SAC焊料中的Ag3Sn在服役过程中会发生选择性氧化,生成纯Ag[10,16]。纯Ag与焊料中的Sn基体形成Ag/Sn电偶。在外加电压的作用下,可能会导致焊料的腐蚀行为发生改变。Zhong等[17]研究表明,在NaCl溶液条件下,当外加电位比较高时,SAC焊料发生腐蚀和电化学迁移,生成的枝晶中含有Ag,该研究表明,SAC焊料中的Ag3Sn发生选择性氧化,导致焊料表面出现纯Ag,在高电位作用下,Ag发生腐蚀,溶出的金属离子在阴极还原形成Ag枝晶。Ag的电极电位比Sn的高,即在热力学上,Ag比Sn更不容易发生腐蚀[13]。在无外加电位条件下,Ag与Sn形成电偶时,Sn作为阳极优先发生腐蚀[18]。对于外加电位的影响,文献中未有详细报道。此外,浸银(ImAg)是一种常用的印刷电路板(PCB)表面处理工艺,焊料中的Sn容易与Ag形成腐蚀电偶[19],电偶腐蚀是一种重要的腐蚀形式[20~22]。因此,研究外加电位下对Ag/Sn电偶的腐蚀行为,揭示其腐蚀机理,对于电子器件的可靠性研究具有重要价值。
微量的Cl-常见于电子器件的服役环境中,因此,本文以0.01 M NaCl为介质,首先通过开路电位和极化曲线测试揭示纯Ag和纯Sn的基本腐蚀行为,在此基础上,采用双极性电化学技术研究Ag/Sn电偶在不同外加电位下的腐蚀行为,并对测试后的样品进行光学显微镜、扫描电子显微镜/能谱(SEM/EDS)表征,分析外加电位对Ag/Sn电偶的腐蚀行为的影响。
1 实验方法
实验材料为纯金属Ag和Sn,纯度均为99.99%。对于极化曲线测试实验,样品的尺寸为12 mm × 12 mm × 3 mm,样品的背面通过焊接与导线连接,再封入环氧树脂中作为工作电极,表面露出作为测试面,控制暴露尺寸为10 mm × 10 mm。在进行电化学实验之前,所有的工作电极用水磨砂纸逐级打磨至2000#,接着使用2.5 μm金刚石抛光膏进行机械抛光,最后依次使用去离子水、乙醇和丙酮清洗样品表面并用冷风吹干。实验溶液为0.01 mol/L NaCl。
采用CHI660E型电化学工作站进行极化曲线测试,测试温度为25℃。实验装置为标准的三电极体系,待测样品作为工作电极,Pt片电极作为对电极,饱和甘汞电极(SCE)作为参比电极。在进行极化曲线测试之前,先将样品浸入实验溶液中,在开路状态下稳定1800 s,记录样品的开路电位(OCP)随时间的变化。随后,从-0.25 VOCP开始,以10 mV/min的速率向阳极方向对样品进行动电位扫描,终止电位为1.5 VSCE。为了保证数据的可重复性,每组极化曲线都进行3组平行实验。
图1
图1
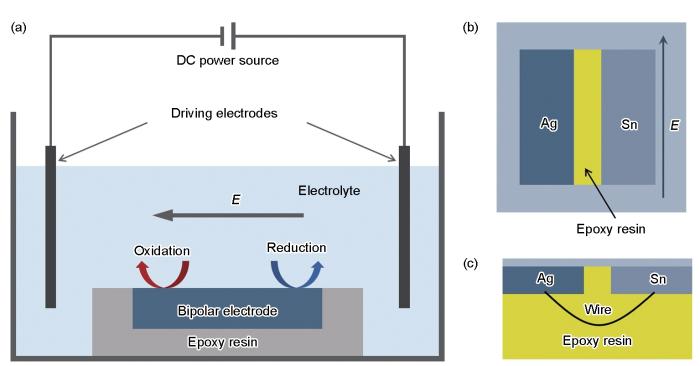
双极性电化学测试装置和样品示意图
Fig.1
Schematic of bipolar electrochemistry test setup (a), top view (b) and side view (c) of bipolar electrode, ‘E’ represents the potential
电化学实验结束后,用ZEISS Axiolab 5光学显微镜观察样品宏观腐蚀形貌,并采用自带能谱分析(EDS)的ZEISS Gemini 300扫描电子显微镜(SEM)进行腐蚀形貌观察及表征。由于光学显微镜所能拍摄的范围有限,对于双极性电化学测试后的样品,其光镜形貌照片是将各个区域的照片拼接后得到的,以展示样品在系列电位下的腐蚀全貌。
2 结果与讨论
2.1 Ag和Sn的开路电位
图2
图2
Ag和Sn在0.01 mol/L NaCl溶液中的OCP
Fig.2
OCP of Ag and Sn in 0.01 mol/L NaCl solution
2.2 Ag和Sn的极化曲线
