金属在人类社会发展进步的过程中起到了不可或缺的重要作用[1~4],而紫铜作为一种常见的金属,具有优异的热力学稳定性,导电性能以及传热性能,并被广泛应用于人类社会的方方面面。此外,其较高的标准电极电位使其具有良好的耐腐蚀性能,但紫铜在含有氧化剂(如O2以及ClO-等等)的介质中也会表现出腐蚀倾向[5,6]。近年来,人们对于紫铜在实际应用时的腐蚀行为做出了研究,比如海洋腐蚀[7~9],大气腐蚀[10~12],土壤腐蚀[13~16]等等。而在工业环境中,紫铜常常面临着腐蚀性溶剂、有机酸和SO2等腐蚀因素的影响[17~21]。这些因素可以通过化学反应对紫铜产生腐蚀作用,从而导致材料的退化。其中,有机酸腐蚀和SO2腐蚀是对紫铜腐蚀影响最为明显的两种腐蚀类型。
本文为了综合探究材料在有机酸和SO2气体的影响下的腐蚀行为,通过暴露实验模拟工业环境。TP2紫铜作业的工业生产环境中,存在着大量带有羧酸根离子的污染物。随着有机酸浓度的改变以及不同污染物的加入,使得TP2紫铜本身在工业应用的过程中面临着严重的腐蚀破坏。甲酸、乙酸作为两种常见的有机酸,探究其对于紫铜的腐蚀影响具有一定的意义。故本文选用甲酸、乙酸作为有机酸气氛并采用Tafel极化测试、电化学阻抗测试、SEM测试以及腐蚀失重测试以探究材料在不同有机酸浓度的气氛中,以及在固定浓度有机酸气氛和不同浓度SO2污染物Na2SO4的溶液中的腐蚀情况,并通过对比研究材料在工业环境中的腐蚀倾向及行为。
1 实验方法
将TP2紫铜样品切割成10 mm × 10 mm × 2 mm,并使用400#和600#砂纸对工作面进行粗磨。样品化学元素含量主要以Cu为主(99.90%),剩余元素如P、Fe、O、Sb等含量都在0.1%以下。
将尺寸为10 mm × 20 mm × 2 mm的TP2紫铜样品固定在玻璃试管中,并将试管以45°的倾角斜靠在封口大玻璃烧杯(烧杯容量为1 L)内部。在烧杯内加入含有一定量有机酸溶液(有机酸浓度为1%,4%,7%和10%),以模拟有机酸腐蚀的暴露环境。同时对体系进行热循环操作,将体系整体置于设定温度为40℃的烘箱中保温12 h;随后,将玻璃烧杯取出,放置于室温环境下12 h。重复上述实验共计30 d,并在第15 d采用JSM-6510LA钨灯丝扫描电镜(SEM)测试对材料进行,第30 d对材料进行腐蚀失重实验测试,以观察材料的微观腐蚀形貌和腐蚀速率,而腐蚀失重实验测试是通过失重公式V = (W1-W2)/(A·t)计算金属的腐蚀速率,其中W1和W2为实验前样品及样品去除的质量(g),A为试样的面积(m2),t为实验周期(h)。
在模拟含硫污染物(SO2)暴露实验中,将TP2紫铜样品以上述暴露实验相同的方式固定在玻璃试管中。固定溶液中有机酸(甲酸/乙酸)的浓度为1%,并改变的Na2SO4浓度(0.01,0.03,0.05和0.10 mol/L),以模拟不同含硫污染物浓度下的环境。同时对体系进行热循环操作,将体系整体置于设定温度为40℃的烘箱中保温12 h;随后,将玻璃烧杯取出,放置于室温环境下12 h。重复上述实验共计30 d,并在第15 d对材料进行SEM测试,第30 d对材料进行腐蚀失重实验测试,以观察材料的微观腐蚀形貌和腐蚀速率。
将上文中打磨好的样品再使用800#、1000#、1500#和2000#的砂纸进行抛光打磨,随后在用铜导线对其进行连接后,使用环氧树脂对其进行封样。电化学测试所用溶液与之前暴露实验所用溶液相对应,且所用样品是经在对应酸性环境中暴露30 d后的样品。Tafel极化测试中,选用的电压范围为-0.3~0.3 V,扫速为1 mV/s。在电化学阻抗测试中,选用的频率范围为105~10-2 Hz,振幅为10 mV。
2 结果与分析
2.1 有机酸的影响
2.1.1 腐蚀现象
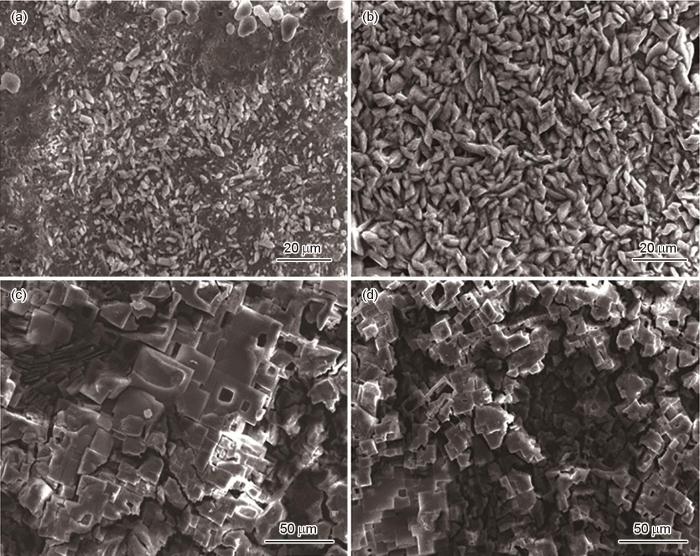
