率,但是并未改变阳极反应过程。当DO为5.5和6.5 mg/L时,随着试样上恒载荷应力的增大,自腐蚀电位发生明显的正移,且同一电位下的阴极电流密度增大,阳极斜率发生较小增大,从而导致自腐蚀电位正向移动。而且随着应力的增加,自腐蚀电流也增大较为明显,从而代表在高含氧量状态下,应力的加载会显著增加金属的腐蚀速率。
图9
图9
施加不同应力的高强钢在含DO的天然海水中浸泡5 d后的极化曲线
Fig.9
Polarization curves of Ni-Cr-Mo-V steel after 5 d immersion under different stresses in natural seawater containing 3.5 mg/L (a), 4.5 mg/L (b), 5.5 mg/L (c) and 6.5 mg/L (d) DO
表2 高强钢在受拉应力作用下含DO的天然海水中浸泡5 d后的极化曲线拟合参数
Table 2
| DO / mg·L-1 | Applied stress | ba / mV·dec-1 | bc / mV·dec-1 | Ecorr / V | Icorr / A·cm-2 |
|---|---|---|---|---|---|
3.5 | 0%Rp0.2 | 57.67 | -295.99 | -0.64 | 3.58 × 10-6 |
| 50%Rp0.2 | 56.89 | -351.79 | -0.64 | 4.46 × 10-6 | |
| 100%Rp0.2 | 57.56 | -396.83 | -0.63 | 5.11 × 10-6 | |
4.5 | 0%Rp0.2 | 73.39 | -319.74 | -0.62 | 4.06 × 10-6 |
| 50%Rp0.2 | 73.92 | -452.67 | -0.61 | 5.62 × 10-6 | |
| 100%Rp0.2 | 71.30 | -483.37 | -0.62 | 7.35 × 10-6 | |
5.5 | 0%Rp0.2 | 75.64 | -369.07 | -0.63 | 5.07 × 10-6 |
| 50%Rp0.2 | 78.54 | -546.61 | -0.60 | 8.33 × 10-6 | |
| 100%Rp0.2 | 79.05 | -724.49 | -0.57 | 9.91 × 10-6 | |
6.5 | 0%Rp0.2 | 78.70 | -489.23 | -0.60 | 7.76 × 10-6 |
| 50%Rp0.2 | 74.75 | -771.37 | -0.56 | 9.48 × 10-6 | |
| 100%Rp0.2 | 108.52 | -1003.2 | -0.55 | 1.18 × 10-5 |
图10分别对比了不同应力条件在不同溶解氧含量天然海水中浸泡5 d后测得的极化曲线。结合表2综合分析可知,Ni-Cr-Mo-V高强钢在不同氧含量的天然海水中均呈现活性溶解特征,阴极斜率明显增加,会导致自腐蚀电位发生正向移动,但是自腐蚀电流密度明显增大。这证明随着溶氧量的增加,金属趋于发生伪钝化现象,钢的自腐蚀电位处于高水平,但是钢处于强极化状态并且具有相对较高的腐蚀速率[27]。并且天然海水中存在氯化物,该物质使腐蚀膜变得疏松和多孔,有利于电解质溶液与基材接触,这可以归因于基于混合电位理论的铁腐蚀产物含量的增加和溶解氧的去极化作用[28]。因为天然海水中的溶解氧增加,导致溶解氧的去极化作用进一步增强,而且随着外加应力的增加,腐蚀产物在应力集中部位会发生开裂,从而加剧了应力集中部位的腐蚀,使得该变化趋势变得愈加明显。因此,电化学的结果和腐蚀形貌相结合,可以看出在高溶解氧含量的天然海水中,对高强钢施加应力,会加剧该材料的腐蚀,而且会在应力集中部位发生更为严重的腐蚀,对使用该材料的装备带来巨大的安全隐患。
图10
图10
高强钢在受力作用下含不同浓度DO的天然海水中浸泡5 d后的极化曲线
Fig.10
Polarization curves of Ni-Cr-Mo-V steel after 5 d immersion in natural seawater containing different concentrations of DO under applied stresses of 0%Rp0.2 (a), 50%Rp0.2 (b) and 100%Rp0.2 (c)
3 结论
(1) 在低温海水环境中,随着溶解氧含量增高,,Ni-Cr-Mo-V高强钢的腐蚀由局部腐蚀变为均匀腐蚀。随着施加应力的增加,应力集中部位腐蚀较为严重。而且施加应力后的腐蚀产物微观结构更为疏松,难以形成致密的保护层保护基体,进而使得腐蚀加剧。
(2) 低温环境中,Ni-Cr-Mo-V高强钢腐蚀产物成分中的Cr和Ni含量不仅随着溶解氧含量的增大而降低,应力的增加也导致Cr和Ni含量的降低,造成其腐蚀产物膜的致密度下降;同时,Cl元素在腐蚀产物中随着溶解氧的增加而富集,使得点蚀和微裂纹的诱发倾向增加,进一步降低了腐蚀产物膜的耐腐蚀作用。
(3) Ni-Cr-Mo-V高强钢的容抗弧半径随着溶解氧含量和施加载荷的增大而减小,而且溶解氧含量和施加应力的增加,均会致使该腐蚀的阴极由混合控制转变为氧的扩散控制,金属趋于伪钝化现象,使得自腐蚀电流密度增大,使高强钢腐蚀严重。因此溶解氧含量和施加应力对于高强钢在低温海水中的腐蚀具有协同作用,溶解氧含量越高,该材料所承受的应力越大,则越容易发生腐蚀破坏。
参考文献
Analysis of the influence of convection heat transfer in circular tubes on ships in a polar environment
[J].Electric heat tracing is the main measure for cold protection of the polar transfer coefficient in marine engineering equipment, but thermal equilibrium is the key problem this technology faces. In this paper, the circular tube was the research object. We studied the influence of convective heat transfer by Fluent software and experiments with a wind speed of 0–40 m/s and temperature of −40–0 °C by constant heat flux heating. The results show that the convective heat transfer increases with increased wind speed and decreased temperature. When the