对于Mg-Li二元合金[12,13],当Li含量小于5.7%(质量分数,下同)时,合金由Li固溶在Mg中的单相α-Mg(HCP)组成;当Li含量超过11%时,合金由Mg固溶在Li中的单相β-Li(BCC)组成;当Li含量在5.7%~11%时,合金由α-Mg和β-Li双相组成。有研究[14,15]指出(α + β)双相Mg-Li合金中存在的腐蚀微电偶对是导致其耐蚀性较差的重要原因。Gu等[16]对Mg-8Li-3Al-2Zn合金的研究表明,合金中添加Y可以在晶界处析出充当阴极保护相的Al2Y颗粒,降低α-Mg和β-Li的电偶腐蚀速率。Morishige等[17]研究表明添加适量的Al可抑制Mg-14Li合金(β单相)的剥落腐蚀,但过量的Al会导致AlLi相的析出,其与基体形成腐蚀微电偶对,降低合金的耐蚀性。这些研究为研制具有优异耐蚀性能的Mg-Li合金指明了方向,即抑制合金中腐蚀微电偶对的形成,提高合金的电化学均匀性。
作者[18]前期对Mg-11Li-3Al-1(Zr, Y)合金的研究表明Mg3Al纳米析出相对提高Mg-Li合金的强度具有重要影响,并基于此进一步设计了具有单相β结构的Mg-15Li-6Al合金(密度仅为1.32 g·cm-3),实验表明该合金的比强度高达311 kN·m·kg-1,超过了绝大多数工程轻质合金;同时,该合金表现出优异的热稳定性,其室温稳定性是商用Mg-14Li-1Al (LA141)合金的两倍多,展现出良好的轻量化应用前景。然而,关于该合金的耐蚀性还未曾有过深入研究。本文选用Mg-15Li-6Al合金作为实验材料,详细研究了经过不同的热处理工艺后合金的耐蚀性能,分析了微观组织对其耐蚀性能的影响机理,旨在为发展新一代具有超轻、高强韧和高耐蚀特征的Mg-Li合金提供实验依据和理论参考。
1 实验方法
选用真空感应熔炼后在坩埚里边缓慢冷却成型的Mg-15Li-6Al (LA156)合金作为实验材料,在LA156铸锭上线切割3个尺寸为20 mm × 10 mm × 7.5 mm的试样块,其中一个不做热处理而保持铸态,对余下的两个先进行固溶处理,固溶温度为400℃,保温1 h,然后分别进行两种冷却处理:空冷和水冷,再用线切割在不同的试样块上切取10 mm × 10 mm × 1.5 mm的试片若干。试片经SiC砂纸从320#逐级研磨至1500#,然后抛光直至表面光亮,采用体积分数为3%的硝酸酒精作为腐蚀剂,腐蚀时间3~5 s,然后清洗吹干备用。
