钢筋混凝土结构耐久性良好,但在恶劣的环境服役会加速混凝土结构劣化,缩短混凝土结构的服役寿命,给混凝土结构带来安全隐患[1]。特别是在滨海盐渍土含有大量的氯化物,氯离子的入侵会导致钢筋表面钝化膜的破坏,钢筋发生腐蚀,进而导致混凝土的开裂[2,3],钢筋腐蚀是造成混凝土劣化的主要原因之一[4,5]。针对于钢筋混凝土腐蚀防护国内外的专家学者做了大量的研究工作[6~9],主要从两方面进行:(1) 增加钢筋混凝土本身的耐蚀性,包括缓蚀剂、高性能混凝土[10]、不锈钢筋[11~13];(2) 表面涂层隔绝腐蚀介质,包括混凝土表面涂层[14]、镀锌钢筋、涂层钢筋[9, 15],以及阴极保护等。在混凝土中添加的缓蚀剂总量较大,成本高,且对混凝土材料还有一定的影响,而不锈钢钢筋、镀锌钢筋等成本较高难以广泛应用。因此,在钢筋表面制备涂层隔绝腐蚀介质,是最经济最有效的防护手段。
目前用于钢筋防护的涂层主要分为有机涂层、有机无机复合涂层、无机涂层3大类,有机涂层主要有环氧树脂涂料[16]、醇酸树脂涂料[17]、聚氨酯涂料[18]和富锌涂料[19]等,其中环氧涂层有着良好的化学稳定性和力学性能。但与混凝土的粘结强度明显降低,且在施工过程中易受磨损破坏,加速了破损处的钢筋的腐蚀[20,21];有机无机复合涂层中有机粘结剂为主的涂层通过添加无机功能性填料提高防护性能、耐磨性、耐候性[22~24],无机功能材料的引入改善了有机涂层的综合性能,但仍具有与混凝土的粘结强度降低的不足,且存在有机溶剂使用的问题。无机涂层中铬酸盐转化膜有毒不再使用[25],而其他诸如磷化层只能起到短时间防锈的作用[26]。硅溶胶涂层具有良好的耐热性、耐酸性、较高的硬度、价格便宜等优点,目前受到广泛关注[27~30]。与有机涂层相比,由于硅溶胶涂层与混凝土组成相近,具有较好的相亲性,硅溶胶涂层应用于钢筋不会降低钢筋与混凝土的粘结强度,同时通过在硅溶胶涂层中引入功能性填料还可以有效抑制侵蚀性离子对钢筋的侵蚀。因此,硅溶胶涂层应用于钢筋是一种理想的防护涂层。用于硅溶胶涂层的制备原料中,水玻璃具有原料来源广、成本低等优点,是一种最适合大批量制备硅溶胶涂层的原料。但是,水玻璃中的Na+在涂层中以游离的形式存在,在服役过程中Na+会溶解导致溶胶涂层的耐水性较差[31]。
本文提出一种用于钢筋表面的硅溶胶涂层,以水玻璃为前驱体,在涂料制备中引入α-TiP填料,利用α-TiP的离子交换功能吸纳水玻璃中存在的游离N+。制备得到的硅溶胶涂层中含有片状α-TiP填料可有效提高涂层的阻隔性能,进而提升硅溶胶涂层对钢筋腐蚀的防护性能,并研究了α-TiP不同添加量对于涂层的改性影响。
1 实验方法
取2.879 g Ti(SO4)2溶于20 mL去离子水中,在搅拌下滴加30 mL 85 %磷酸。超声分散10 min,将混合溶液转入有聚四氟乙烯内衬的不锈钢反应釜中,分别在120℃反应12 h (a1)、120℃反应20 h (a2), 150℃反应12 h (b1)、150℃反应16 h (b2)、150℃反应20 h (b3),180℃反应12 h (c1)、180℃反应16 h (c2)、180℃反应20 h (c3),反应后冷却至室温,将所得产物10000 r/min离心分离,取下层沉淀用去离子水充分洗涤,在60℃干燥得到α-TiP粉末。
采用Q235钢板替代钢筋,将5 cm × 7 cm的Q235钢板依次用400#、600#、800#、1000#、1500#打磨,用石油醚、丙酮清洗除油,酒精超声10 min、去离子水超声5 min,酸洗5 min,去离子水冲掉表面残留的酸,放入磷化液(Zn(NO3)2 56 g/L,Mn(NO3)2 20 g/L,Mn(H2PO4)2·2H2O 24 g/L,C6H8O7·H2O 2 g/L)中70℃磷化15 min,磷化完成后去离子水冲洗吹干表面备用。
