由于水泥水化产物构成复杂,将C-S-H从水化产物中剥离出来较为困难,因此,诸多学者通过合成C-S-H或分子动力学模拟的方法,来探究其对Cl-的吸附作用。勾密峰等[7]在混凝土模拟孔隙液中加入不同钙硅比的合成C-S-H,表明钙硅比为0.80~1.75时,C-S-H吸附Cl-能力随钙硅比的增加先增加后减少。Zhou等[8]为深入研究C-S-H对氯化物的吸附机制,进行了C-S-H的分子动力学模拟计算,表明钙离子可以明显影响C-S-H的表面体系,较高Ca/Si的C-S-H可以结合更多Cl-。Jain等[9]研究表明在混凝土模拟孔隙溶液中,pH值为11.6和12.6时,OH-的存在对C-S-H结合Cl-能力有积极影响。彭小芹等[10]将C-S-H粉体掺入水泥浆中,可见C-S-H粉体对水泥水化进程有显著提升,外掺的C-S-H粉体充当水泥水化产物的成核位点,能降低水泥水化反应的成核势垒。Nicoleau[11]也研究表明纳米级C-S-H能促进水泥水化和快速提高砂浆试件的力学性能。上述研究多基于混凝土模拟孔隙液和分子动力学计算来研究C-S-H和Cl-结合性能以及内掺C-S-H对水泥水化进程的影响,然而,内掺C-S-H到水泥基材料中对Cl-结合性能的影响未展开系统研究。因此,有必要开展C-S-H在水泥基材料中对Cl-结合性能影响的研究,不仅可对模拟试验研究有进一步的补充和验证,还可为C-S-H在水泥基材料中的应用提供理论支持。
在混凝土模拟溶液中对合成C-S-H Cl-结合性能的研究已经开展,并已撰写相关论文。本文试验为在上述研究基础上,利用合成的C-S-H制备了不同C-S-H掺量的水泥净浆,探究了合成C-S-H对水泥净浆Cl-结合性能的影响。通过测试分析净浆试件自由Cl-含量以及pH值随龄期的演变规律来探讨C-S-H对水泥净浆Cl-结合的影响。利用X射线衍射(XRD)、热重(TG-DTG)、扫描电子显微镜(SEM)以及自带能谱(EDS)深入分析了C-S-H对水泥水化过程中物相演变和水泥水化产物微观形貌的影响,为探究Cl-结合机理,改善水泥基材料的Cl-结合能力提供理论基础。
1 实验方法
1.1 C-S-H的制备
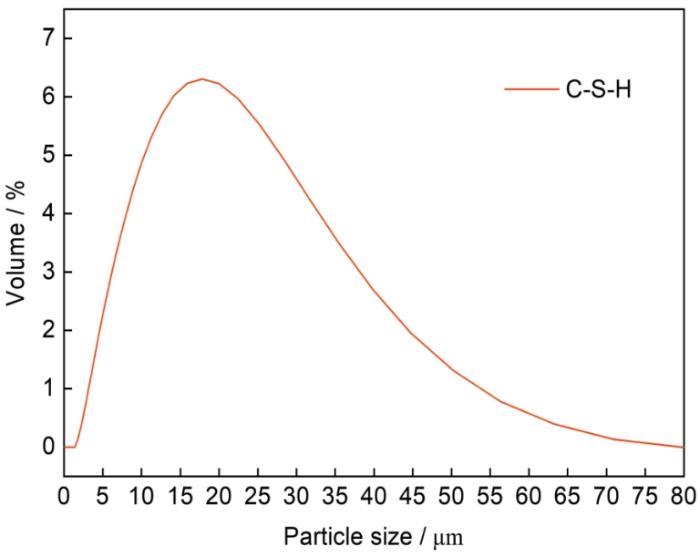
纯硅酸盐水泥的Ca/Si范围为1.2~2.3,掺加硅灰的混合体系Ca/Si为1.1~1.5,掺加矿渣的混合体系Ca/Si为0.7~2.4,通常,混合材料水化产物的平均Ca/Si比纯硅酸盐水泥的小[12]。本试验水泥为复合水泥,故采用Ca/Si为1.5的合成C-S-H。