采用CS31OH电化学工作站测试试样在3.5%NaCl溶液中的动电位极化曲线,测试过程采用三电极体系,辅助电极为Pt电极,参比电极为饱和甘汞电极(SCE),工作电极为用环氧树脂封装的待测试样,工作面积为1 cm2,动电位极化曲线扫描速率为5 mV/s。
采用失重法测试不同状态合金的腐蚀速率,腐蚀介质为3.5%NaCl溶液,试样在其中的浸泡时间为40 h。浸泡实验结束后,把样品放入铬酸溶液中15 min,去除附着在表面的腐蚀产物,清洗干净,冷风吹干,采用型号为AB104-S的电子天平(精度0.1 mg)称量腐蚀前后合金质量的变化。采用数码相机拍摄样品的宏观腐蚀形貌,并用KH-7700三维体式显微镜测试腐蚀坑的深度。采用场发射扫描电镜(FESEM,FEI Navo450)观察铸态、空冷和水冷3种状态试样的显微组织,采用X射线衍射仪(XRD,Bruker D8 ADVANCE)分析合金基体和腐蚀产物的相组成。
2 结果与讨论
2.1 不同处理方式合金的微观组织
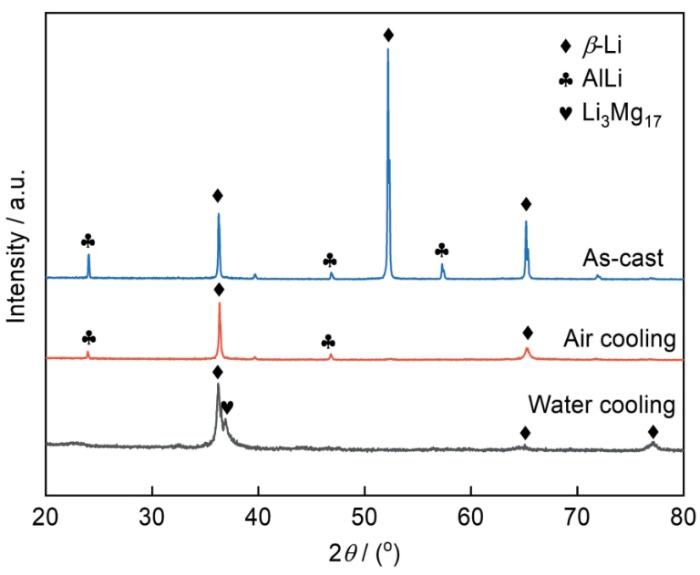
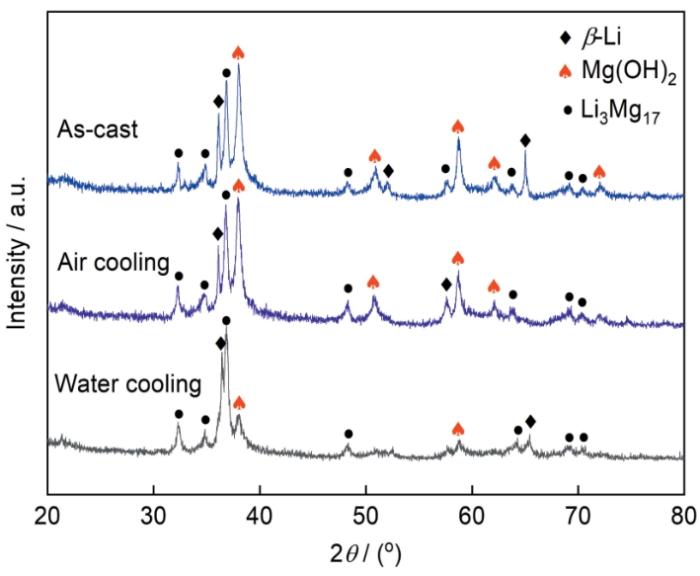
图1所示为铸态LA156合金以及其固溶处理并分别经过空冷和水冷后合金的XRD谱。可以看出,铸态合金由大量β-Li和少量AlLi相组成;经过固溶处理并空冷后,合金中依然存在β-Li相和AlLi相,但AlLi相的衍射峰明显降低;而固溶处理并水冷后,合金中AlLi相的衍射峰消失,而出现少量的Li3Mg17,即Li在α-Mg中的固溶相。该结果表明,随着固溶处理后冷却速率的加快,合金中的AlLi相逐渐减少,以致XRD已检测不到水冷试样中的AlLi相。
图1
图2
图2
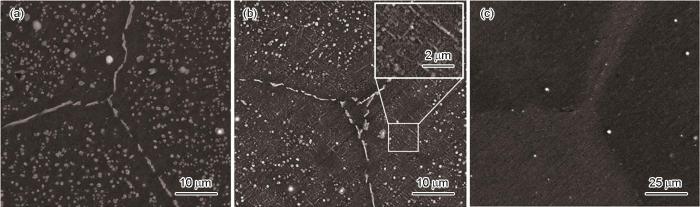
不同状态LA156合金的微观组织
Fig.2
Microstructures of different state LA156 alloys: (a) as-cast, (b) air cooling, (c) water cooling
2.2 不同处理方式合金在3.5%NaCl水溶液中的腐蚀行为
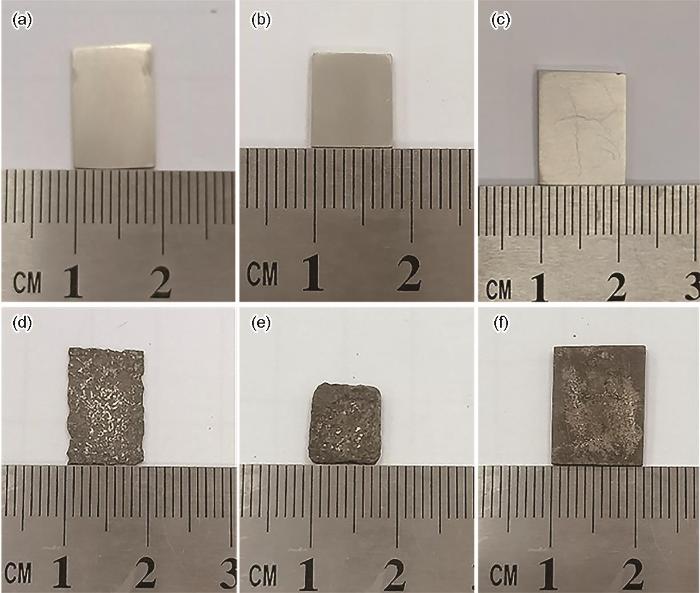
图3所示为铸态LA156合金以及其固溶处理并分别经过空冷和水冷后在3.5%NaCl水溶液中浸泡40 h去除腐蚀产物后的宏观形貌。可以看出,铸态试样和固溶空冷态试样发生了较为严重的腐蚀,试样边缘已经溶解,表面呈现明显的凹凸不平;而固溶水冷态试样则基本仍维持了其浸泡前较规则的外形,且试样表面较平整,说明其腐蚀程度在这3种试样中最轻,耐蚀性最佳。
