由于无法采用水溶液电镀的形式制备铝镀层,目前镀铝工艺包括热浸镀铝、化学气相渗铝、粉末渗铝及离子液体镀铝等[10, 13]。热浸镀铝由于铝的熔点高(800℃)、活性大,因此热浸镀铝的工艺难度较高,造成大量能源浪费[14,15]。化学气相渗铝工艺能够对叶片微小通道内表面进行防护,且对各式各样涂层的化学和形态学方面具有适应性,但该工艺成本高,其中涉及有Cl2、HCl和H2等易燃易爆、有毒气体,生产过程中气体的泄露易造成灾难性事故[16,17]。粉末渗铝制备渗铝层表面渗剂容易出现粘结现象,渗铝周期较长,催渗剂氯化铵对皮肤和粘膜具有刺激性,分解出氨气对渗铝过程造成毒化作用[18,19]。离子液体镀铝工艺能够制备表面高平整度、致密的铝涂层,但工艺采用有机离子铝溶剂,成本高、条件苛刻,废液需特殊处理[20~22]。
本实验采用AIP在不同偏压参数(0、-50、-100、-200和-300 V)下制备铝涂层,通过宏观、微观形貌和粗糙度测试等手段表征不同偏压下镀铝涂层的结构特点,采用电化学测试及盐雾腐蚀实验研究不同偏压对镀铝碳钢腐蚀行为的影响,进而分析不同偏压参数对AIP沉积铝涂层结构及防腐性能的影响。
1 实验方法
实验使用MS-3型多功能电弧离子镀膜机,其为圆柱形沉积内腔,尺寸为ϕ450 mm ×500 mm。实验采用单靶施镀,为纯金属Al靶(纯度99.9%),靶材尺寸为ϕ96 mm ×25 mm。采用Ar(纯度99.99%)作为保护气体。选用规格为25 mm × 16 mm × 2 mm的45#碳钢片作为基材,其化学成分(质量分数,%)为:C 0.42~0.50,Ni ≤ 0.25,Cr ≤ 0.25,Mn 0.50~0.80,Si 0.17~0.37,P ≤0.035,S ≤0.035。实验前对碳钢基材用不同的砂纸进行逐级打磨,采用无水乙醇进行除油清洗,烘干后置于干燥皿中备用。处理后的试样片装入离子镀膜设备制样室中的样品架上,保持试样片平行于中心转轴,样品竖直放置,与靶材间距离约为100 mm。镀膜时试样架转速为12 r/min,制样室温度为200℃,当真空度达到5.0 × 10-2 Pa后,通入Ar,使得真空室压力维持在0.9~1.0 Pa,随后调整基材负偏压分别在实验值(0、-50、-100、-200和-300 V)进行铝镀层的制备,沉积时间为30 min。相关参数如表1所示。
表1 Al涂层的沉积参数
Table 1
| Parameter | Value |
|---|---|
| Bias voltage / V | Experimental value |
| Flow of Ar / m3·s-1 | 1.67 × 107 |
| Arc current / A | 55 |
| Deposition time / min | 30 |
| Background pressure / Pa | 0.9~1.0 |
| Chamber temperature / oC | 200 |