分别将0.2、0.4和0.6 g的α-TiP粉末分散在3.5 mL水中超声分散30 min,纳米ZnO粉末0.5 g分散在3.5 mL水中超声分散30 min。掺入适量的氧化锌可以填补涂层的间隙增加涂层的韧性、致密性,也有助于减少Na+的析出增加涂层耐水性[36]。取10 mL的水玻璃(SiO2含量27.5%,NaO含量8.78%,模数3.23),按(氧化锌、α-TiP)顺序在磁力搅拌下将各分散好的填料逐滴加入到水玻璃中,机械搅拌3 h,超声分散30 min,制备出α-TiP改性硅溶胶涂料。取0.7 mL涂料均匀的刷涂在5 cm× 7 cm的磷化板上,室温干燥4 h,真空干燥箱120℃干燥2 h。所得涂层厚度约为20 μm。空白对比样品除不添加α-TiP外,其他步骤与上述相同。
采用扫描电子显微镜(FESEM,JSM-6510)观察α-TiP形貌,采用X射线衍射仪(XRD, D8 Advance)分析样品组成结构,以单色Cu Kα辐射,在15 kV、20 mA的条件下,扫描速率为2.4(2θ) (o)/min,扫描范围为10°~40°。采用红外光谱分析仪(FT-IR,PerkinElmera)分析α-TiP和涂层的组成,扫描范围为2000~400 cm-1。
将0.1 g α-TiP与50 mL的5 × 10-4 mol/L碳酸钠溶液混合,搅拌3 h达到离子交换平衡,采用离子色谱仪测试交换前后Na+浓度。
Na+交换量qe (mg·g-1)通过以下方程式计算[37]:
式中:qe为离子交换量,C0为交换溶液的Na+初始浓度,CE为交换溶液的Na+平衡后的浓度,m为用于交换的α-TiP的质量,V用于交换的溶液体积。
采用电化学工作站(Interface1000)对涂层进行电化学测试,所用溶液为含有3.5%(质量分数) NaCl的饱和氢氧化钙溶液(pH = 12.6)。采用三电极体系,铂网作为对电极,饱和甘汞电极为参比电极,涂层样品为工作电极,在一种电化学测试防腐涂层性能的便捷检测装置中进行[38],暴露面积为7 cm2。测试前,将样品浸泡在溶液中30 min获得稳定的开路电位(OCP),随后进行电化学阻抗谱(EIS)和极化曲线测试。EIS的测量的频率范围为105~10-2 Hz,正弦扰动幅值为10 mV。动电位极化扫描范围为-100~+100 mV (vs. OCP),扫描速率0.1667 mV/s;用CView软件进行最小二乘法拟合动电位极化测试结果,用ZSimpWin软件结合等效电路对EIS数据进行解析。
2 结果与讨论
2.1 合成条件
2.1.1 反应时间和温度的影响
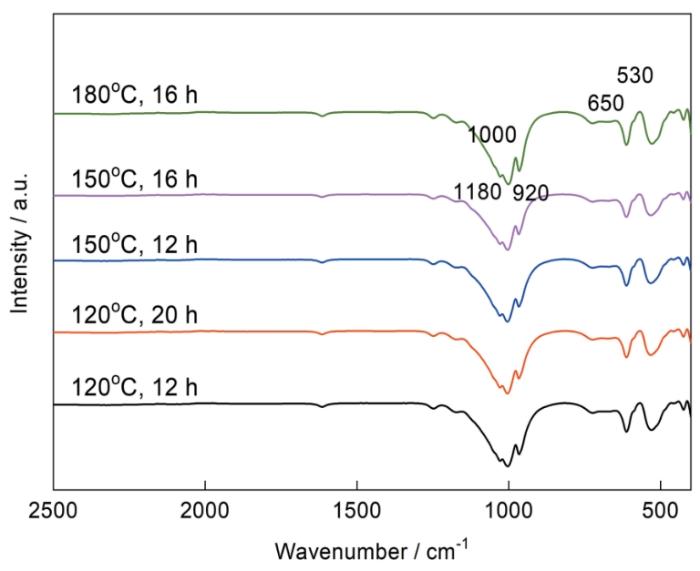
图1a为不同温度和时间下所制备的α-TiP的XRD谱,水热法制备的α-TiP衍射峰峰形规整而尖锐,表明试样有良好的结晶度,样品在2θ = 11.54°、20.85°、25.75°处有3个主强衍射峰,对应(002)、(110)、(112)晶面的特征衍射峰,其最强峰(002)出现在2θ = 11.54°,对应的层间距为0.7667 nm[39]。在水热时间一定的情况下,水热温度越低对应(002)晶面的衍射峰越低且较宽(见a1、b1、c1的对比),表明水热温度低生成的晶粒尺寸更小,结晶度较差。图1b为所制备的α-TiP的XRD谱中(002)峰与(112)峰的峰强比。在水热温度不变的情况下,随着水热时间的延长衍射峰的强度增加,(002)晶面与(112)晶面的峰强比变大,而在水热温度为180℃时这种增长更加明显。XRD谱表明,在180℃温度下增加水热时间使得合成α-TiP更有利于(002)晶面的生长,水热时长为20 h时最好。
图1
图1
不同温度和时间下合成的α-TiP的XRD谱及(002)与(112)峰峰强比
Fig.1
XRD patterns (a) and peak to peak ratio between (002) and (112) peaks (b) of α-TiP synthesized at different condition
图2
图2