temperature is below −30 °C, the effect of temperature is increased; when the wind speed is greater than 25 m/s and the temperature is lower than −20 °C, the effect of temperature on the convective heat transfer coefficient of the circular tube increases. Based on the simulation data, we established a prediction model, and the rationality of the prediction model was verified by tests. The model provides reference for the design of electric heat tracing of circular tubes on polar ships.
Research on China's participation in the polar and deep sea's governance
[J].
我国参与极地深海治理问题研究
[J].
Current situations and development measures of equipment industry of Arctic oil and gas development in China
[J].
中国极地油气资源开发装备产业现状及发展策略建议
[J].
Development strategy for polar equipment in China
[J].
我国极地装备技术发展战略研究
[J].
Welding procedure of FH40 low-temperature high-strength steel for polar ships
[J].
极地船用FH40低温高强钢焊接工艺
[J].
Development and microstructure analysis of high strength steel plate used for polar icebreaker and polar transport ships
[A].
Development of cryogenic steel for polar ships
[A].
极地船舶用低温钢发展
[A].
Distribution of oxygen-minimum layer and factors controlling on it in areas adjacent to South Shetlands and North of Adelaide, Antarctica, in Summer
[J].
南设得兰群岛及阿得雷德岛以北海域夏季溶解氧含量最小层分布及其控制因素
[J].
The effect of dissolved CO2 and O2 on the corrosion of iron
[J].
Combined effect of hydrostatic pressure and dissolved oxygen on the electrochemical behavior of low-alloy high-strength steel
[J].
静水压与溶解氧耦合作用对低合金高强钢腐蚀电化学行为的影响
[J].
Investigation on stress corrosion cracking behavior of welded high strength low alloy steel in seawater containing various dissolved oxygen concentrations
[J].
Comparative study on the stress corrosion cracking of X70 pipeline steel in simulated shallow and deep sea environments
[J].
Effect of chloride ion on corrosion behavior of low carbon steel in 0.1 M NaHCO3 solution with different dissolved oxygen concentrations
[J].
The effect of hydrogen on stress corrosion behavior of X65 steel welded joint in simulated deep sea environment
[J].
Corrosion evolution and stress corrosion cracking of E690 steel for marine construction in artificial seawater under potentiostatic anodic polarization
[J].
Development of a numerical model for simulating stress corrosion cracking in spent nuclear fuel canisters
[J].Prediction and detection of the chloride-induced stress corrosion cracking (CISCC) in Type 304 stainless steel spent nuclear fuel canisters are vital for the lifetime extension of dry storage canisters. This paper conducts a critical review that focuses on the numerical modeling and simulation on the research progress of the CISCC. The numerical models emphasizing the residual stress, susceptible microstructure, and corrosive environment are summarized individually. Meanwhile, the simulation studies on the role of hydrogen-assisted cracking are reviewed. Finally, a multi-physical numerical model, which combines the different fields is proposed based on our recent investigation.