采用宏观照片对镀层表面整体状态进行观察,采用S-3400N扫描电子显微镜(SEM)观察涂层的表面微观形貌和截面形貌,采用INCA 250 X-Max 50能量色散谱仪(EDS)对涂层表面及截面进行元素成分表征。采用SDR990型粗糙度仪对镀层表面进行粗糙度测试,以评价涂层致密性。使用CHI920型电化学工作站进行电化学测试,均采用三电极体系,试样作为工作电极(测试面积1 cm2),铂电极(1 cm × 1 cm)作为辅助电极,饱和甘汞电极(SCE)作为参比电极,模拟电解液为3.5%(质量分数)NaCl溶液,室温测试[21]。其中开路电位(OCP)测试时间为5 min;阻抗测试频率范围为104~10‒1 Hz,方向从高频到低频,扰动电位幅度为5 mV[23],在进行这些测试之前,保证试样OCP的变化小于± 0.1 V[23];极化曲线测试电位范围为OCP ± 200 mV,扫描速率为1 mV/s[22]。依据GB/T 10125进行中性盐雾实验(5%NaCl溶液,35℃),评价不同偏压下镀铝层防腐蚀性能。
2 结果与讨论
2.1 形貌结构与成分组织
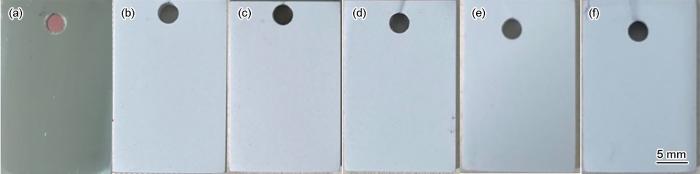
图1是各偏压下45#碳钢基体及镀铝涂层的宏观形貌。抛光后的碳钢表面光滑平整,呈镜面状态;经不同偏压镀铝30 min后,试样表面呈亮白色,无镜面光泽,为漫反射,说明试样表面具有一定粗糙度。
图1
图1
不同偏压下镀铝涂层表面宏观形貌
Fig.1
Surface macro-morphologies of 45# steel substrate (a) and aluminium coatings deposited under different bias-voltages: (b) 0 V, (c) -50 V, (d) -100 V, (e) -200 V, (f) -300 V
图2
图2
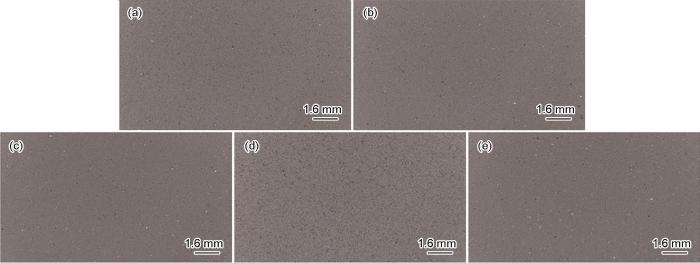
不同偏压镀铝试样的微观形貌
Fig.2
Microscopic morphologies of aluminium coatings deposited at different bias-voltages: (a) 0 V, (b) -50 V, (c) -100 V, (d) -200 V, (e) -300 V
图3
图3
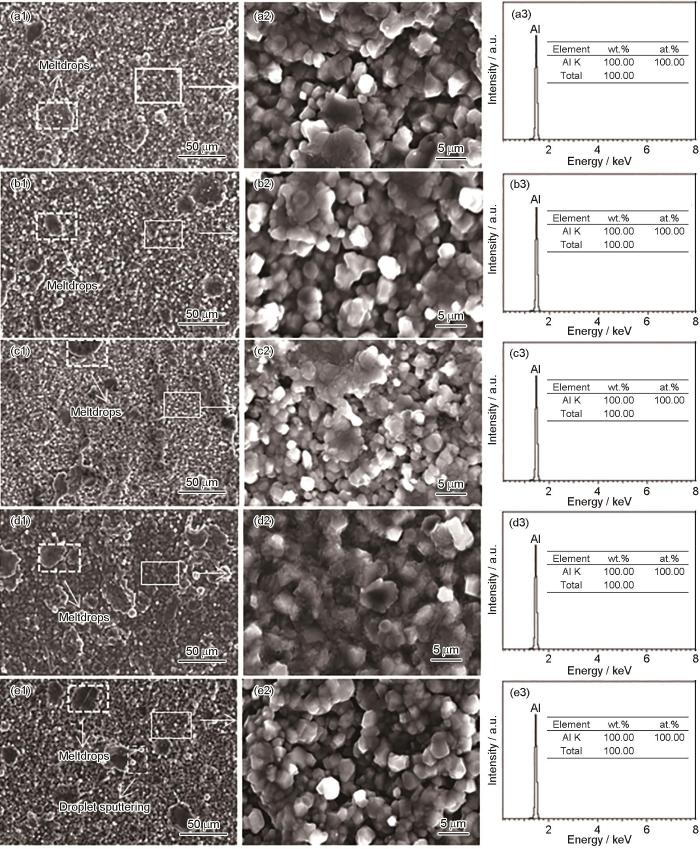
不同偏压下镀铝涂层表面微观形貌及能谱结果
Fig.3
Surface micro-morphologies (a1-e1, a2-e2) and EDS results (a3-e3) of aluminium coatings deposited at different bias-voltages: (a1-a3) 0 V, (b1-b3) -50 V, (c1-c3) -100 V, (d1-d3) -200 V, (e1-e3) -300 V
从局部放大图中看出,0与-50 V偏压下的试样,整体颗粒分布较均匀,大部分颗粒粒径在5~50 μm之间,熔滴现象轻微,说明-50 V偏压对Al的沉积未产生明显影响;当偏压增加到-100 V时,离化效果增加,沉积粒径变小[24, 25],大部分颗粒粒径在2~30 μm之间,但同时开始出现明显的熔滴现象,且熔滴形状不规则,这是由于部分靶材激发的大粒子还未完全离化,在较高偏压影响下快速迁移到试样表面,形成熔滴[26, 27]。在偏压为-200 V时,靶材离化及激发的速率进一步加快,多数还未离化的熔滴沉积到试样表面后容易相互融附在一起,部分覆盖与小粒径颗粒表面,使得颗粒增大,部分填补小粒径Al颗粒的空隙,使得表面更加致密;在偏压升高到-300 V时,表面颗粒状态与-200 V较为接近,但由于偏压的进一步增大,使得多数还未离化的熔滴高速沉积到试样表面,发生明显的熔滴溅射现象,熔滴的溅射导致镀层存在一定空隙。