TP2紫铜在含有不同浓度的两种有机酸(甲酸、乙酸)的气氛中的腐蚀形貌如图1和2所示。由图可知,随着有机酸浓度逐渐增加,TP2紫铜的受腐蚀程度也逐步加剧。紫铜样品在浓度为1%的甲酸气氛中时,腐蚀就已经发生了,有短条状的腐蚀产物出现在了样品的表面上,但其表面由制备所导致划痕仍然可见。当浓度增加到4%时,短条状腐蚀产物数量呈倍数增加,并相互堆叠在紫铜样品表面。而当其浓度增加到7%之后,样品表面的腐蚀形貌由短条状转变为了立方晶态,这种多孔疏松的立方晶态腐蚀形貌极大的增加了腐蚀介质对于材料基体的渗透,加速了材料的腐蚀速度。除此之外,疏松多孔的结构,还会劣化材料整体的强韧性,对于工件的强度造成了不可逆的损伤。乙酸气氛对于紫铜样品的腐蚀影响规律与甲酸气氛基本相同,都是随着气氛中有机酸的浓度的增加使得材料表面的腐蚀情况愈加严重,但不同的是乙酸并未像甲酸一般在浓度达到某个临界点后,在样品表面所造成的腐蚀形貌会发生明显改变。这与TP2紫铜在不同有机酸环境中腐蚀机理有很大关联。在含有甲酸的气氛中,当有水蒸气凝结于紫铜表面形成液膜后,铜单质则会与溶解于水中的H+以及O2相互作用发生吸氧反应并被转变为Cu+,一部分Cu+形成了Cu2O,另一部继续被O2氧化从而形成了Cu(OH)2。当气氛中的甲酸浓度不是很大时,Cu(OH)2的水合物则会与甲酸反应生成羟基甲酸铜Cu(OH)(HCOO),从而在样品表面呈现出短条状的腐蚀产物;在此有机酸浓度足够大或者反应时间足够长的条件下,Cu(OH)(HCOO)会进一步与甲酸发生反应生成甲酸铜Cu(HCOO)2晶体,从而在样品表面呈现出立方晶态的腐蚀形貌。而在含有乙酸气氛中,紫铜的腐蚀产物主要为氧化亚铜Cu2O和乙酸铜的水合物Cu(CH3COO)2·2H2O,其过度产物是碱式乙酸铜Cu4OH(CH3OO)7·2H2O。当气氛中乙酸浓度不高(1%和4%)时,样品表面腐蚀产物主要以细小的颗粒为主,且此时样品表面残留的划痕仍然可见。当其浓度增加至7%之后,样品表面划痕消失,且腐蚀产物也会相互团聚并形成一层新的腐蚀层。
图1
图1
TP2紫铜在浓度为1%、4%、7%和10%的甲酸气氛中的SEM像
Fig.1
SEM images of the TP2 copper in the formic acid atmosphere with the concentration of 1% (a), 4% (b), 7% (c) and 10% (d)
图2
图2
TP2紫铜在浓度为1%、4%、7%和10%的乙酸气氛中的SEM像
Fig.2
SEM images of the TP2 copper in the acetic acid atmosphere with the concentration of 1% (a), 4% (b), 7% (c) and 10% (d)
表1 有机酸环境中暴露30 d后的紫铜试样腐蚀速率结果
Table 1
| Corrosion medium | Concentration | Mass loss / g | Corrosion rate / mm·a-1 |
|---|---|---|---|
| Formic acid | 1% | 0.0067 | 0.0963 |
| 0.0074 | |||
| 4% | 0.0129 | 0.1859 | |
| 0.0143 | |||
| 7% | 0.0198 | 0.2761 | |
| 0.0206 | |||
| 10% | 0.0254 | 0.3383 | |
| 0.0241 | |||
| Acetic acid | 1% | 0.045 | 0.0724 |
| 0.0061 | |||
| 4% | 0.0113 | 0.1619 | |
| 0.0124 | |||
| 7% | 0.0145 | 0.2098 | |
| 0.0162 | |||
| 10% | 0.0203 | 0.2884 | |
| 0.0219 |
图3
图3