不同水热条件下α-TiP的红外光谱
Fig.2
Infrared spectrum of α-TiP under different hydrothermal conditions
图3为不同条件下水热合成的α-TiP的形貌图,主要为六边形片层状结构,尺寸从100 nm到600 nm不等,厚度分布在20~100 nm之间。图3a为120℃/12 h合成所得样品的SEM图,图3b为120℃/20 h合成所得样品的SEM图,对比图3a,120℃/20 h所得样品厚度增加,粒径尺寸没有增加,120℃条件下合成的粒径小于300 nm,层状厚度分布范围30~100 nm。图3c与图3a对比可见,粒径尺寸没有显著变化,粒径均小于300 nm,厚度分布范围由原来的30~100 nm增长到60~100 nm,层状结构更加明显,边缘更加清晰。同样合成时间下,180℃相较于150℃厚度没有明显的增长,但是在180℃条件下而随着时间的延长20 h时其粒径分布从150 nm到600 nm,合成的α-TiP片层状结构明显,粒径分布更均匀。180℃条件下随着水热时间的增加,粒径尺寸逐渐增加。120℃下水热时间的增加层状α-TiP的厚度增加,而180℃条件下更有利于层状α-TiP的横向增长。
图3
图3
不同水热条件合成的α-TiP形貌图
Fig.3
SEM image of the α-TiP sample synthesized at 120oC/12 h (a), 120oC/20 h (b), 150oC/12 h (c), 150oC/16 h (d), 150oC/20 h (e), 180oC/12 h (f), 180oC/16 h (g) and 180oC/20 h (h)
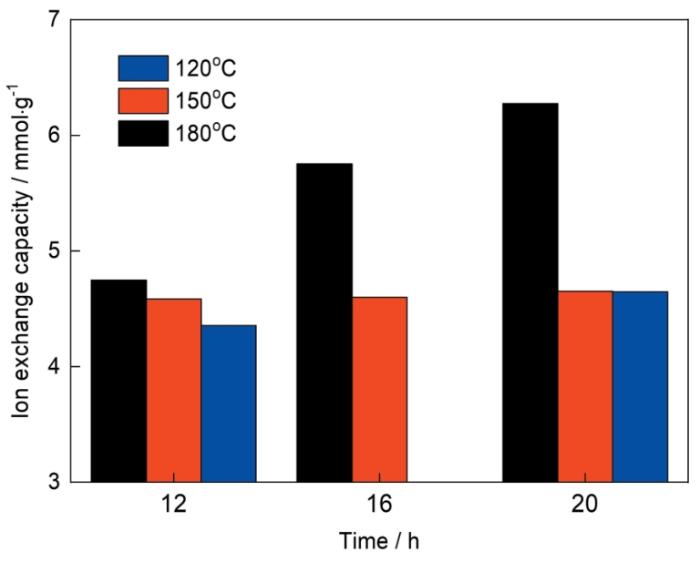
2.1.2 α-TiP的离子交换能力
图4
图4
不同合成条件合成的α-TiP的离子交换容量
Fig.4
Ion exchange capacity of α-TiP synthesized under different synthesis conditions
2.2 α-TiP改性涂层的性能
图5
图6
2.3 涂层耐腐蚀性
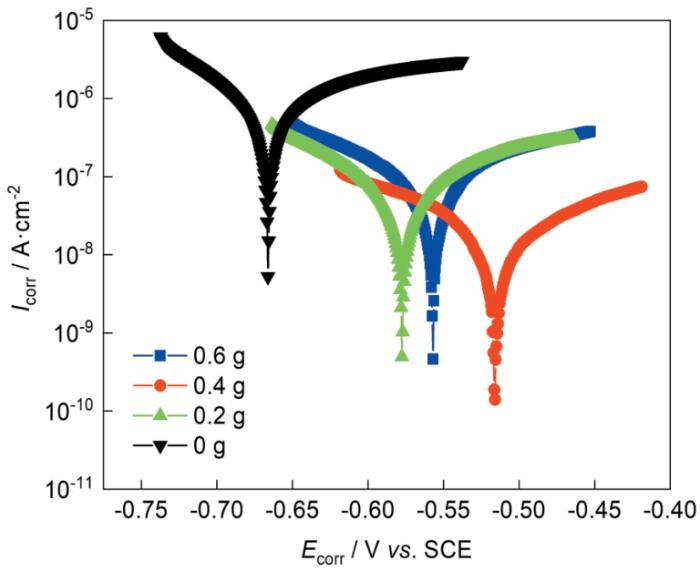
2.3.1 极化曲线测量
图7
表1 极化曲线拟合值
Table 1
| Sample | Ecorr / V vs SCE | I0 / A·cm-2 |
|---|---|---|
| 0 | -0.667 | 2.27 × 10-6 |
| 0.2 | -0.577 | 4.15 × 10-8 |
| 0.4 | -0.516 | 8.817 × 10-9 |
| 0.6 | -0.557 | 8.26 × 10-8 |
表2 不同添加量的涂层的拟合值
Table 2
| Sample | Time d | CPEf nΩ-1·Sn·cm-2 | n1 | Rf Ω·cm2 | CPEdl nΩ-1·Sn·cm-2 | n2 | Rct Ω·cm2 |
|---|---|---|---|---|---|---|---|
| 0 g | 1 | 1.237 × 10-7 | 0.7564 | 1.808 × 105 | 2.479 × 10-7 | 0.7136 | 1.72 × 105 |
| 3 | 1.817 × 10-6 | 0.683 | 4314 | 3.425 × 10-5 | 0.6116 | 1.096 × 104 | |
| 5 | 2.38 × 10-5 | 0.5271 | 2517 | 7.609 × 10-6 | 0.6536 | 903.4 | |
| 0.2 g | 1 | 4.772 × 10-10 | 0.9257 | 8.495 × 106 | 2.138 × 10-8 | 0.5833 | 2.451 × 107 |