采用人工合成方式制备C-S-H,将分析纯硅酸钠(Na2SiO3⸱9H2O)和硝酸钙(Ca(NO3)2⸱4H2O)按照钙硅摩尔比1.5称取,采用去除CO2的去离子水,水固比取20,将称取好的固体试剂分别溶解于去离子水中,之后进行混合搅拌,然后使用分析纯NaOH调节pH值到13左右,再将溶液置于干燥的三角瓶中密封,密封后将三角瓶置入恒温摇床(30℃,120 r/min)中水化反应14 d,然后将产物洗涤并且进行抽滤,放入真空干燥箱中50℃干燥至恒重,最后存放于真空干燥皿中备用。制备好的C-S-H如图1所示,为白色粉末状,其比表面积为45.8 m2/g,对于水化基本完成的水泥浆体,C-S-H比表面积为25~700 m2/g[13],因此,制备所得C-S-H比表面积相对较小。图2为制备C-S-H的粒径分布图,C-S-H由于其具有多种形貌,如纤维状粒子、网络状粒子、不规则扁平粒子等,其粒子大小一般在0.2~0.5 μm之间[14],由此可见,制备所得C-S-H粒径相对较大。采用Malvern粒度仪对C-S-H进行测试,测试结果如图2所示。
图1
图2
采用Perkin Elmer PE Frontier红外光谱仪变换红外光谱(FT-IR)对制备的C-S-H进行测试,测试范围为4000~400 cm-1。C-S-H在红外光谱中含有O-Si-O、Si-O-Si、Si-O(Q1)、Si-O(Q2)、O-Ca-O、H2O、-OH等基团,其峰值位置分别在450、650、816、970、1445、1640和3440 cm-1左右。图3为合成C-S-H的Fourier红外光谱图,从图中可以看出,制备的C-S-H样品在3447 cm-1处显示-OH振动峰,1638 cm-1处显示H2O振动峰,1383 cm-1处显示O-Ca-O振动峰,971 cm-1处显示Si-O(Q2)振动峰,816 cm-1处显示Si-O(Q1)振动峰,658 cm-1处显示Si-O-Si振动峰,457 cm-1处显示O-Si-O振动峰,与上述C-S-H各基团的波数范围相对应,这表明所制备的样品为相对纯净的C-S-H结构。
图3
1.2 配合比设计及试件制备
水泥采用P⸱C 42.5复合硅酸盐水泥,主要化学成分为CaO、Al3O2、SiO2、Fe2O3、MgO、SO3、TiO2等,各成分占水泥质量比分别为57.34%、6.73%、27.82%、4.36%、3.32%、3.70%、0.56%。为在净浆试件中引入Cl-,采用NaCl浓度为0.5 mol/L的去离子水作为拌合水。水泥净浆的水灰比为0.45(质量比),C-S-H添加比例见表1。根据实验配合比制备净浆,之后浇筑于70.7 mm × 70.7 mm × 70.7 mm的模具中,待试件成型24 h后拆模,置于标准养护箱(相对湿度≥95%,温度为20 ± 3℃)中养护至相应龄期。
表1 水泥试件中C-S-H添加比例 (mass fraction / %)