TP2紫铜分别在不同浓度的甲酸和乙酸气氛中的失重变化趋势图
Fig.3
Trend graph of mass loss for the TP2 copper in different concentrations of formic acid (a) and acetic acid (b) atmosphere
2.1.2 电化学行为
图4
图4
在有机酸环境中TP2紫铜在不同浓度的甲酸和乙酸气氛中暴露30 d后的的Tafel极化曲线图
Fig.4
Tafel polarization curves of TP2 copper after exposure 30 d in the atmospheres containing different concentrations (1%, 4%, 7% and 10%) of formic acid (a) and acetic acid (b)
图5
图5
TP2紫铜在不同浓度的甲酸和乙酸溶液中暴露30 d后的紫铜的自腐蚀电流变化趋势图
Fig.5
Self-corrosive currents of TP2 copper after exposure 30 d in the solutions containing different concentrations (1%, 4%, 7% and 10%) of formic acid and acetic acid
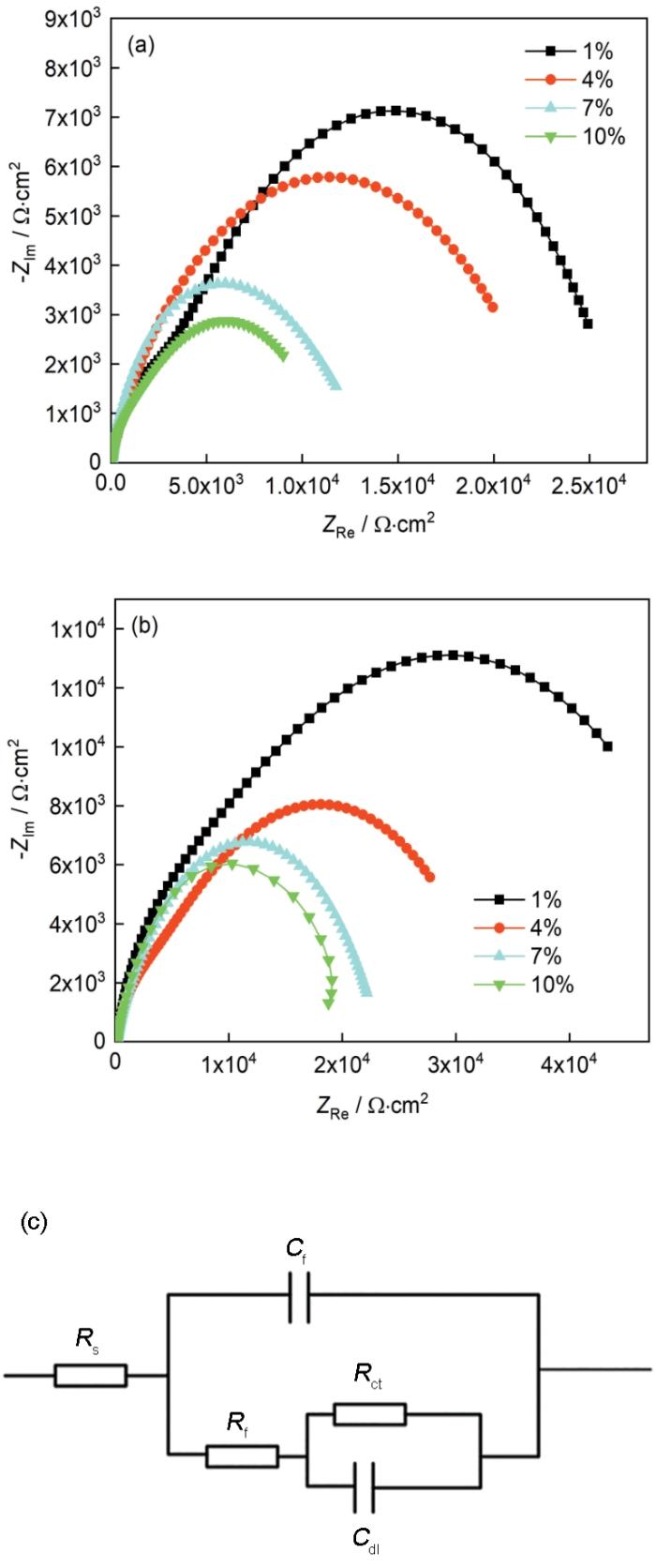
图6分别是紫铜在不同浓度甲酸和乙酸气氛中暴露30 d的电化学阻抗谱及其等效电路。其中,Rs是溶液电阻,Rf和Qf是膜电阻及其对应的常相位角元件,Rct和Cdl分别是电荷转移电阻以及代表双电层电容的常相位角元件[27]。图中,材料在高频区的容抗弧半径都随着气氛中有机酸浓度的增加而减小;但当气氛中有机酸的浓度大于7%后,材料的容抗弧半径减小趋势逐步放缓,这表明外界对于材料基体的影响已经逐渐逼近最恶劣的情况。而在图7中所示的Rct的变化趋势也是如此,这表明由于有机酸浓度的增加,材料表面电荷转移速率加快,从而使得整体的腐蚀速率加快。当浓度达到10%之后,两种气氛的Rct值都降至很小,这证明此时实验中所用的这两种有机酸都对材料表现出了较强的腐蚀性,极大的对材料进行破坏。甲酸相比于乙酸拥有更强的酸性,这使得其在水膜中更容易电离出H+,再加之其对于紫铜基体疏松多孔的微观腐蚀形貌,使其获得了比乙酸更小的Rct值,展现出了更加严重的腐蚀状态。
图6
图6
TP2紫铜分别在不同浓度的甲酸和乙酸溶液中暴露30 d后的电化学阻抗谱图及其对应等效电路图
Fig.6
Electrochemical impedance spectra of TP2 copper after exposure 30 d in the solutions containing different concentrations (1%, 4%, 7% and 10%) of formic acid (a) and acetic acid (b), and the relating equivalent circuit diagram (c)
图7
图7
TP2紫铜在不同浓度的甲酸,乙酸中暴露30 d后的Rct数值随有机酸浓度变化趋势图
Fig.7
Rct values of TP2 copper after exposure 30 d in the solutions containing different concentrations (1%, 4%, 7% and 10%) of formic acid (a) and acetic acid (b)
2.2 SO2 污染物的影响
2.2.1 腐蚀现象