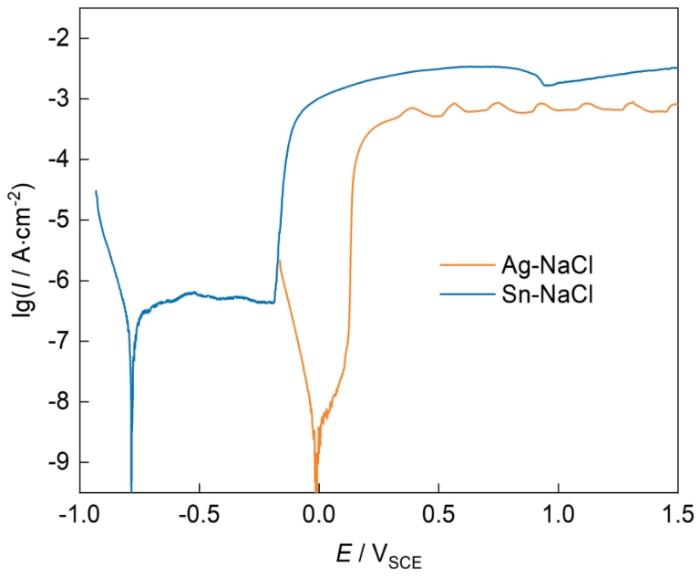
Ag和Sn在0.01 mol/L NaCl溶液中的动电位极化曲线如图3所示。在NaCl溶液中,随着电位升高,Ag的阳极电流密度迅速增大,电位高于0.25 VSCE后,电流密度保持稳定,约在
图3
图3
Ag和Sn在0.01 mol/L NaCl溶液中的动电位极化曲线
Fig.3
Polarization curves of Ag and Sn in 0.01 mol/L NaCl solution
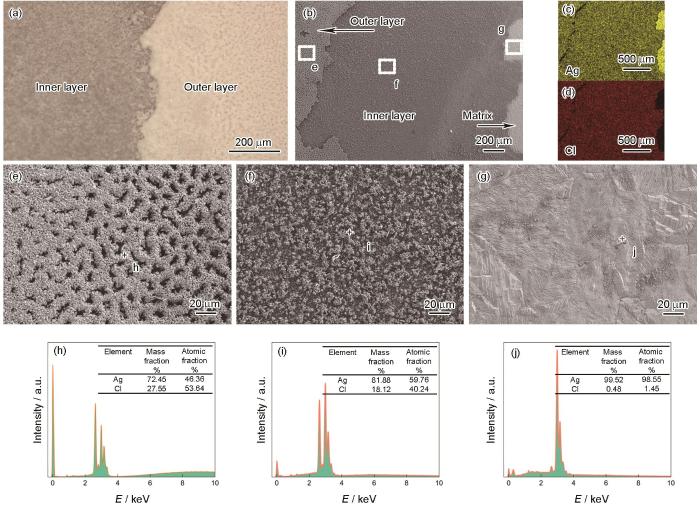
Ag在0.01 mol/L NaCl溶液中极化曲线测试后的形貌和SEM/EDS表征结果如图4所示。光镜和SEM结果(图4a和b)显示,Ag表面有腐蚀产物覆盖,主要成分是AgCl (图4c和d)。腐蚀产物对基体粘附力比较弱,水流冲洗后部分产物发生脱落。产物膜呈现双层结构,外层疏松多孔(图4e),内层较为致密(图4f),二者的EDS成分结果有所区别,可能是因为厚度不同,导致基体Ag的信号强度不一样(图4h和i)。产物膜下方是Ag基体,表面因为腐蚀而变得不平整(图4g),Cl含量很少(图4j)。上述结果表明,Ag在NaCl溶液中极化曲线测试时形成AgCl产物膜覆盖在基体表面,阻碍了离子的扩散。在极化曲线上表现为电位高于0.25 VSCE后,Ag的电流密度基本不变,即达到了极限扩散电流密度。
图4
图4
Ag在0.01 mol/L NaCl溶液中极化曲线测试后的形貌和SEM和EDS表征
Fig.4
Optical image (a), SEM image (b), element distribution maps (c, d) and magnified views (e‒g) of Fig.4b, EDS results of points in Fig.4e-g (h‒j) of Ag after polarization in 0.01 mol/L NaCl solution
图5
图5
Sn在0.01 mol/L NaCl溶液中极化曲线测试后的形貌和SEM/EDS表征
Fig.5
Optical image (a), SEM images (b, f), element distribution maps of Fig.5b (c‒e) and EDS result of points in Fig.5f (g, h) of Sn after polarization in 0.01 mol/L NaCl solution
反映在极化曲线上是高电位下,Sn的电流密度较高。
2.3 Ag/Sn电偶的双极性电化学测试
图6
图6
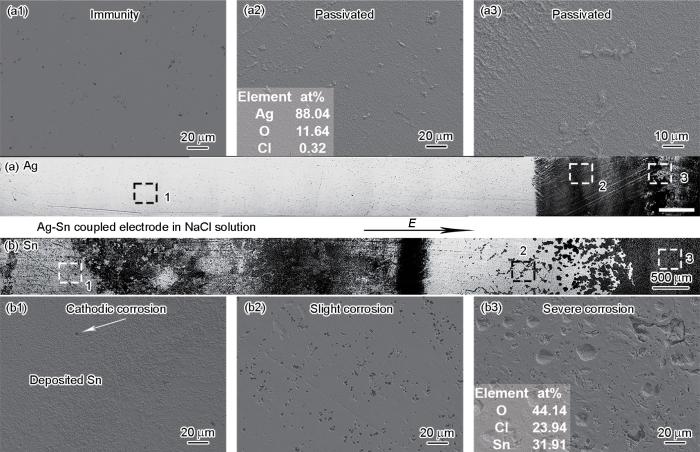
Ag和Sn在0.01 mol/L NaCl溶液中经过10 min双极性电化学测试后的光镜形貌和SEM和EDS结果
Fig.6
SEM images and EDS results of the Ag (a1-a3) of zones 1, 2, and 3 in Fig.6a and Sn (b1-b3) of zones 1, 2, and 3 in Fig.6b after the BPE test for 10 min in 0.01 mol/L NaCl solution
2.4 讨论
AgCl在水溶液中的溶解度较低(Ksp(AgCl) =
电位降低,Sn进入免蚀状态。当电位进一步降低时,SnH4生成:
导致Sn发生阴极腐蚀。
因此,在NaCl溶液中,Ag/Sn电偶中的Sn在高电位和
3 结论
本文针对SAC焊点材料的腐蚀,采用单金属Ag和Sn,结合开路电位和极化曲线测试、双极性电化学测试方法研究外加电位下Ag/Sn电偶的腐蚀行为,得到以下结论:
(1) Sn在NaCl溶液中的OCP低于Ag,表明Sn的热力学腐蚀倾向高于Ag。
(2) 在NaCl溶液中,Sn的腐蚀电流始终大于Ag,Ag表面生成较为致密的AgCl膜,阻碍基体溶解。
(3) 双极性电化学测试能够同时揭示Ag/Sn电偶在系列外加电位下的腐蚀形貌,具有高通量的特性。Sn在低电位下发生阴极腐蚀,在高电位下,NaCl溶液中,由于
参考文献
A reliability assessment guide for the transition planning to lead-free electronics for companies whose products are RoHS exempted or excluded
[J].
Effects of Ag contents in Sn–xAg lead-free solders on microstructure, corrosion behavior and interfacial reaction with Cu substrate
[J].
A critical review on performance, microstructure and corrosion resistance of Pb-free solders
[J].In the quest for viable alternatives to lead-based solders, electronic industries have started to focus on several potential lead-free solder alloys. After reviewing more than 100 recent studies, the present paper attempts to report the feasibility of various lead-free solder alloys in terms of performance, microstructure, corrosion resistance, and cost analysis. It reveals that there is a lack of scientific data based on which solid decisions on reliable lead-free solders, particularly their composition, can be made. Laboratory tests and data from actual services suggest that Sn-Ag-Cu solder alloys are perhaps the best choices for electronic industries. However, some problems in their practical usage must be addressed. This comprehensive review presents the major shortcomings of lead-free solders and possible solutions. Finally, a number of issues have been reported, which need to be confirmed in further studies. (C) 2018 Published by Elsevier Ltd.
Mechanical properties of Pb-free SnAg solder joints
[J].