图3表面形貌结果表明,随着偏压参数的增大,一方面可以加大离化程度,细化镀层的颗粒粒径,偏压为-100 V时粒径最小。另一方面,偏压的增大同时也会加速熔滴的沉积,偏压为-200 V时,熔滴能良好填充颗粒之间的空隙并不发生明显溅射现象。
图4为不同偏压下镀铝涂层的截面形貌。偏压0、-50、-100、-200和-300 V参数下的涂层厚度分别约为14、18、15、22和38 μm,相应的扩散层厚度分别约为2、2、4、7和5 μm。根据截面形貌及能谱结果可知,采用真空离子镀技术制备的镀铝涂层与碳钢基体界面发生明显的互扩散,为冶金结合。随着偏压增大,粒子运动速率增大,涂层厚度增厚,且扩散层也随之增厚,但超过一定偏压后,扩散层有所降低,其中200 V偏压下的扩散层最厚。
图4
图4
不同偏压下45#碳钢镀铝涂层截面形貌及能谱结果
Fig.4
Cross-sectional morphologies (a1-e1, a2-e2) and EDS results (a3-e3) of aluminium coatings deposited on 45# carbon steel at 0 V (a1-a3), -50 V (b1-b3), -100 V (c1-c3), -200 V (d1-d3) and -300 V (e1-e3) bias-voltages
2.2 镀层表面粗糙度
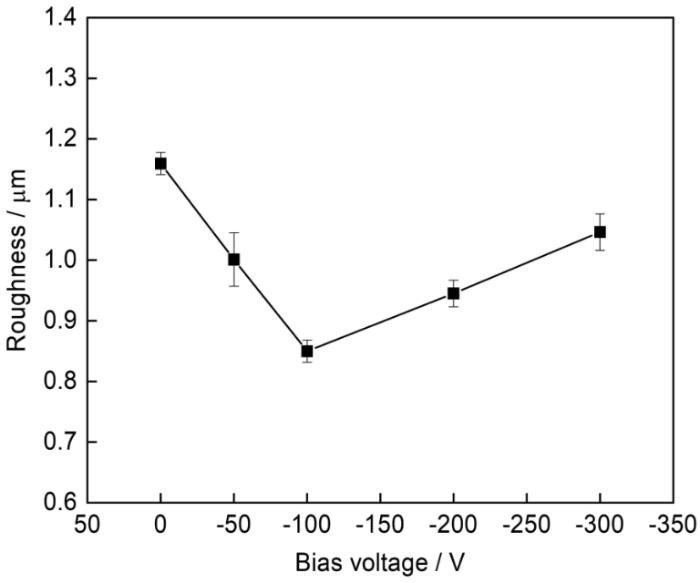
图5为45#碳钢不同偏压下镀铝涂层的粗糙度变化关系。可以看出,-100 V参数下的粗糙度最低,其次是-200 V参数下的试样。通过表面微观形貌图及分析也从侧面印证了偏压的适当增大,颗粒变小,镀层的粗糙度降低;但偏压再增大,则会使得镀层出现熔滴,表面状态变差,粗糙度升高。
图5
图5
不同偏压下镀铝涂层的粗糙度变化关系
Fig.5
Dependence of surface roughness of aluminium coating on deposition bias-voltage
2.3 电化学测试
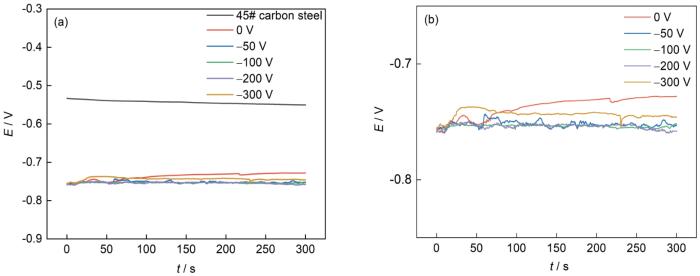
开路电位和体系的阻抗可在一定程度上反应腐蚀倾向,电解液使用浓度为3.5%NaCl溶液。图6为45#碳钢及不同偏压下镀铝涂层的开路电位曲线图。可以看出,碳钢空白试样的开路电位在-0.52~-0.54 V之间,不同偏压参数下的镀铝涂层试样的开路电位在-0.72~-0.77 V之间。开路电位结果说明,不同参数下镀铝膜层覆盖完整,表面状态均匀,无漏镀现象。
图6
图6
不同偏压下镀铝涂层及基体的开路电位
Fig.6