图8和9分别是TP2紫铜在固定浓度有机酸(甲酸和乙酸)气氛和SO2污染物Na2SO4的共同作用下的微观腐蚀形貌图片。在甲酸气氛中,当Na2SO4的浓度仅为0.01 mol/L时(图8a),就已经出现了大量的腐蚀产物,在部分裂缝处甚至出现了较大的腐蚀产物,但基体表面的划痕依然可以辨认。当浓度增加到0.03 mol/L时(图8b),划痕已基本不见,取而代之的是更多的腐蚀产物。在随后的SEM形貌中(图8c、d),紫铜表面的腐蚀产物越来越多,并伴随着腐蚀晶状物和分层现象的出现。在乙酸气氛中,当Na2SO4的浓度为0.01 mol/L时(图9a),材料表面划痕附近分布着许多细小的腐蚀产物。随着Na2SO4的浓度进一步增加(图9b),表面划痕仍然可以辨认,但腐蚀产物体积明显增大。当浓度增至0.05 mol/L时(图9c),材料的腐蚀形貌变得十分复杂并出现了腐蚀产物相互连接的现象;并当浓度变为0.10 mol/L时(图9d),紫铜的腐蚀表面出现了新的锈层,腐蚀产物相互连接成了片状,但其与基体之间的连接并不是十分紧密的,这使得新的锈层并不具备对于基体的保护作用。这两组腐蚀形貌相比于同浓度的有机酸气氛,都表现出了更为严重的腐蚀状况,这表明工业大气环境中的SO2气体极易对于TP2紫铜工件产生不利影响。
图8
图8
TP2紫铜在不同Na2SO4浓度的甲酸气氛中暴露15 d后的SEM像
Fig.8
SEM images of the TP2 copper after exposure for 15 d in the formic acid atmosphere with 0.01mol/L (a), 0.03 mol/L (b), 0.05 mol/L (c) and 0.10 mol/L (d) Na2SO4
图9
图9
TP2紫铜在不同Na2SO4浓度的乙酸气氛中暴露15 d后的SEM像
Fig.9
SEM images of the TP2 copper after exposure for 15 d in the acetic acid atmosphere with 0.01 mol/L (a), 0.03 mol/L (b), 0.05 mol/L (c) and 0.10 mol/L (d) Na2SO4
表2 紫铜在含Na2SO4的有机酸环境暴露30 d后的腐蚀速率结果
Table 2
| Corrosion medium | Na2SO4 concentration / mol·L-1 | Mass loss / g | Corrosion rate / mm·a-1 |
|---|---|---|---|
| Formic acid | 0.01 | 0.0083 | 0.1052 |
| 0.0071 | |||
| 0.03 | 0.0101 | 0.1462 | |
| 0.0113 | |||
| 0.05 | 0.0159 | 0.2057 | |
| 0.0142 | |||
| 0.1 | 0.0206 | 0.2898 | |
| 0.0218 | |||
| Acetic acid | 0.01 | 0.0051 | 0.0773 |
| 0.0063 | |||
| 0.03 | 0.0062 | 0.0923 | |
| 0.0074 | |||
| 0.05 | 0.0101 | 0.1305 | |
| 0.0090 | |||
| 0.1 | 0.0145 | 0.1934 | |
| 0.0138 |
图10
图10
TP2紫铜分别在甲酸及乙酸气氛中不同Na2SO4浓度暴露30 d后的腐蚀失重变化曲线
Fig.10
Corrosion mass loss curves of copper after exposure for 30 d in formic acid (a) and acetic acid (b) atmosphere with different Na2SO4 concentrations (0.01, 0.03, 0.05 and 0.10 mol/L)