Electrochemical corrosion of SAC alloys: a review
[J].Tin–silver–copper (SAC) solder alloys are the most promising candidates to replace Sn–Pb solder alloys. However, their application is still facing several challenges; one example is the electrochemical corrosion behaviour, which imposes a risk to electronics reliability. Numerous investigations have been carried out to evaluate the corrosion performance of SAC lead-free alloys, regarding the effect of the corrosive environment, the different manufacturing technologies, the effect of fluxes, the metallic contents within the SAC alloys themselves, and the different alloying elements. In these studies, widely used electrochemical techniques are applied as accelerated corrosion tests, such as linear sweep voltammetry and electrochemical impedance spectroscopy. However, there is lack of studies that try to summarise the various corrosion results in terms of lead-free solder alloys including low-Ag and composite solders. This study aims to review these studies by showing the most important highlights regarding the corrosion processes and the possible future developments.
Ag3Sn compounds coarsening behaviors in micro-joints
[J].As solder joints are being scaled down, intermetallic compounds (IMCs) are playing an increasingly critical role in the reliability of solder joints, and thereby an in-depth understanding of IMCs microstructure evolutions in micro-joints is of great significance. This study focused on coarsening behaviors of Ag3Sn compounds in Sn-3.0Ag-0.5Cu (SAC305) micro-joints of flip chip assemblies using thermal shock (TS) tests. The results showed that the Ag3Sn compounds grew and rapidly coarsened into larger ones as TS cycles increased. Compared with such coarsening behaviors during thermal aging, TS exhibited a significantly accelerating influence. This predominant contribution is quantitatively determined to be induced by strain-enhanced aging. Moreover, based on observations for Ag3Sn microstructure evolutions during TS cycling, one particular finding showed that there are two types of coarsening modes (i.e., Ostwald ripening and Necking coalescence) co-existing in the Ag3Sn coarsening process. The corresponding evolutions mechanism was elucidated in a combination of simulative analysis and experimental validation. Furthermore, a kinetic model of the Ag3Sn coarsening was established incorporating static aging and strain-enhanced aging constant, the growth exponent (n) was calculated to be 1.70, and the predominant coarsening mode was confirmed to be the necking coalescence.
Corrosion of electronic materials and devices
[J].Electronic materials and devices corrode in the same ways as automobiles, bridges, and pipelines, but their typically small dimensions make them orders of magnitude more susceptible to corrosion failure. As elsewhere, the corrosion involves interactions with the environment. Under control, these interactions can be put to use, as in the formation of protective and functional oxide films for superconducting devices. Otherwise, they cause damage, as in the electrolytic dissolution of conductors, even gold, in the presence of humidity and ionic contamination from atmospheric particles and gases. Preventing corrosion entails identifying the damaging interactions and excluding species that allow them to occur.
Pitting corrosion behavior of Sn-0.7Cu lead-free alloy in simulated marine atmospheric enviroment and the effect of trace Ga
[J].
人工模拟海洋大气环境下Sn-0.7Cu无铅焊料的点蚀行为及微量Ga的影响
[J].
Corrosion of Sn-Ag-Cu lead-free solders and the corresponding effects on board level solder joint reliability
[A].
Effect of Ag3Sn intermetallic compounds on corrosion of Sn-3.0Ag-0.5Cu solder under high-temperature and high-humidity condition
[J].
Corrosion behavior of Sn-3.0Ag-0.5Cu lead-free solder joints
[J].
Corrosion behavior of Sn-3.0Ag-0.5Cu alloy under chlorine-containing thin electrolyte layers
[J].
Effect of micromorphology on corrosion and mechanical properties of SAC305 lead-free solders
[J].
Microstructure induced galvanic corrosion evolution of SAC305 solder alloys in simulated marine atmosphere
[J].Motivated by the increasing use of Sn-3.0Ag-0.5Cu (SAC305) solder in electronics worked in marine atmospheric environment and the uneven distribution of Ag3Sn and Cu6Sn5 intermetallic compounds (IMCs) in β-Sn matrix, comb-like electrodes have been designed for in-situ EIS measurements to study the microstructure induced galvanic corrosion evolution of SAC305 solder in simulated marine atmosphere with high-temperature and high-humidity. Results indicate that in-situ EIS measurement by comb-like electrodes is an effective method for corrosion evolution behavior study of SAC305 solder. Besides, the galvanic effect between Ag3Sn IMCs and β-Sn matrix can aggravate the corrosion of both as-received and furnace-cooled SAC305 solder as the exposure time proceeds in spite of the presence of corrosion product layer. Pitting corrosion can be preferentially found on furnace-cooled SAC305 with larger Ag3Sn grain size. Moreover, the generated inner stress during phases transformation process with Sn3O(OH)2Cl2 as an intermediate and the possible hydrogen evolution at local acidified sites are supposed to be responsible for the loose, porous, cracked, and non-adherent corrosion product layer. These findings clearly demonstrate the corrosion acceleration behavior and mechanism of SAC305 solder, and provide potential guidelines on maintenance of microelectronic devices for safe operation and longer in-service duration.