Open circuit potentials of 45# carbon steel substrate and aluminium coatings deposited at different bias-voltages (a), and enlarged view of the open circuit potential of the aluminium coatings (b)
图7
图7
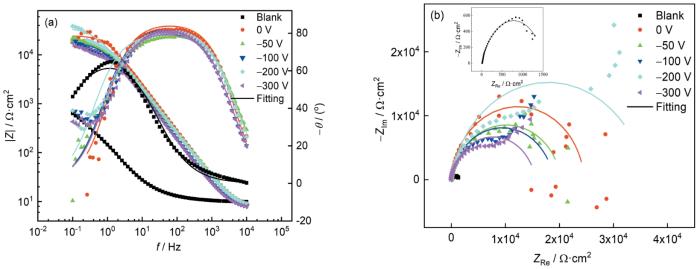
不同偏压下镀铝涂层的EIS测试结果
Fig.7
Bode (a) and Nyquist (b) plots of aluminium coatings deposited at different bias-voltages
图8
图8
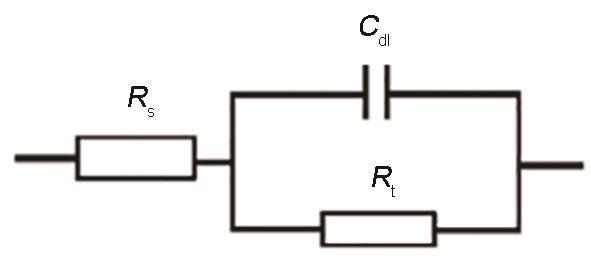
镀铝试样的阻抗谱等效电路模型
Fig.8
Equivalent circuit model for fitting impedance spectroscopies in Fig.7
涂层的耐蚀性和Rt有着密切的关系,拟合后的对应参数如表2所示。可知,45#碳钢的Rt为1.53 × 103 Ω·cm2,不同偏压镀铝涂层的Rt在(1.50~2.98) ×104 Ω·cm2之间。相比碳钢基体,镀铝涂层在3.5%NaCl溶液中表现出良好的耐蚀性,其中,-200 V的Rt较大,说明其耐蚀性相对较好。不过,铝镀层为两性金属,易于发生反应导致表面变性。由于低频极化时间较长,在低频阴极极化过程与阳极极化过程中,所有试样表面均容易发生变化,失去稳态,造成低频测试紊乱。
表2 不同偏压参数下镀铝涂层EIS测量结果的拟合参数
Table 2
Bias V | Rs Ω·cm2 | Cdl Ω·cm-2·S n | Rt Ω·cm2 | Chsq |
|---|---|---|---|---|
| Blank | 10.47 | 1.72 × 10-3 | 1.53 × 103 | 2.40 × 10-3 |
| 0 | 7.177 | 1.38 × 10-5 | 2.46 × 104 | 2.12 × 10-2 |
| -50 | 6.805 | 1.30 × 10-5 | 2.03 × 104 | 6.15 × 10-3 |
| -100 | 7.309 | 1.49 × 10-5 | 2.29 × 104 | 2.43 × 10-2 |
| -200 | 7.412 | 1.31 × 10-5 | 2.98 × 104 | 2.69 × 10-2 |
| -300 | 7.023 | 1.56 × 10-5 | 1.50 × 104 | 1.98 × 10-2 |
图9
图9
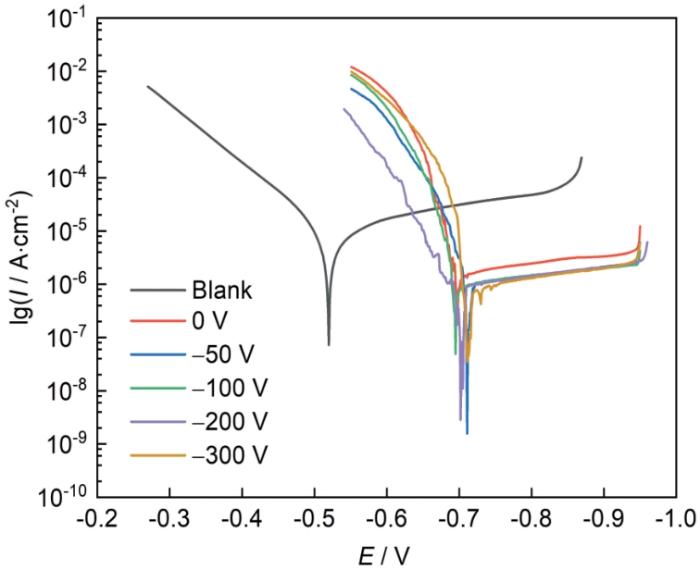
不同偏压下镀铝涂层的极化曲线
Fig.9
Electrochemical polarization curves of aluminium coatings deposited under different bias-voltages
表3 不同偏压下的镀铝涂层自腐蚀电流密度拟合结果
Table 3
| Bias / V | Icorr / A·cm-2 |
|---|---|
| Blank | 1.19 × 10-5 |
| 0 | 5.49 × 10-6 |
| -50 | 1.41 × 10-6 |