Effects of dissolved oxygen and temperature on the stress corrosion of O6Cr17Ni12Mo2Ti stainless steel in supercritical water
[J].
溶解氧和温度对O6Cr17Ni12Mo2Ti不锈钢在超临界水中应力腐蚀的影响
[J].
Stress corrosion and cathodic protection of X80 pipeline steel weldment in acidic seawater
[J].
X80管线钢焊接件在酸性海水中的应力腐蚀及阴极保护
[J].
Pitting corrosion of mild steel in marine immersion environment-Part 2: Variability of maximum pit depth
[J].
Discussion: statistical characterization of pitting corrosion—Part 1: data analysis and Part 2: probabilistic modeling for maximum pit depth
[J].
Influence of seawater on the carbon steel initial corrosion behavior
[J].
Effects of Cr, Ni and Cu on the corrosion behavior of low carbon microalloying steel in a Cl- containing environment
[J].
Corrosion behavior of 300M ultra high strength steel in simulated marine environment
[J].
300M超高强度钢在模拟海洋环境中的腐蚀行为研究
[J].
Corrosion characteristics of carbon steel in simulated marine atmospheres
[J].
基于海洋大气环境因素影响下的碳钢腐蚀特征研究
[J].
Effect of hydrostatic pressure on the corrosion behaviors of two low alloy steels
[J].In this paper, the effect of hydrostatic pressure on the corrosion behaviors of two steels was investigated by weight loss test, electrochemical measurements, rust layer analysis and morphology observation in natural seawater environment with high–pressure in lab. The results showed that X steel was inclined to being corroded. With the increase of hydrostatic pressure, the corrosion resistance of two steels were deteriorated, which were attributable to the increase of the anodic reaction rate. Hydrostatic pressure had little effect on oxygen diffusion reductin process of X steel. Howeve, the cathodic current density of Y steel under higher hydrostatic pressure decreased because the coosion products were directly involved in cathodic reduction reaction. And with the increase of hydrostatic pressure, the morphology of two steels changed significantly and dfferently. Under high pressure, some shallo–dish shape localized corrosion appeared on the surface of X steel whereas on the surface of Y steel some tunnel localized crrsion appeared.
模拟深海压力对2种低合金钢腐蚀行为的影响
[J].
Characterisation of passive films formed on low carbon steel in borate buffer solution (pH 9.2) by electrochemical impedance spectroscopy
[J].
Investigation of pseudo-passivation of mild steel in CO2 corrosion
[J].The iron carbonate corrosion product layer formed on mild steel in carbon dioxide (CO2) environments is known to retard corrosion. When not fully covering the steel surface, it may also lead to initiation of localized corrosion, due to a galvanic effect. In this work, the stability of a protective iron carbonate layer has been studied at 80°C over a relatively wide range of bulk pH. Experiments were done in a glass cell using a three-electrode system. Electrochemical techniques such as linear polarization resistance (LPR) and potentiodynamic polarization (PP) were used. Surface analysis techniques (scanning electron microscopy [SEM], x-ray diffraction [XRD], and transmission electron microscopy [TEM]) were used to confirm the composition and structure of the protective layer. Experimental results confirmed a pseudo-passive behavior, indicated by a positive shift in the open-circuit potential and a significantly retarded corrosion rate for systems at pH 6.0 and above. However, a stable and protective pseudo-passive layer could not be formed at pH 5.6 or lower.
Corrosion evolution of low alloy steel in deaerated bicarbonate solutions
[J].Corrosion evolution during immersion tests (up to 43 days) of NiCu steel in deaerated 0.1 mol/L bicarbonate solutions was investigated by electrochemical measurements, scanning electron microscopy (SEM) and X-ray diffraction (XRD). Results show that NiCu steel transformed from the anodic dissolution in the early stage of immersion to a metastable passive state in the final stage as the open-circuit potential value shifted positively, which was aroused by the precipitation of corrosion products. This process was mainly promoted by the trace amount of oxygen. Simultaneously, dominant cathodic reaction transformed from the hydrogen evolution in early stage to reduction processes of corrosion products in later stages. Possible corrosion processes were discussed with the assistance of a corresponding Pourbaix diagram.