图3
图3
不同状态LA156合金在3.5%NaCl溶液中浸泡40 h前后的宏观形貌
Fig.3
Macroscopic morphologies of different state LA156 alloys before (a-c) and after (d-f) 40 h immersion in 3.5%NaCl solution: (a, d) as-cast, (b, e) air cooling, (c, f) water cooling
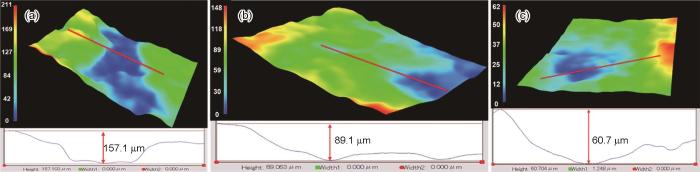
图4所示为用三维体视显微镜观察上述3种不同状态的LA156合金试样经过40 h浸泡后的表面局部腐蚀形貌。由图可见,铸态试样、固溶空冷态试样和固溶水冷态试样表面的腐蚀坑最大深度分别为157.1、89.1 和60.7 μm,表明固溶处理降低了合金的局部腐蚀程度,提高了合金的耐蚀性,且固溶水冷态试样耐蚀性能最好。
图4
图4
不同状态LA156合金在3.5%NaCl溶液中浸泡40 h后的局部腐蚀形貌
Fig.4
Localized morphologies of different state LA156 alloys after 40 h immersion in 3.5%NaCl solution: (a) as-cast, (b) air cooling, (c) water cooling
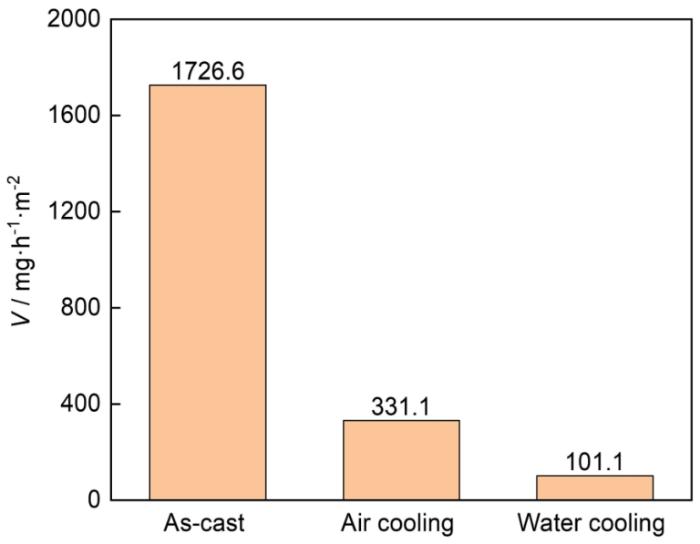
图5所示为上述3种不同状态的LA156合金试样经过40 h浸泡后,利用失重法计算的腐蚀速率柱状图。由图可知,铸态试样的腐蚀速率最大,固溶空冷态试样和固溶水冷态试样的腐蚀速率分别为铸态试样的19.2%和5.9%。该结果也表明铸态试样的耐蚀性最差,而通过固溶处理之后的空冷试样和水冷试样的耐蚀性有所提高,且水冷试样耐蚀性最好。
图5
图5
不同状态LA156合金在3.5%NaCl溶液中的腐蚀速率
Fig.5
Corrosion rates of different state LA156 alloys in 3.5%NaCl solution