| -100 | 1.66 × 10-6 |
| -200 | 1.18 × 10-6 |
| -300 | 1.62 × 10-6 |
2.4 盐雾实验
图10为2、18、48、96和336 h中性盐雾实验后不同偏压镀铝涂层试样的腐蚀形貌照片。2 h中性盐雾实验后,无涂层45#碳钢出现红锈,发生明显腐蚀现象,各偏压下镀铝涂层试样均未观察到明显的腐蚀现象;18 h后,45#碳钢腐蚀进一步加剧,各偏压下镀铝涂层边缘区域变暗,铝层开始发生轻微腐蚀;48 h后,45#碳钢整个表面完全发生腐蚀,各偏压下镀铝涂层的表面出现黑斑和白锈,腐蚀程度严重;96 h后,45#碳钢及各偏压下镀铝涂层的表面腐蚀未明显进一步加重;336 h中性盐雾试验后,45#碳钢腐蚀非常严重,已被红锈完全覆盖,各偏压下镀铝涂层腐蚀加重,其中-100和-300 V出现轻微红锈,说明此偏压下45#碳钢基体已发生明显腐蚀,镀铝涂层已失去防护效果;而0和-200 V未观察到明显红锈,说明这两个偏压下涂层依旧具有一定防护性能。
图10
图10
45#碳钢和不同偏压下沉积的铝涂层经不同时间盐雾后的表面形貌
Fig.10
Corrosion morphologies of 45# carbon steel (a1-e1) and aluminium coatings deposited at the bias-voltages of 0 V (a2-e2), -50 V (a3-e3), -100 V (a4-e4), -200 V (a5-e5) and -300 V (a6-e6) after neutral salt spray test for 2 h (a1-a6), 18 h (b1-b6), 48 h (c1-c6), 96 h (d1-d6) and 336 h (e1-e6)
图11
图11
不同偏压下沉积的铝涂层经盐雾实验336 h后的腐蚀形貌
Fig.11
Corrosion morphologies of aluminium coatings deposited at the bias-voltages of 0 V (a), -50 V (b), -100 V (c), -200 V (d) and -300 V (e) after neutral salt spray test for 336 h
2.5 腐蚀机理分析
为了进一步判断镀铝涂层的防护机理,对偏压-200 V试样盐雾48 h和偏压0 V试样盐雾336 h后的腐蚀微观形貌进行观察。
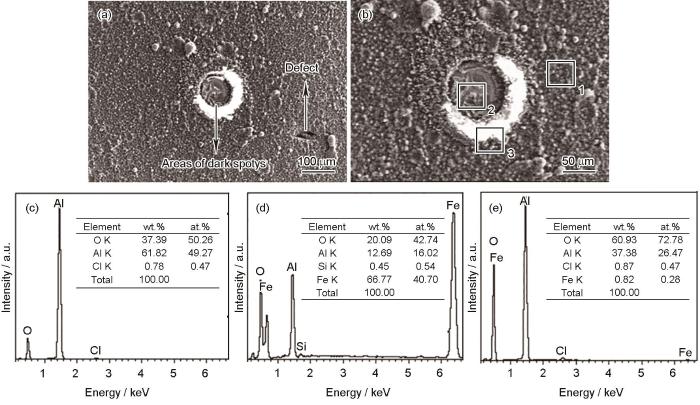
图12为偏压-200 V试样经盐雾腐蚀48 h后的黑斑区域的微观形貌及能谱分析。从图12a中可以看出,对于存在部分孔隙缺陷的非完全致密覆盖膜层,这些孔隙缺陷即为腐蚀发生的源区,此腐蚀形貌为明显的点腐蚀结构。通过对黑斑区域放大观察及能谱分析,可以进一步阐明镀铝涂层在盐雾中的初期腐蚀行为。其中,图12b区域1形貌与镀铝涂层形貌一致,无明显腐蚀特征,但能谱结果(图12c)显示,此区域除了Al以外,还含有50.26% (原子比)的O,说明腐蚀初期镀铝涂层表面会与水及氧生成Al的氧化物质,即铝镀层表面在腐蚀初期会生成一层氧化膜;图12b区域2膜层结构与区域1镀铝涂层结构存在明显差别,并非为镀铝涂层的颗粒状,通过能谱结果(图12d)显示,此区域除了含有Al和O外,还含有大量Fe,说明区域2膜层为铁铝互扩散层。图12b区域3为区域2外延区域,表面存在明显腐蚀产物,能谱结果(图12e)表明此腐蚀产物主要以Al的氧化物为主,含有少量Fe的氧化物。