2.2.2 电化学行为
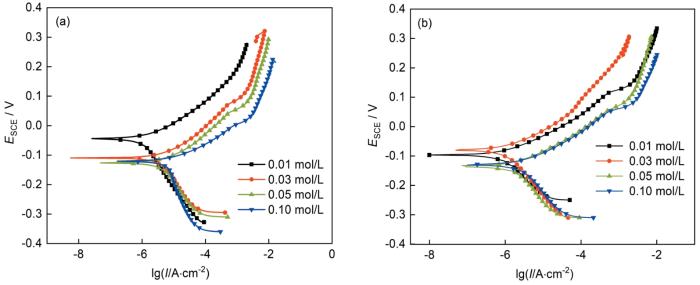
TP2紫铜在两种固定浓度(1%)有机酸气氛中随Na2SO4浓度变化的Tafel曲线如图11所示,图12是相对应的自腐蚀电流密度变化曲线。随着溶液中SO2污染物浓度的增加,材料的Tafel曲线出现了不同程度的右移以及下移趋势。相较于只有有机酸影响的情况下,与Na2SO4的共同作用大大提升了材料的自腐蚀电流密度,当Na2SO4浓度仅为0.01 mol/L时,材料在甲酸以及乙酸的自腐蚀电流密度已经达到了1.22和0.839 μA/cm2,而相同浓度的有机酸气氛的自腐蚀电流密度仅为0.785和0.437 μA/cm2。当Na2SO4浓度达到0.1 mol/L后,材料在甲酸以及乙酸中的自腐蚀电流密度更是达到了6.01和2.75 μA/cm2,展现出了对材料基体严重的腐蚀破坏能力。
图11
图11
TP2紫铜分别在甲酸及乙酸气氛中添加不同Na2SO4浓度中暴露30 d后的Tafel极化曲线
Fig.11
Tafel polarisation curves of TP2 copper after exposure for 30 d in formic acid (a) and acetic acid (b) atmosphere with different Na2SO4 concentrations (0.01, 0.03, 0.05 and 0.10 mol/L)
图12
图12
TP2紫铜分别在甲酸及乙酸气氛中添加不同Na2SO4浓度中暴露30 d后的自腐蚀电流密度变化曲线
Fig.12
Variation of self-corrosion current density of TP2 copper after exposure for 30 d in formic acid and acetic acid atmosphere with different Na2SO4 concentrations (0.01, 0.03, 0.05 and 0.10 mol/L)
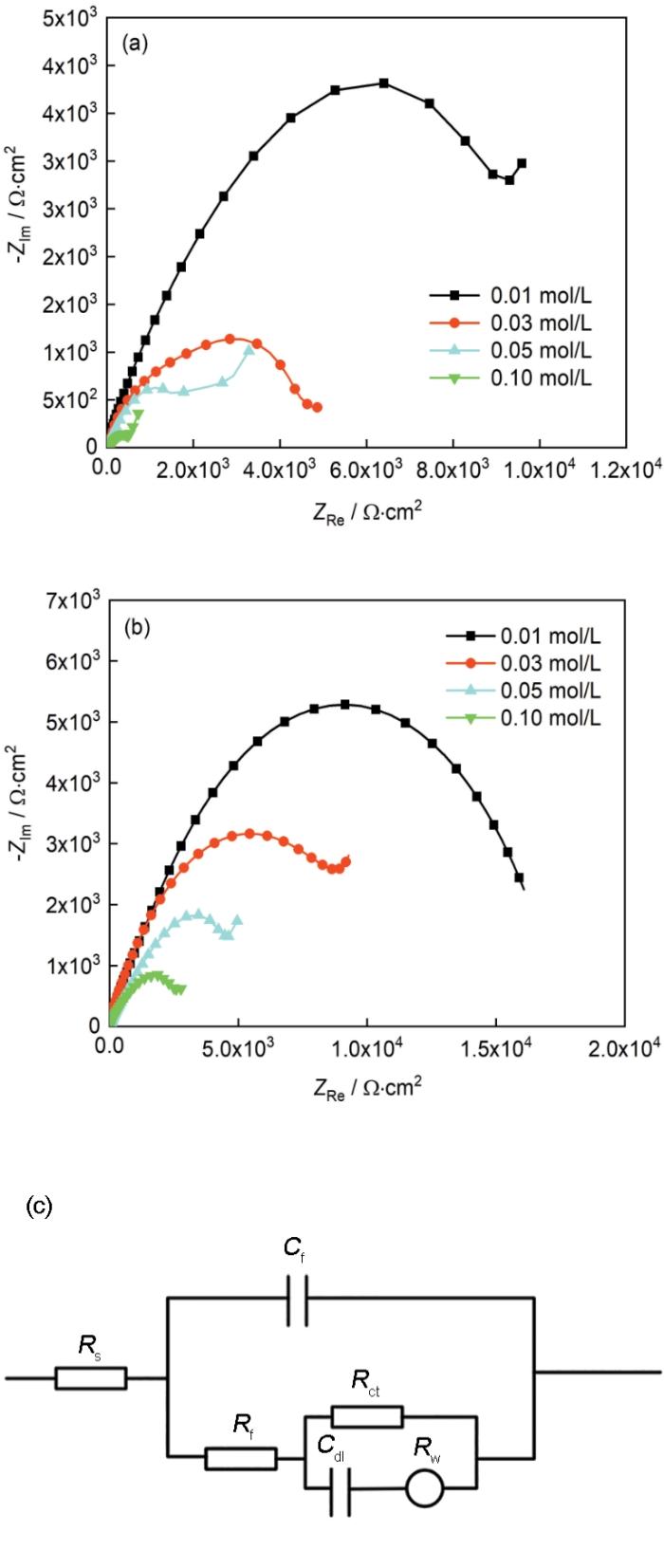
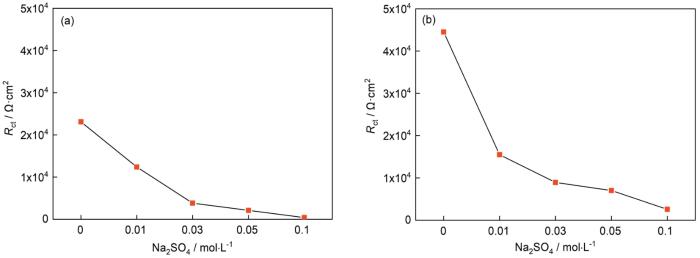
图13为材料在甲酸、乙酸与SO2污染物Na2SO4的复合作用下的电化学阻抗谱及其相对应的等效电路图。其等效电路图与上文中的不同之处在于,添加了Rw电阻作为低频区数据的拟合电路元器件之一,其代表了Warburg电阻并用于表征材料表面的离子扩散能力[28]。由图中可以明显观察到,材料的容抗弧半径随着Na2SO4浓度的增大逐渐减小。由等效电路拟合得到的电荷转移电阻Rct随Na2SO4浓度变化趋势如图14所示,当其浓度达到0.01 mol/L时,材料在甲酸、乙酸气氛中的Rct值分别为12400和15500 Ω,已经远小于没有Na2SO4复合作用影响的情况(甲酸、乙酸的Rct值分别为23100和44510 Ω·cm2)。当浓度达到0.1 mol/L时,Rct值则是分别减小到了407和2570 Ω·cm2。随着Na2SO4浓度增加而急剧下降的Rct值,说明了材料在有机酸气氛与SO2污染物的共同作用下展示出较强的腐蚀倾向,且SO2污染物的存在极大的加快了材料在有机酸环境里的腐蚀速率。
图13
图13
TP2紫铜在甲酸和乙酸中添加不同浓度的Na2SO4中暴露30 d后的阻抗谱图及其对应的等效电路图
Fig.13
Impedance spectra of TP2 copper after exposure for 30 d in formic acid (a) and acetic acid (b) after addition of different concentra-tions of Na2SO4 (0.01, 0.03, 0.05 and 0.10 mol/L), and the relating equivalent circuit diagram (c)