图6为上述3种不同状态的LA156合金试样经过40 h浸泡后的腐蚀产物XRD谱。3种试样腐蚀产物的主要成分均为Mg(OH)2,其中,铸态试样的Mg(OH)2衍射峰最强,其次为固溶空冷态试样,而固溶水冷态试样的Mg(OH)2衍射峰最低。该结果表明,铸态试样腐蚀产物最多,即腐蚀最严重,而固溶空冷态试样和固溶水冷态试样耐蚀性比铸态试样更好,并且水冷试样的Mg(OH)2的衍射峰最低,表明该试样形成的腐蚀产物最少,即固溶水冷态试样的耐蚀性最好。Mg(OH)2呈现疏松多孔的结构,无法有效保护合金基体[12,20]。此外,在3种试样的腐蚀产物中均没有检测到Li2CO3,这可能与3.5%NaCl水溶液是由去离子水配制有关,CO2在去离子水中的溶解度非常有限,并且NaCl能够降低CO2在水溶液中的溶解度[21]。
图6
图6
不同状态LA156合金在3.5%NaCl溶液中浸泡40 h后的XRD谱
Fig.6
XRD patterns of different state LA156 alloys after 40 h immersion in 3.5%NaCl solution
2.3 不同处理方式合金在3.5%NaCl水溶液中的极化行为
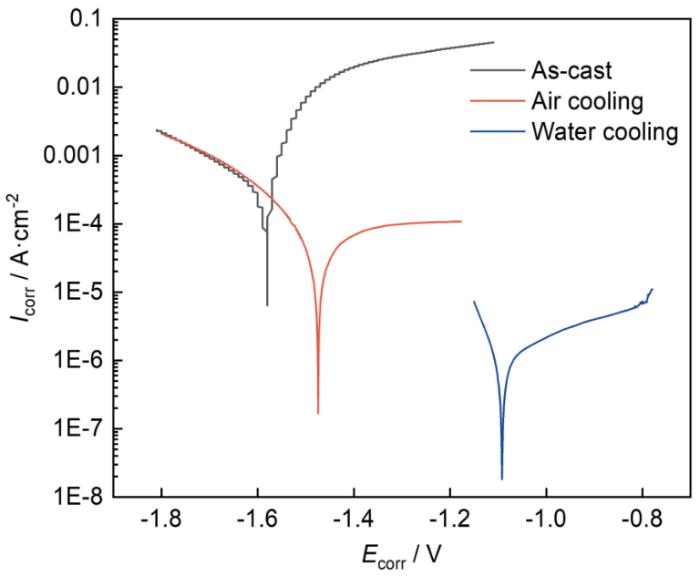
图7所示为3种不同状态的LA156合金试样的动电位极化曲线,表1为通过Tafel外推法获得的腐蚀电位(Ecorr)和腐蚀电流密度(Icorr)。由于金属Mg和Li的电极电位均低于析氢反应的平衡电极电位,所以Mg-Li合金的阴极反应主要为析氢反应,阳极反应为合金的活性溶解[22,23]。从图7和表1可知,铸态试样的腐蚀电位为-1.57 V,空冷试样的腐蚀电位为-1.46 V,水冷试样的腐蚀电位为-1.09 V。固溶处理后,空冷试样和水冷试样的腐蚀电位相比铸态试样分别提高了110和480 mV。铸态试样腐蚀电流密度为0.90 × 10-3 A·cm-2,空冷试样为1.50 × 10-4 A·cm-2,水冷试样为1.48 × 10-6 A·cm-2。固溶处理后,空冷试样和水冷试样的腐蚀电流密度比铸态试样分别降低了大约1个和3个数量级。该电化学测试结果表明LA156合金经固溶处理后耐蚀性大幅提高,且水冷试样比空冷试样具有更优异的耐蚀性能。
图7
图7
不同状态LA156合金在3.5%NaCl溶液中的极化曲线
Fig.7
Polarization curves of different state LA156 alloys in 3.5%NaCl solution
表1 极化曲线拟合结果
Table 1
| Sample state | Ecorr (vs. SCE) /V | Icorr / A·cm-2 |
|---|---|---|
| As-cast | -1.57 | 0.90 × 10-3 |
| Air cooling | -1.46 | 1.50 × 10-4 |