图12
图12
盐雾实验48 h后镀铝涂层的微观表面形貌及能谱分析
Fig.12
Microscopic surface morphologies (a, b) and EDS results (c-e) of aluminium coating deposited at the bias-voltage of -200 V after 48 h neutral salt spray test
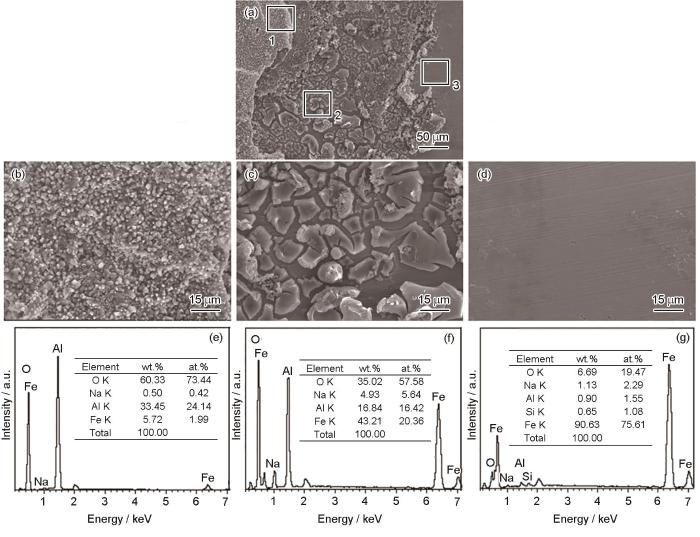
图13为0 V偏压试样经盐雾腐蚀336 h后的微观表面形貌及能谱分析。可以看出,试样表面(图13a)最终腐蚀形成了3类不同的区域。区域1(图13b)显示试样表面存在残余的颗粒状镀铝层,能谱(图13e)显示主要为Al的氧化物和少量Fe的氧化物,说明残余镀铝层发生了较大程度的腐蚀。区域2(图13c)形貌与区域1(图13b)镀铝层形貌明显不同,能谱(图13f)显示主要为Fe和Al的氧化物,其中,Fe的含量大大超过Al的含量,说明区域2(图13c)为铁铝扩散层,盐雾腐蚀336 h后铁铝扩散层产生明显腐蚀裂纹,已无法良好覆盖基体。区域3(图13d)表面较为平整,无明显腐蚀痕迹,能谱(图13g)结果显示为Fe基体表面。因此,此阶段为镀层快速腐蚀阶段,镀铝涂层及铁铝扩散层均作为腐蚀过程中的阳极相,对Fe基体进行阴极保护。
图13
图13
盐雾实验336 h后镀铝涂层的微观表面形貌及能谱分析
Fig.13
Microscopic surface morphologies (a-d) and EDS results (e-g) of aluminium coating deposited at the bias-voltage of 0 V after 336 h neutral salt spray test
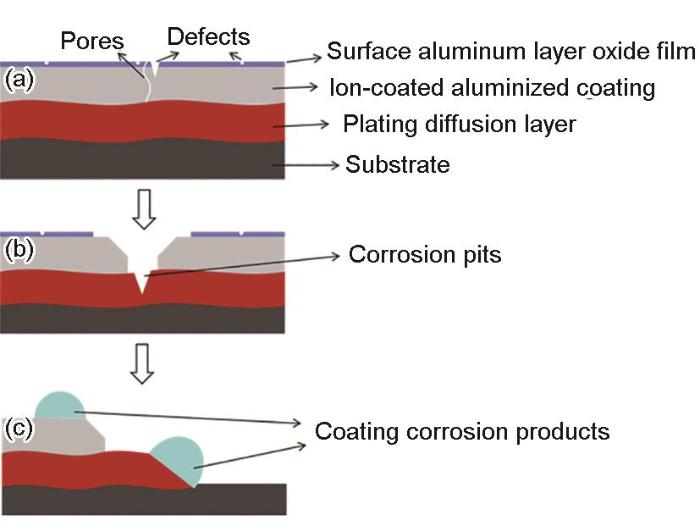
图14
图14
镀铝层在不同阶段的腐蚀机理图
Fig.14
Corrosion mechanism of AIP deposited aluminium coatings during corrosion at different stages: (a) initial stage, (b) intermediate stage, (c) later stage
在氯化物溶液中由于Cl-的存在,所以在较高活性的局部区域发生腐蚀(阳极溶解)[28]:
图14c为腐蚀后期(快速腐蚀阶段),此时,镀铝涂层及铁铝扩散层已经无法完全覆盖基体,基体发生裸露,但由于镀铝及铁铝扩散层的腐蚀电位更低,在此阶段作为阳极发生腐蚀,从而对Fe基体进行阴极保护,表现为基体表面光亮平整,腐蚀轻微。
3 结论
(1) 不同偏压下涂层镀覆完整,表面无漏镀现象,各偏压下铝镀层均与45#碳钢基体发生互扩散,有明显扩散层存在。偏压为-200 V时,镀铝涂层表面致密、均匀,无明显空隙;涂层与基体的扩散层最厚,约为7 μm。