Table 1
| Specimen number | Cement | C-S-H |
|---|---|---|
| OC | 100.00 | 0.00 |
| CC-2.00% | 98.00 | 2.00 |
| CC-2.25% | 97.75 | 2.25 |
| CC-2.50% | 97.50 | 2.50 |
| CC-2.75% | 97.25 | 2.75 |
| CC-3.00% | 97.00 | 3.00 |
1.3 测试方法
为了分析掺加C-S-H对水泥净浆结合Cl-的影响,对龄期为7、14、21、28和35 d时的净浆试件进行了自由Cl-含量和pH值测试。使用混凝土磨粉机取净浆试件距表面1~4 mm的粉末,将样品用0.63 mm的标准筛过筛,在烘箱中烘干2 h,温度设置为105 ± 5℃,随后放入真空干燥皿中冷却至室温。采用JTJ270-98中的化学滴定法,测量各组粉样中的自由Cl-含量。测试粉样pH值,取2 g粉样加入20 mL去离子水,振荡30 min,以加速离子浸出,24 h后,采用上海雷磁pH计(PHS-3C)测量各组粉样上清液pH值。
取相应龄期净浆试件,敲碎选取部分颗粒,在异丙醇中浸泡24 h,更换新的异丙醇后再继续浸泡6 d以充分置换样品中的自由水,最后将样品放置于真空干燥箱中连续抽真空使异丙醇从样品中挥发。干燥完成后在SU8100型扫描电镜上进行SEM-EDS测试。敲碎相应龄期净浆试件,用玛瑙研钵对部分敲碎的颗粒加入异丙醇进行研磨,过0.08 mm标准筛,为使异丙醇和自由水蒸发并且尽量不破坏样品中化合物,本试验样品统一置于40℃真空干燥箱中干燥3 d[15,16],完成后采用PANalytical X-PERT 3型XRD进行测试,测试范围5°~70°,扫描速度2°/min。采用NetzschTG209F3型TG-DTG进行TG-DTG测试,升温区间为室温至1000℃,升温速率10℃/min,测试气氛为N2。
2 结果与讨论
2.1 C-S-H对水泥净浆Cl- 结合性能的影响
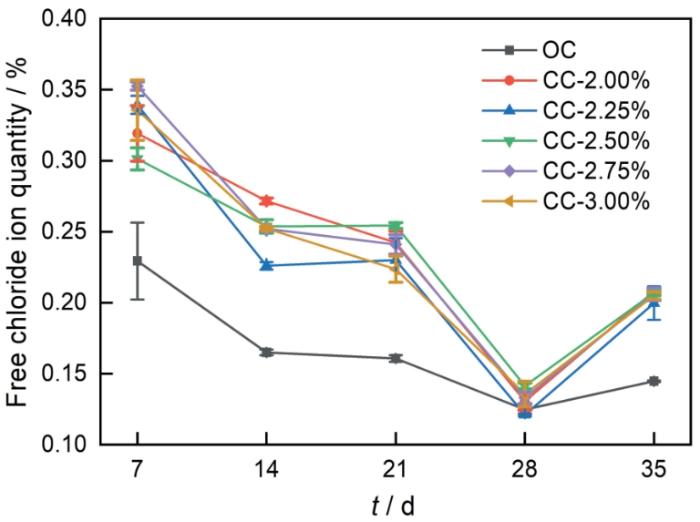
图4为各组水泥净浆中自由Cl-含量随龄期的变化规律,从图中可见,各组净浆试件中自由Cl-含量随龄期的增加皆呈先下降后上升的趋势,相比于OC组,掺加C-S-H组后期上升趋势更为明显。在养护龄期为7、14、21和35 d时,加入C-S-H组净浆的自由Cl-含量皆高于OC组,而在龄期为28 d时,掺加C-S-H组净浆的自由Cl-含量和OC组较为相近。由此可见,掺加C-S-H并没有有效提高水泥净浆的Cl-结合性能。通常在水泥基材料中掺加偏高岭土、矿渣等矿物掺合料时,提高了水泥浆体中C-S-H生成量,使得水泥基材料的Cl-结合量增加[17,18],但由上述试验结果可知,掺加C-S-H于水泥基材料中并没有达到增加Cl-结合量的效果。通过粒径和比表面积测试可知,掺加到净浆中的合成C-S-H粒径相对较大,比表面积相对较小,故造成水泥净浆中C-S-H总比表面积的降低,不利于水泥净浆对Cl-的结合。