| Water cooling | -1.09 | 1.48 × 10-6 |
图8
图8
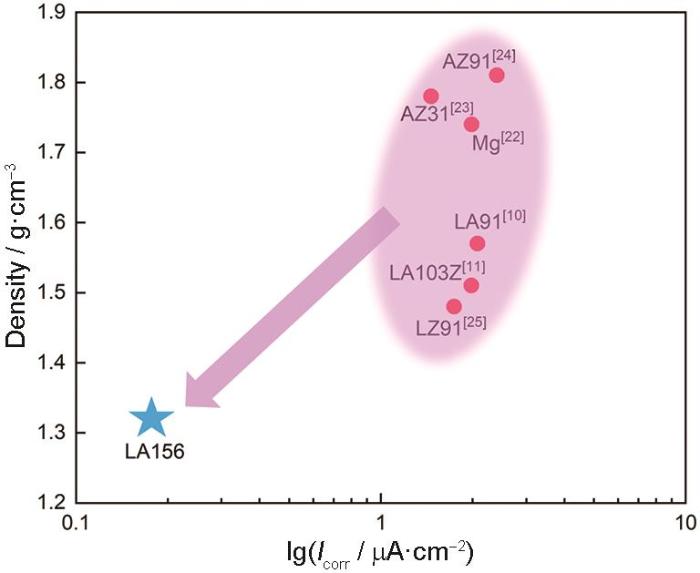
水冷态LA156合金与纯Mg及其它Mg(-Li)合金密度与腐蚀电流密度的比较
Fig.8
Comparison of density and corrosion current density of water cooling LA156 with pure Mg and some Mg(-Li) alloys
通过对浸泡后试样的宏观形貌观察、腐蚀速率计算以及极化曲线结果分析,固溶处理能够有效地提高LA156合金的耐蚀性能,并且固溶处理后水冷处理试样的耐蚀性要比空冷处理试样的耐蚀性更好。根据显微组织观察和XRD分析,这与固溶处理后合金微观组织的变化有关。研究[12,16]指出,AlLi颗粒可以与β-Li基体形成腐蚀微电偶对,且AlLi的标准电极电位比β-Li基体更低,腐蚀微电偶对的存在显著增大了合金的腐蚀速率。在本研究中,铸态试样的晶粒内部和晶界处均析出了大量的AlLi,合金发生严重的电偶腐蚀,导致其耐蚀性较差。固溶处理使更多的Al固溶于β-Li中,空冷处理后AlLi析出相的数量和尺寸相比铸态试样均有所减小,AlLi与β-Li两相间腐蚀微电偶对的数量也相应减少,所以其腐蚀速率降低。固溶处理后进行水冷处理,绝大部分Al仍固溶于β-Li中,这是由于高冷却速率显著抑制了AlLi相的析出。一方面,基体中固溶更多的Al有助于提升合金的自腐蚀电位;另一方面,AlLi相体积分数的显著减小也极大地降低了AlLi与β-Li之间的电偶腐蚀速率[17,26]。
3 结 论
(1) Mg-15Li-6Al铸态合金主要由β-Li和AlLi析出相组成,通过固溶处理可以减少合金中AlLi析出相的体积分数,尤其是固溶处理后进行水冷处理的合金基体组织基本由单相β-Li组成。
(2) 3.5%NaCl水溶液中浸泡验和极化曲线测试表明3种不同状态Mg-15Li-6Al合金的耐蚀性由低到高的顺序为:铸态<固溶空冷态<固溶水冷态,且固溶处理后进行水冷使合金的自腐蚀电位提高了480 mV,自腐蚀电流密度降低了3个数量级。
(3) 固溶处理使得Al有效回溶到β-Li基体中,随后的快速冷却有效抑制了AlLi相的析出,提高了合金组织的电化学均匀性,导致AlLi与β-Li两相间腐蚀微电偶对的数量减少,合金的耐蚀性显著提高。
参考文献
Alloying and application of Mg-Li alloys: a review
[J].