图14
图14
紫铜在甲酸,乙酸中暴露30 d后Rct随Na2SO4浓度变化趋势图
Fig.14
Graph of the trend of Rct with Na2SO4 concentration after exposure for 30 d in the formic acid (a) and acetic acid (b)
3 结论
在有机酸气氛及SO2污染物两种因素共同作用下,紫铜基体表现出了复杂的腐蚀行为。其腐蚀产物相互团聚成块,在乙酸气氛中甚至相互连接形成片状结构并构成了新的锈层。在电化学测试中发现,相较于相同浓度的有机酸气氛,材料在添加Na2SO4之后展现出了更为严重的腐蚀倾向。其自腐蚀电流密度要远远大于未添加Na2SO4实验组的数值,且其电荷转移电阻也远小于未添加Na2SO4实验组的数值。气氛中有机酸的浓度和溶液中的污染物浓度也是重要的影响因素,分别随着两者浓度的增加,材料基体都会遭到更为严重的腐蚀破坏。除此之外,甲酸由于具有较强的酸性,比较容易在腐蚀介质中电离出H+,从而在相同的条件下展现出了比乙酸更强的腐蚀能力。TP2紫铜工件在工业环境中难免会遇到有机酸及SO2等有害气体的侵蚀,如何有效的隔绝或者降低这些腐蚀因素对于材料的影响,提升材料的使用寿命是一个值得继续研究的方向。
参考文献
Improvement of protection performance of Mg-rich epoxy coating on AZ91D magnesium alloy by DC anodic oxidation
[J].
Investigating the influence of hydrogen on stress corrosion cracking of 2205 duplex stainless steel in sulfuric acid by electrochemical impedance spectroscopy
[J].As a nondestructive and sensitive method, electrochemical impedance spectroscopy (EIS) can be used to investigate the passivation and breakdown of passive films on steel. In this study, EIS, combined with slow strain rate test and scanning electron microscopy, was employed to study the stress corrosion cracking (SCC) behavior of 2205 duplex stainless steel in 0.5 m sulfuric acid solution under hydrogen-charging conditions. Results showed that the corrosion resistance of passive film on the hydrogen-charged specimen was lower than that for the specimen with no hydrogen charge. Hydrogen-induced cracking was evident after the specimens had been charged for 24 h. The phase shift in EIS, calculated from frequencies between 0.1 and 10 Hz, could be used to monitor the SCC process.