| 3 | 4.90 × 10-10 | 0.9237 | 2.826 × 106 | 3.685 × 10-8 | 0.3659 | 2.371 × 107 | |
| 5 | 6.49 × 10-10 | 0.9183 | 1.18 × 106 | 1.136 × 10-7 | 0.4644 | 1.038 × 107 | |
| 7 | 9.152 × 10-10 | 0.9194 | 3.027 × 105 | 8.179 × 10-8 | 0.6124 | 3.373 × 105 | |
| 0.4 g | 1 | 4.288 × 10-10 | 0.8967 | 4.449 × 106 | 5.79 × 10-8 | 0.3801 | 1.679 × 108 |
| 3 | 7.397 × 10-13 | 1 | 9.33 × 106 | 1.305 × 10-9 | 0.235 | 2.536 × 107 | |
| 5 | 4,474 × 10-12 | 0.8967 | 8.067 × 106 | 6.125 × 10-9 | 0.2186 | 1.943 × 108 | |
| 7 | 8.368 × 10-9 | 0.2985 | 7.732 × 106 | 1.072 × 10-12 | 0.9819 | 1.166 × 108 | |
| 0.6 g | 1 | 5.674 × 10-8 | 0.9090 | 5.207 × 106 | 3.027 × 10-8 | 0.5474 | 2.932 × 107 |
| 3 | 4.03 × 10-10 | 0.9429 | 5.762 × 105 | 4.983 × 10-8 | 0.2792 | 3.865 × 107 | |
| 5 | 6.14 × 10-10 | 0.9154 | 6.066 × 106 | 2.273 × 10-7 | 0.6547 | 3.146 × 107 | |
| 7 | 6.599 × 10-10 | 0.9152 | 2.159 × 106 | 3.976 × 10-8 | 0.4733 | 6.076 × 107 |
2.3.2 EIS
图8
图8
不同添加量涂层的Bode图
Fig.8
Bode diagram of coatings with different addition amounts: (a1, a2) 0 g, (b1, b2) 0.2 g, (c1, c2) 0.4 g, (d1, d2) 0.6 g
在相角图中,添加α-TiP样品在浸泡初期高频处的相角均高于80°,而未添加样品的高频相角为50°,相角的高频响应反映了涂层的阻隔性能,高频相角越大说明涂层的阻隔性能越好。在浸泡开始涂层的响应主要集中在高频,涂层随着浸泡时间的增加,未添加样品在中频低频响应增强,低频响应的出现表明电解质溶液到达涂层/基板界面发生反应。而添加后的涂层随着浸泡时间的增加,高频处仍保持较高的相角,在10-2~10-1 Hz频率下的响应代表由于扩散产生的Warburg阻抗,0.4 g样品在浸泡的第7 d出现因电解质到达基板反应导致的响应。阻隔性能随α-TiP添加量的增加而增加,当添加量增加到0.6 g时,涂层的阻隔性能反而下降,可能是α-TiP添加过多容易造成α-TiP的团聚,造成涂层不均匀产生缺陷,降低了涂层的阻隔性能。
图9
根据图9中的等效电路对于不同添加量的涂层的阻抗谱进行解析,得到Rf、Rct随时间变化,如图10所示。由图10可见,添加量为0.2 g的涂层的Rf都随着浸泡时间的增加而减小,添加量为0.4和0.6 g的涂层的Rf都随着浸泡时间的增加先增加后减小,α-TiP添加量0.4 g时涂层具有更高的膜电阻。0.4 g样品电荷转移电阻最大,相比于其他样品大一个数量级。电荷转移电阻反映了基体界面发生反应的速率,电荷转移电阻越大代表基体界面发生腐蚀反应的速率越小。由于层状结构α-TiP在涂层中可以延长溶剂和侵蚀性离子向基体的路径,有效地起到阻隔溶剂分子和侵蚀性离子向基体的扩散,延缓基体发生腐蚀。数据表明0.4 g样品的腐蚀防护能力最好,对电解质液有着更好的阻隔性能,而较少的添加量则起不到明显的阻隔作用,而较多的添加则可能会引起团聚而产生更多的缺陷,导致其阻隔能力下降。