Study on corrosion behavior of β-Sn and intermetallic compounds phases in SAC305 alloy by in-situ EC-AFM and first-principles calculation
[J].
A perspective on effect by Ag addition to corrosion evolution of Pb-free Sn solder
[J].
Evidence for Ag participating the electrochemical migration of 96.5Sn-3Ag-0.5Cu alloy
[J].
Structure and corrosion behavior of the oxide film formed on Sn-based Pb-free solder
[D].
锡基无铅焊料表面氧化膜膜层结构及腐蚀性能表征
[D].
Research progress of galvanic corrosion in marine environment
[J].
海洋环境中金属电偶腐蚀研究进展
[J].
Galvanic corrosion behavior of 20# steel/tin bronze couple in flowing seawater
[J].
20#钢/锡青铜偶对在流动海水中的电偶腐蚀行为研究
[J].20#钢穿舱件和锡青铜阀电偶腐蚀是船舶海水管路系统严重腐蚀部位之一。为控制20#钢/锡青铜电偶腐蚀延长海水管路系统寿命,本文通过原位测量20#钢管材和ZCuSn5Pb5Zn5锡青铜管材在静态以及1、3和5 m/s流速海水中的电偶电位和电偶电流,分析电偶腐蚀速率随时间和流速的变化规律;同时采用扫描电镜 (SEM) 和激光Raman光谱仪分析腐蚀形貌和腐蚀产物组分。结果表明,在不同流速海水中,20#钢与ZCuSn5Pb5Zn5合金间存在明显的电偶腐蚀倾向,20#钢作为阳极加剧腐蚀,ZCuSn5Pb5Zn5合金作为阴极受到保护;相比于静态海水,20#钢阳极极化电流密度和ZCuSn5Pb5Zn5合金阴极极化电流密度在流动海水中显著增加,电偶腐蚀显著加剧,1 m/s流速下的电偶腐蚀速率是静态下的17.5倍;当海水流速达到5 m/s后,20#钢表面形成了致密性较高、活性低的腐蚀产物沉积层,电偶腐蚀速率减小。
Influence of seawater flow speed on galvanic corrosion behavior of B10/B30 alloys coupling
[J].
海水流速对B10/B30电偶腐蚀行为影响规律研究
[J].B10和B30铜镍合金分别为船舶海水管路和冷却器的主要材料,二者由于镍含量不同腐蚀电位不同,管路与冷却设备连接后,B10和B30存在电偶腐蚀风险,特别是在流动海水加速腐蚀介质和腐蚀产物扩散工况条件。为控制B10/B30电偶腐蚀以延长海水管路系统使用寿命,本文通过电化学法测试了B10和B30管状偶对在静态以及1、3和5 m/s流速海水中的电偶电位和电偶电流,分析电偶腐蚀速率随时间和流速的变化规律。研究结果表明:在静态海水中,B10与B30的电偶腐蚀倾向较小,试验初期B10作为阳极腐蚀略有增加,实验40 h后电偶电流趋近于零;流动海水中,B10阳极极化电流密度和B30阴极极化电流密度显著增加,B10始终作为阳极电偶腐蚀显著加剧,1 m/s流速下的电偶腐蚀速率是静态下的79倍,且随着海水流速的增大,B10/B30电偶电流密度增大,电偶腐蚀速率加快,混合电位理论分析表明B10/B30电偶腐蚀速率是由B10阳极反应动力学和B30阴极反应动力学共同控制。
Selective corrosion of β-Sn and intermetallic compounds in an Ag–Sn alloy at different potentials in NaCl and Na2SO4 solutions
[J].
Bipolar electrochemistry for high-throughput corrosion screening
[J].
Corrosion characterization of tin-lead and lead free solders in 3.5wt.% NaCl solution
[J].
Electrochemical corrosion study of Sn-3Ag-3Cu solder alloy in NaCl solution
[J].
Potentiodynamic behaviour of tin in different buffer solutions
[J].
Effect of Ag addition on the corrosion properties of Sn-based solder alloys
[J].