镁锂合金的合金化及其应用
[J].
Wang J S. Research progress on corrosion and protection of corrosion-resistant Mg-Li alloys
[J].
耐腐蚀Mg-Li合金的腐蚀与防护及其性能研究进展
[J].高比强度和高比刚度Mg-Li合金,由于其出色的减重效果,是理想的航空航天金属结构工程材料。然而,由于其耐腐蚀性能较差,严重制约了其在一些服役环境中的广泛应用。因此,耐腐蚀Mg-Li合金的成分、工艺研制是未来发展的重要方向。本文综述了其腐蚀机理及不同类型表面防护 方法 (金属涂层、阳极氧化、导电性-屏蔽性聚合物涂层和智能涂层)、研究现状与未来应用。同时,分析了不同类型腐蚀防护方法的优势与局限性问题,讨论了耐腐蚀Mg-Li合金腐蚀与防护的未来研究趋势,以期为耐腐蚀Mg-Li合金开发提供新思路。
Aging behavior of Mg-Li-Al alloys
[J].
Natural ageing responses of duplex structured Mg-Li based alloys
[J].Natural ageing responses of duplex structured Mg-6%Li and Mg-6%Li-6%Zn-1.2%Y alloys have been investigated. Microstructural analyses revealed that the precipitation and coarsening process of α-Mg particles could occur in β-Li phases of both two alloys during ageing process. Since a certain amount of Mg atoms in β-Li phases were consumed for the precipitation of abundant tiny MgLiZn particles, the size of α-Mg precipitates in Mg-6%Li-6%Zn-1.2%Y alloy was relatively smaller than that in Mg-6%Li alloy. Micro hardness measurements demonstrated that with the ageing time increasing, the α-Mg phases in Mg-6%Li alloy could have a constant hardness value of 41 HV, but the contained β-Li phases exhibited a slight age-softening response. Compared with the Mg-6%Li alloy, the age-softening response of β-Li phases in Mg-6%Li-6%Zn-1.2%Y alloy was much more profound. Meanwhile, a normal age-hardening response of α-Mg phases was maintained. Tensile results indicated that obvious ageing-softening phenomenon in terms of macro tensile strength occurred in both two alloys. Failure analysis demonstrated that for the Mg-6%Li alloy, cracks were preferentially initiated at α-Mg/β-Li interfaces. For the Mg-6%Li-6%Zn-1.2%Y alloy, cracks occurred at both α-Mg/β-Li interfaces and slip bands in α-Mg and β-Li phases.
A high-specific-strength and corrosion-resistant magnesium alloy
[J].Ultra-lightweight alloys with high strength, ductility and corrosion resistance are desirable for applications in the automotive, aerospace, defence, biomedical, sporting and electronic goods sectors. Ductility and corrosion resistance are generally inversely correlated with strength, making it difficult to optimize all three simultaneously. Here we design an ultralow density (1.4 g cm(-3)) Mg-Li-based alloy that is strong, ductile, and more corrosion resistant than Mg-based alloys reported so far. The alloy is Li-rich and a solute nanostructure within a body-centred cubic matrix is achieved by a series of extrusion, heat-treatment and rolling processes. Corrosion resistance from the environment is believed to occur by a uniform lithium carbonate film in which surface coverage is much greater than in traditional hexagonal close-packed Mg-based alloys, explaining the superior corrosion resistance of the alloy.
Microstructure and mechanical behavior of Mg-10Li-3Al-2.5Sr alloy
[J].
Characterisation of Mg-Li alloy processed by solution treatment and large strain rolling
[J].The effects of solution treatment and large strain rolling (LSR) on the microstructure and mechanical properties of Mg–10.73Li–4.49Al–0.52Y alloy were investigated. Results showed that after solution treatment, α-phase and AlLi phase were dissolved into β matrix, which led to the increase of strength. With the increased temperature of LSR, new phase MgLi2Al (θ-phase) and AlLi phase precipitated from the matrix in turns. The Mg–Li alloy rolled at 623 K with 75 reduction showed the ultimate tensile strength of 328 MPa, which was more superior to many other Mg–Li alloys. The excellent strength could be explained by the mechanisms of solution strengthening and fine grain strengthening.
Phase transformations in an ultralight BCC Mg alloy during anisothermal ageing
[J].