图10
图10
Rf和Rct随浸泡时间变化曲线
Fig.10
Curve of Rf (a) and Rct (b) during different immersion time
当涂层在模拟液中浸泡7 d,0.4 g样品观察到其电化学性能逐渐下降表明金属表面的已开始发生腐蚀,涂层的防护性能逐渐衰减。
3 结论
使用水热法制备了α-TiP,研究了不同水热时间及水热温度对α-TiP晶面取向及离子交换能力的影响。并利用α-TiP离子交换性能以水玻璃为前驱体制备硅溶胶涂层。探究了不同添加量的影响,利用扫描电镜观察α-TiP及涂层截面形貌,采用XRD和红外对α-TiP、涂层成分进行物相分析,最后利用电化学测试来评估涂层的耐蚀性能。得出以下结论:
(1) 利用水热法合成了α-TiP,在120~180℃范围内温度越高越有利于薄片状结构的生长,离子交换能力越高。
(2) α-TiP改性硅溶胶涂层,有效提升了涂层的阻隔性能。添加量为0.4 g的涂层电化学阻隔性能最好,当添加量高于0.4 g,容易造成α-TiP的团聚产生缺陷,涂层的防护性能下降。
(3) 在Ca(OH)2模拟液的环境中,α-TiP的加入可以使硅溶胶涂层在碱性环境下稳定存在,涂层可以在混凝土环境下提供良好的防护性能。
参考文献
The cost of corrosion in China
[J].Corrosion is a ubiquitous and costly problem for a variety of industries. Understanding and reducing the cost of corrosion remain primary interests for corrosion professionals and relevant asset owners. The present study summarises the findings that arose from the landmark “Study of Corrosion Status and Control Strategies in China”, a key consulting project of the Chinese Academy of Engineering in 2015, which sought to determine the national cost of corrosion and costs associated with representative industries in China. The study estimated that the cost of corrosion in China was approximately 2127.8 billion RMB (~ 310 billion USD), representing about 3.34% of the gross domestic product. The transportation and electronics industries were the two that generated the highest costs among all those surveyed. Based on the survey results, corrosion is a major and significant issue, with several key general strategies to reduce the cost of corrosion also outlined.