(2) 3.5%NaCl溶液中45#碳钢的Rt为1.53 × 103 Ω·cm2,自腐蚀电流密度为1.19 × 10-5 A·cm-2,而不同偏压下镀铝涂层Rt在(1.50~2.98) × 104 Ω·cm2之间,自腐蚀电流密度在(1.18~5.49) × 10-6 A·cm-2之间。电化学测试结果表明,不同偏压下镀铝涂层的耐蚀性明显优于碳钢基体。
(3) 中性盐雾实验2 h后,45#碳钢基体已发生明显腐蚀;不同偏压下镀铝涂层盐雾实验48 h后出现明显白锈及黑斑;336 h后,碳钢基体已完全被锈覆盖,腐蚀严重;-200 V偏压条件下,在336 h盐雾腐蚀后无红锈且其黑斑较少,表现出优异的防护性能。
(4) 在盐雾实验过程中,电弧离子镀铝层前后的腐蚀机理不同。首先,镀层发生腐蚀而露出的扩散层,破裂形成了蚀坑,即镀层表现为局部腐蚀;腐蚀后期,其镀层以及铁铝扩散层发生严重腐蚀而破裂,而基体表面较完整,腐蚀程度轻微,镀铝层及铁铝扩散层在腐蚀后期对基体为阴极保护机制。
(5) 当靶电流55 A、Ar压力1.0 Pa、偏压-200 V时,真空离子镀铝涂层结构致密,与基体结合良好,具有优异的耐蚀性。
参考文献
Study on erosion and corrosion behavior and protection of steel in flowing artificial seawater
[J].
钢在流动人造海水中的冲刷腐蚀行为与防护研究
[J].
Research progress of carbon steel corrosion in seawater full lmmersion zone
[J].
碳钢在海水全浸区腐蚀的研究进展
[J].
Study on corrosion behavior of Q235 carbon steel in seawater environment
[D].
海水环境下Q235碳钢的腐蚀行为研究
[D].
Study on multiprincipal CoCrNi coating on 45 steel surface by plasma cladding
[D].
45钢表面等离子熔覆多主元CoCrNi涂层的研究
[D].
Comparison of properties of Ni-Co-P coating and Ni-W-P coating prepared by electroless plating on surface of 45 steel rod-shaped parts
[J].
45钢杆件化学镀Ni-Co-P及Ni-W-P镀层的性能比较
[J].
Localized electrochemical corrosion behavior of the interface of hot-dip galvanized coating
[J].
热浸镀锌层界面的微区电化学腐蚀行为
[J].
Study on replacement of Cd or Cd-Ti electroplating by metallic-ceramic anticorrosive coating
[J].
金属陶瓷防腐蚀涂层替代镀镉或镉-钛工艺的研究
[J].