Corrosion behavior of three super austenitic stainless steels in a molten salts mixture at 650~750oC
[J].
超级奥氏体不锈钢的热腐蚀行为及机理研究
[J].研究了254SMo、904L和317L超级奥氏体不锈钢在650、700和750 ℃下30%Na<sub>2</sub>SO<sub>4</sub>+30%K<sub>2</sub>SO<sub>4</sub>+20%NaCl+20%KCl混合熔盐中的热腐蚀行为。通过腐蚀动力学以及腐蚀产物成分和形貌分析,探究了3种不锈钢在熔融混合盐中的高温腐蚀机理。结果表明,3种不锈钢在不同温度下均表现为失重,耐蚀性顺序为254SMo>904L>317L不锈钢;熔融的氯盐加速腐蚀,主要遵循“电化学腐蚀+氯活性腐蚀”腐蚀机制,硫酸盐通过“碱性助溶”机制溶解和破坏腐蚀层,从而造成严重的内部和晶间腐蚀;在两种腐蚀机制中,以氯腐蚀为主,硫腐蚀为辅;提高Mo和Ni含量可以在一定程度上改善奥氏体不锈钢的耐高温腐蚀性。
Corrosion characteristics of 5A06 Al-alloy exposed in natural deep-sea environment
[J].
深海环境5A06铝合金腐蚀行为与表面特性
[J].通过自主研制的高效串型深海环境试验装置在西太平洋深海自然环境下开展了5A06铝合金的腐蚀行为实验,分析了5A06铝合金在500,800,1200和2000 m深海环境下暴露1 a的腐蚀形貌、腐蚀规律和电化学行为。实海结果显示,5A06铝合金的腐蚀形式以点蚀为主,平均腐蚀速率随海水深度增加先升高后降低,最大值出现在水深500 m处,为17 μm/a,是浅表海水环境下的3.1倍;而在800~2000 m水深范围,5A06铝合金腐蚀状况大大减弱,腐蚀速率在0.9~1.4 μm/a水平,800 m时仅为浅表海水的0.21倍,2000 m时则为0.14倍。电化学测试结果显示,试样自腐蚀电位随海水深度增加而正移,2000 m深度下达到-0.640 V (vs. Ag/AgCl);电荷转移阻抗随着试验深度的增加而显著增大,2000 m深度下达到了最大值,为1.91×10<sup>8</sup> Ω·cm<sup>2</sup>。
Corrosion behavior of copper in cyclic dry-wet environment with gas mixture of SO2 and H2S
[J].
干湿交替环境中SO2和H2S混合气体对紫铜T2的腐蚀行为研究
[J].
Corrosion protection of copper and copper alloys in different corrosive medium using environmentally friendly corrosion inhibitors
[J].The corrosion mechanism of copper and copper alloy is reviewed. A number of scientific papers have been investigated to determine the corrosion mechanism and protection techniques of copper and copper alloy corrosion. Results have shown that copper can be corroded in an acidic or an alkaline environment, and oxide formation is the corrosion initiation process. The use of corrosion inhibitors is one of several ways of controlling metal corrosion. There are inorganic (toxic) and organic (green) corrosion inhibitors invented so far. Nowadays, environmental issue is a concern of several scientists in the world. From the results of recent scientific papers, green corrosion inhibitors can be used for copper corrosion protection and they are both economical and environmentally safe. Furthermore, future researches are needed to determine more efficient, environmentally friendly corrosion inhibitors for copper and copper alloys.
Corrosion behavior of purple copper and brass H62 in real sea navigation marine atmosphere
[A].
紫铜与黄铜H62在实海航行海洋大气中的腐蚀行为研究
[A].
Insight into the mechanism of alloying elements (Sn, Be) effect on copper corrosion during long-term degradation in harsh marine environment
[J].
Numerical simulation of galvanic corrosion of TP2Y copper pipes coupled with steel pipes in a seawater pipe systems of ships
[J].
舰船海水管系中紫铜/钢制管道耦接后电偶腐蚀的数值模拟研究
[J].基于流场、浓度场与电化学动力学过程的耦合,采用COMSOL Multiphysics软件,模拟研究了紫铜 (TP2Y) 与#20钢管道耦接后在静态、流动3.5% (质量分数) NaCl溶液中的电偶腐蚀行为,并预测了静态条件下电偶腐蚀的发展趋势。结果表明,在耦接的异种金属管道中,TP2Y管道作为阴极受到保护,#20钢管道作为阳极受到腐蚀,其腐蚀长度均受管径大小、介质流动以及时间的影响。当管径增大时,电偶对中阴、阳极金属管道电位变化长度逐渐增大;当管道内介质流速增大时,阴阳极管道的内表面电位较静态下开始正移。同时,在靠近耦接位置时,紫铜 (TP2Y) 管道内表面电位急剧变负,#20钢管道内表面电位急剧变正,电流密度最大。在静态条件下,电偶对中金属管道内表面电位随着时间发生负移。在48 h后,电位基本不再发生变化,阴阳极的内表面进入稳定状态;在30 d后,在靠近阳极金属管道法兰处的总厚度减薄约8.87 μm,阳极金属管道的腐蚀长度约为800 mm。
Effect of an alternating electric field on the atmospheric corrosion behaviour of copper under a thin electrolyte layer
[J].