Rebar corrosion detection, protection, and rehabilitation of reinforced concrete structures in coastal environments: a review
[J].
Research progress on corrosion and anti-corrosion technology of ribbed steel
[J].
带肋钢腐蚀及其防腐蚀技术研究进展
[J].
Interactions between chloride and cement-paste materials
[J].
Durability characteristics and quantification of ultra-high strength alkali-activated concrete
[J].
Protection of steel reinforcement for concrete- a review
[J].
Soy-protein and corn-derived polyol based coatings for corrosion mitigation in reinforced concrete
[J].
Fabrication of amphiphobic concrete coating with good abrasion resistance and anti-oil adhesion properties by using waste clam powder
[J].
Corrosion behavior of epoxy/zinc duplex coated rebar embedded in concrete in ocean environment
[J].
Long-term performance of concrete surface coatings in soil exposure of marine environments
[J].
Structural performance of stainless steel reinforced concrete members: a review
[J].
Experimental and analytical assessment of the behavior of stainless steel reinforced concrete beams
[J].
Corrosion behavior of duplex stainless steel reinforcement in ternary binder concrete exposed to natural chloride penetration
[J].The corrosion behavior duplex stainless steel rebars embedded in concrete exposed to natural chloride penetration has been carried out. Three duplex stainless steels (DSSs) differentiated in the Ni, Mo and Mn content and two types of concretes elaborated with different binders, Portland cement and Portland cement blended with Slag and Limestone, were employed. The corrosion behavior was followed through E-corr and Rp measurements along the exposure period. The tests were extended up to corrosion detection or to 2 years, and the chloride contents accumulated at the rebar level were determined. The DSS with lower Ni content has demonstrated to show lower corrosion resistance in presence of chlorides and pitting corrosion at lower chloride contents accumulated at the rebar level were detected. The DSSs embedded in OPC concrete resist better to corrosion in presence of chlorides than those rebars embedded in concrete containing blast furnace slag and limestone. (C) 2018 Elsevier Ltd. All rights reserved.
Influence of SiO2 content and exposure periods on the anticorrosion behavior of epoxy nanocomposite coatings
[J].Epoxy coating formulations containing 1%, 3%, and 5% SiO2 nanoparticles were produced and applied on a mild steel substrate to achieve the objective of high performance corrosion resistance. The electrochemical impedance spectroscopy (EIS) technique was employed to measure the anticorrosive properties of coatings. The corrosion tests were performed by exposing the coated samples in a solution of 3.5% NaCl for different periods of time, varied from 1 h and up to 30 days. Fourier transform infrared spectroscopy (FT-IR) and X-ray diffraction (XRD) analyses revealed the presence of nanoparticles in the final cured samples. Establishing the incorporation of the nanoparticles in the coating formulations was confirmed by employing both of XRD and FT-IR techniques. The FT-IR spectra have proved to be satisfactory indicating that there was a complete reaction between the epoxy resin with the hardener. EIS measurements confirmed that the presence and the increase of SiO2 nanoparticles greatly improved the corrosion resistance of the epoxy coating. The highest corrosion resistance for the coatings was obtained for the formulation with 5% SiO2 nanoparticles content, particularly with prolonging the immersion time to 30 days.