Study on the mechanism of hydrogen permeation in the process of cadmium electroplating in high strength steel
[D].
高强度钢电镀镉过程中渗氢机理研究
[D].
Corrosion behavior of aluminized steel sheets in 50-year outdoor exposure test
[J].Aluminized steel sheets are very resistant to corrosion in the outdoor exposure environment. We evaluated the corrosion behavior of aluminized steel sheets with a Type 1 coating containing approximately 10% Si and a Type 2 coating not containing Si in a 50-year outdoor exposure test. Both specimens had strong perforation resistance, but those with Type 2 coating exhibited excellent perforation resistance. Type 2 aluminized steel sheets are known as a superior corrosion resistant materials compared to Type 1 aluminized steel sheets due to their thicker intermediate layer and higher coating weight. The Type 2 aluminized steel sheets in this exposure test included two sublayers composed of Fe2Al5 and FeAl2 as the intermediate layer between the aluminized layer and the steel substrate. The FeAl2 phase has less noble potential than the steel substrate and the Fe2Al5 phase in an artificial rain environment. As a result, this layer provided sacrificial corrosion protection for the steel substrate, and was the reason why the specimens with Type 2 coating exhibited better perforation resistance than those with Type 1 coating.
Comparison study on performance of IVD and ILEp aluminum coatings
[J].
离子镀铝与离子液体电镀铝涂层性能对比研究
[J].
Research on aluminum plating technology by ion vapor deposition
[J].
离子气相沉积镀铝技术研究
[J].
Protective performance comparison of aluminium-based coatings on 300M steel surface
[J].
300M钢表面铝基涂层防护性能对比研究
[J].
Aluminizing steel surface for providing protection in marine environment: a technological review
[J].
海洋环境下钢材表面镀铝工艺的研究进展
[J].
Effect of hot-dip aluminizing on surface microstructure and high temperature oxidation resistance of 316L stainless steel
[J].
热浸镀铝对316L不锈钢表面组织和抗高温氧化性的影响
[J].
Long-term deterioration mechanism of hot-dip aluminum coating exposed to a coastal-atmospheric environment
[J].
Aluminum diffusion in aluminide coatings deposited by the CVD method on pure nickel
[J].
Preparation of aluminide coating on hollow-blade inner-cavity by CVD method
[J].
采用CVD法制备空心叶片内腔铝化物涂层
[J].
Structure analysis of aluminized coatings on P92 steel prepared by pack cementation aluminizing and vapor aluminizing
[J].
P92耐热钢粉末包埋渗铝与化学气相渗铝涂层组织结构研究
[J].
Research progress on surface state and element diffusion mechanism of steel with surface coating prepared by pack aluminizing method
[J].
钢表面粉末包埋渗铝的表面状态及元素扩散机理研究进展
[J].
Electrochemically active species in aluminum electrodeposition baths of AlCl3/glyme solutions
[J].
Electropolishing of Al and Al alloys in AlCl3/trimethylamine hydrochloride ionic liquid
[J].
Effect of deposition time on corrosion resistance of Al-coated AZ31 magnesium alloy
[J].
沉积时间对镀铝层AZ31镁合金耐蚀性的影响
[J].
Corrosion property at marine atmosphere of Al based coatings replacing Cd on high strength steels
[J].
高强度钢代镉铝基涂层耐海洋环境腐蚀性能评价
[J].
Effect of arc ion plating technological parameters on the deposition and properties of Cr coatings
[J].
电弧离子镀工艺参数对Cr涂层沉积及性能的影响
[J].
Effect of arc ion plating bias on structure and properties of TiAlSiN films
[J].
电弧离子镀偏压对TiAlSiN涂层结构及性能的影响
[J].
Effect of bias voltage on the properties of TiAlN coating by shielded arc ion plating
[J].
偏压对挡板电弧离子镀TiAlN涂层性能的影响
[J].
Preparation and properties of CrN film by arc ion plating
[J].
电弧离子镀CrN薄膜的制备及性能研究
[J].
Corrosion behavior of cold-sprayed aluminum coating in seawater
[J].
冷喷涂铝涂层在海水中的腐蚀行为研究
[J]. 利用电化学测试手段研究冷喷涂铝涂层在海水环境中的腐蚀行为,并研究了冷喷涂铝涂层在中性盐雾中腐蚀速度的变化规律. 结果表明,海水环境中冷喷涂铝涂层表面覆盖致密稳定的腐蚀产物,有效阻止了腐蚀介质向涂层内部的渗透,腐蚀速度随腐蚀时间的增加迅速降低.