Mechanical properties and corrosion behavior of an extruded dilute Mg-alloy Mg-0.5Bi-0.5Sn-0.5Ca
[J].
挤压态低合金化Mg-0.5Bi-0.5Sn-0.5Ca合金的力学性能及腐蚀行为研究
[J].探究了复合添加微量Sn与Ca对挤压态Mg-0.5Bi基合金的微观组织、力学性能及腐蚀行为的影响。结果表明:挤压态Mg-0.5Bi-0.5Sn-0.5Ca (质量分数,%) 合金主要由α-Mg、Mg<sub>2</sub>Bi<sub>2</sub>Ca以及Mg<sub>2</sub>Sn相组成,合金表现出晶粒尺寸均匀分布的完全动态再结晶组织。合金的抗拉强度 (UTS) 为191 MPa,伸长率 (EL) 高达31.5%,腐蚀速率 (P<sub>i</sub>) 为0.51 mm/a,极化阻抗 (R<sub>p</sub>) 为707.19 Ω·cm<sup>2</sup>。此外,挤压态合金在腐蚀过程中生成了含Ca以及含Sn的腐蚀产物中间层,从而提升了腐蚀产物层的保护作用,导致析氢速率随着浸泡时间的增加先增大后减小。最后由于腐蚀产物膜的破裂,析氢速率达到了2.43 mL/d。
Research on the preparation and corrosion resistance of the low energy plasma electrolysis oxide coating of magnesium lithium alloy
[J].
镁锂合金低能耗等离子电解氧化膜层的制备与耐蚀性研究
[J].
Effect of KOH concentration on the growth process and corrosion resistance of micro-arc oxidation (MAO) coatings on LA103Z Mg-Li alloy
[J].
KOH浓度对LA103Z镁锂合金微弧氧化成膜过程及膜层耐蚀性的影响
[J].
Research progress on corrosion behavior of magnesium-lithium alloys
[J].
镁锂合金腐蚀行为研究进展
[J].
Corrosion characterization of Mg-8Li alloy in NaCl solution
[J].
Comparison on corrosion resistance and surface film of pure Mg and Mg-14Li alloy
[J].
Corrosion resistant and high-strength dual-phase Mg-Li-Al-Zn alloy by friction stir processing
[J].
Influence of yttrium addition on the corrosion behaviour of as-cast Mg-8Li-3Al-2Zn alloy
[J].
Effect of Al composition on the corrosion resistance of Mg-14 mass% Li system alloy
[J].
Precipitation strengthening in an ultralight magnesium alloy
[J].Body-centred cubic magnesium-lithium-aluminium-base alloys are the lightest of all the structural alloys, with recently developed alloy compositions showing a unique multi-dimensional property profile. By hitherto unrecognised mechanisms, such alloys also exhibit exceptional immediate strengthening after solution treatment and water quenching, but strength eventually decreases during prolonged low temperature ageing. We show that such phenomena are due to the precipitation of semi-coherent D0-MgAl nanoparticles during rapid cooling followed by gradual coarsening and subsequent loss of coherency. Physical explanation of these phenomena allowed the creation of an exceptionally low-density alloy that is also structurally stable by controlling the lattice mismatch and volume fraction of the MgAl nanoparticles. The outcome is one of highest specific-strength engineering alloys ever developed.
Ultrahigh specific strength in a magnesium alloy strengthened by spinodal decomposition
[J].Spinodal decomposition is discovered to be an effective way to strengthen magnesium alloy.
Corrosion mechanism and surface protection of Mg-Li alloy
[J].
Mg-Li合金的腐蚀机理与表面防护
[J].
Solubility of CO2 in water and NaCl solution in equilibrium with hydrate. Part I: experimental measurement
[J].
Corrosion behavior of pure Mg and Mg-Li alloy in 3.5%NaCl of pH = 7
[J].
纯镁和镁锂合金在中性 3.5%NaCl溶液中的腐蚀行为
[J].
Corrosion behavior of Cu-containing AZ31 magnesium alloy
[J].
含铜AZ31镁合金的腐蚀行为
[J].
Effects of rare earths on corrosion behavior of AZ91 Mg alloy
[J].
稀土对AZ91镁合金腐蚀行为的影响
[J].
Influence of deformation on the corrosion behavior of LZ91 Mg-Li alloy
[J].