Influence of kaolin and dolomite as filler on bond strength of polyurethane coated reinforcement concrete
[J].
Bond properties of epoxy-coated reinforcement to seawater coral concrete
[J].
环氧涂层钢筋与海水珊瑚混凝土的黏结性能
[J].
Research progress on modified alkyd resins in China
[J].
国内改性醇酸树脂研究进展
[J].
Test and comparison of various types of coatings for concrete
[J].
不同种类混凝土涂料的性能测试及对比研究
[J].
Corrosion resistance of three zinc-rich epoxy coatings
[J].
环氧富锌涂层防腐蚀性能研究
[J].制备了添加纳米SO<sub>2</sub>材料的纳米复合环氧富锌涂层,通过测试金属Zn含量、拉开法附着力、浸泡实验、盐雾实验和电化学阻抗谱实验,并与两种未添加纳米材料的环氧富锌涂层防腐蚀性能进行对比研究,结果表明,纳米复合环氧富锌涂层具有良好的附着力和内聚力,前期可以作为有机屏蔽层,中期阴极保护作用温和,持续时间长,后期Zn的反应产物又可以提供涂层良好的屏蔽,耐腐蚀性能显著,而未添加纳米材料的两种富锌涂层由于起泡和锈蚀而失效。
The effective factors of bond and anchorage performances of epoxy-coated reinforcement
[J].
影响环氧涂层钢筋粘结锚固性能的因素分析
[J].
Research on the progress in bonding and anchoring mechanism of coated steel bar to concrete
[J].
涂层钢筋与混凝土粘结锚固机理研究进展
[J].
Modification of epoxy resins with functional silanes, polysiloxanes, silsesquioxanes, silica and silicates
[J].
Scratch, wear and corrosion resistant organic inorganic hybrid materials for metals protection and barrier
[J].
Nanostructured poly(methyl methacrylate)-silica coatings for corrosion protection of reinforcing steel
[J].
Silica-based coating for corrosion protection of electrogalvanized steel
[J].
Apply suitable phosphating films
[J].
磷化层的正确选用
[J].
Improvement of barrier properties of a hybrid sol–gel coating by incorporation of synthetic talc-like phyllosilicates for corrosion protection of a carbon steel
[J].
Research and application of silicate adhesive
[J].
硅酸盐胶黏剂的研究与应用
[J].
Corrosion and fire protective behavior of advanced phosphatic geopolymeric coating on mild steel substrate
[J].
Transformation of silicate gels during heat treatment in air and in argon – Spectroscopic studies
[J].
The analysis of the solidification mechanismes & water resistance improvement accesses of the water-glass
[J].
水玻璃的固化机理及其提高耐水性途径分析
[J].
Inorganic ion-exchange pellicles obtained by delamination of α-zirconium phosphate crystals
[J].
Preparation and ion-exchange properties of a new phase of the crystalline titanium phosphate, Ti(HPO4)2·2H2O
[J].
Functionalized porous aromatic framework for efficient uranium adsorption from aqueous solutions
[J].
Chapter 2 - Ion exchangers, their structure and major properties
[A].
Low cost fabrication of anti-corrosive coatings on metal surfaces
[J].
低成本制备铁基金属表面防腐蚀无机涂层
[J].
Synthesis of titanium phosphates from unconventional solid precursor and their ion-exchange and electrochemical properties
[J].
Convenient testing device and assembly method for electrochemical testing of anti-corrosion coating performance
[P].
电化学测试防腐涂层性能的便捷检测装置及组装方法
[P].
Mild syntheses and surface characterization of amorphous TiO(OH)(H2PO4)·H2O ion-exchanger
[J].
Hydrothermal synthesis and characterization of the layered compound of α-titanium hydrophosphate
[J].
层状化合物α-磷酸钛的水热合成与表征
[J].
Crystalline insoluble salts of polybasic metals—I Ion-exchange properties of crystalline titanium phosphate
[J].
Ion exchange on α-titanium phosphate and formation of new crystalline phases by hydrolysis of the ion-exchanged phases
[J].
Effect of formulation of silica‐based solution on corrosion resistance of silicate coating on hot‐dip galvanized steel
[J].