Study on the mechanism of the photoelectrochemical effect on the initial NaCl-induced atmospheric corrosion process of pure copper exposed in Humidified Pure Air
[J].
A corrosion failure analysis of copper wires used in outdoor terminal boxes in substation
[J].
The effect of hematite on the corrosion behavior of copper in saturated red soil solutions
[J].
Galvanic corrosion behavior of Q235 steel-red copper in acid red soil of different water content
[J].
不同湿度的酸性红壤中Q235钢-紫铜电偶腐蚀行为研究
[J].
Corrosion behavior of the dominant actinomycetes in soil on copper
[J].
土壤优势放线菌菌群对紫铜的腐蚀
[J].
Corrosion behavior of copper in a simulated grounding condition in electric power grid
[J].
铜在电网接地工况下的腐蚀行为研究
[J].通过室内模拟加速实验、电化学测试以及X射线衍射 (XRD)、扫描电子显微镜 (SEM) 等手段对通电工况下铜接地网材料在酸性土壤环境中的腐蚀特征进行了研究。结果表明,通电条件对Cu的腐蚀行为有较大影响,Cu的腐蚀速率随外加电流密度的增大而逐渐增大;腐蚀产物以CuO和Cu<sub>2</sub>O为主,Cu<sub>2</sub>O的占比随外加电流密度的增大而减小。
Intergranular erosion corrosion of pure copper tube in flowing NaCl solution
[J].
Comparison of the corrosion behavior of copper tubes in formic acid and acetic acid environment
[J].
Corrosion of copper and steel alloys in a simulated underground storage-tank sump environment containing acid-producing bacteria
[J].
An investigation on the mechanisms of ant nest corrosion of copper tube in formic acid environment
[J].
Interactive effect of ant nest corrosion and stress corrosion on the failure of copper tubes
[J].
Investigation on the electrochemical corrosion behavior of TP2 copper and influence of BTA in organic acid environment
[J].In this work, the corrosion behavior of copper in a simulated organic acid environment containing formic acid and acetic acid was investigated by electrochemical testing and surface characterization. In addition to deducing the corrosion mechanism of copper in the organic acid corrosion environment, the corrosion inhibitor BTA was also used to slow down the corrosion of copper by organic acid. The results show that the corrosion rate of copper in the two groups of organic acids first decreases and then increases with the immersion time. Microelectrochemical (Scanning Vibrating Electrode Technique) results shows that the anodic peak of the sample is higher in formic acid. Formic acid is more corrosive. The corrosion products of red copper gradually increased in the two groups of organic acid atmospheres, and the final corrosion products were cuprous oxide, copper formate particles and copper acetate hydrate, respectively. When the concentration of BTA is 0.5 g/L, the electrochemical activity of TP2 copper is weakened, the surface of the sample is relatively smooth, there are no large corrosion pits, and the corrosion rate is reduced.
Quantitative determination of corrosion products and adsorbed water on copper in humid air containing SO2 by IR-RAS measurements
[J].
Creeping corrosion of copper on printed circuit board assemblies
[J].
Corrosion of Cu under Highly Corrosive Environments
[J].
The atmospheric corrosion of quaternary bronzes: an evaluation of the dissolution rate of the alloying elements
[J].
Comparison of electrochemical corrosion behavior of copper in liquid film in atmosphere containing SO2 or H2S
[J].
大气环境中SO2和H2S对铜的电化学腐蚀行为比较
[J].
Electrochemical corrosion behavior of micrometer-sized porous Ti3SiC2 compounds in NaCl solution
[J].The electrochemical corrosion behavior of reactively synthesized porous Ti3SiC2 in 1 M NaCl solution was investigated compared with that of porous TiAl. Porous Ti3SiC2 and TiAl have high-purity single phase characteristics with open porosities of 48% and 41% and maximum apertures of 6.9 and 16.3 mu m, respectively, which show positive direction shift behavior of open circuit potential at the early immersion stage with stable potential ranges of 0.25 similar to 0.27 and -0.36 similar to-0.38 V (vs. saturated calomel), respectively. The electrochemical corrosion procedures of the two porous compounds exhibit three-stage characteristics of activation, passivation, and breakdown. Porous Ti3SiC2 has a much larger ohmic resistance and Warburg coefficient than TiAl. The passivation films of the two porous compounds consist of an inner layer with a larger resistance and a porous outer layer. Porous Ti3SiC2 has a significantly larger resistance and relatively smaller capacitance for the inner passivation film, indicating better corrosion resistance stability.