图4
图4
水泥净浆自由Cl-含量随龄期变化规律
Fig.4
Variations of the contents of free chloride ions in cement pastes with cement age
2.2 C-S-H对水泥净浆pH值的影响
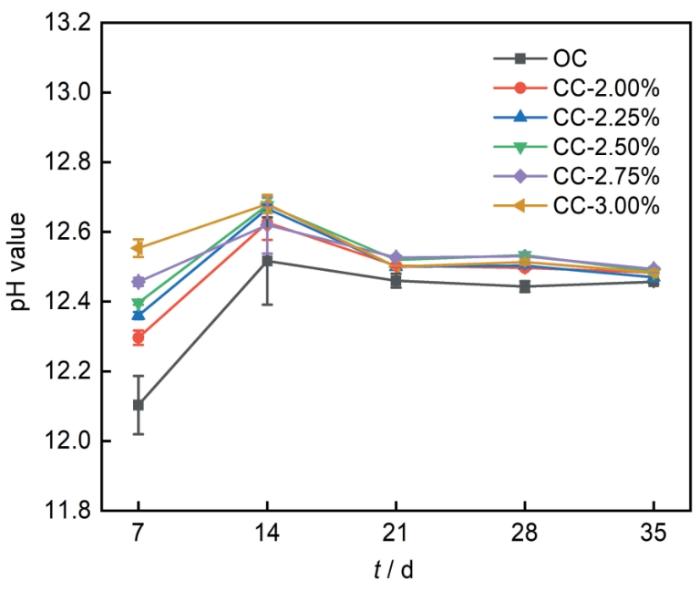
各组水泥净浆随龄期的pH值变化规律如图5所示,由图5可以看出,随着龄期的增加,水泥净浆pH值皆呈现先上升后下降最后趋于平稳的趋势。随着龄期的增加,差异逐渐缩小,最后趋于一致。值得注意的是,在龄期为7、14、21和28 d时,OC组pH值低于掺加C-S-H组,而龄期35 d时,各组试件pH值基本一致。此外,龄期为7 d时,随着C-S-H掺加量的增加,水泥净浆pH值随之增加。pH值降低会促进羟基(OH-)基团的去质子化,从而促进Ca2+在带负电荷C-S-H表面或层间的吸附,Ca2+在带负电荷C-S-H表面或在层间的吸附有利于Cl-结合[19]。因此,掺加C-S-H水泥净浆较高pH值反而不利于C-S-H对Cl-的结合。
图5
图5
水泥净浆pH值随龄期变化规律
Fig.5
Variations of pH values of cement pastes with cement age
水泥水化初期,C3S和C2S与H2O反应生成C-S-H和Ca(OH)2,Ca(OH)2的生成提高了水泥孔隙液的pH值,因此,各组水泥净浆水化7 d~14 d的pH值呈上升趋势。在水泥净浆中加入C-S-H之后,水泥水化早期,除水泥颗粒表面外,孔隙中的C-S-H颗粒成为了水化产物新的成核位点,与在水泥液相成核相比,在C-S-H颗粒表面成核有更低的成核表面能,降低了水泥水化反应的成核势垒,促进了C-S-H的生长[11,12,20]。因此,加入C-S-H促进了水泥净浆的早期水化进程,在促进C-S-H生长的同时,生成的Ca(OH)2也随之增加,故C-S-H组的pH值略高于OC组,且龄期7 d时pH值随C-S-H掺量的增加而增加。
火山灰反应是硅酸盐玻璃体SiO2等与水泥水化产生的Ca(OH)2发生化学反应,生成水化硅酸钙凝胶[21]。由于复合水泥中SiO2含量较高,SiO2与Ca(OH)2和高碱性的C-S-H凝胶产生火山灰反应,生成低碱性的C-S-H凝胶,如
2.3 微观分析
2.3.1 XRD分析
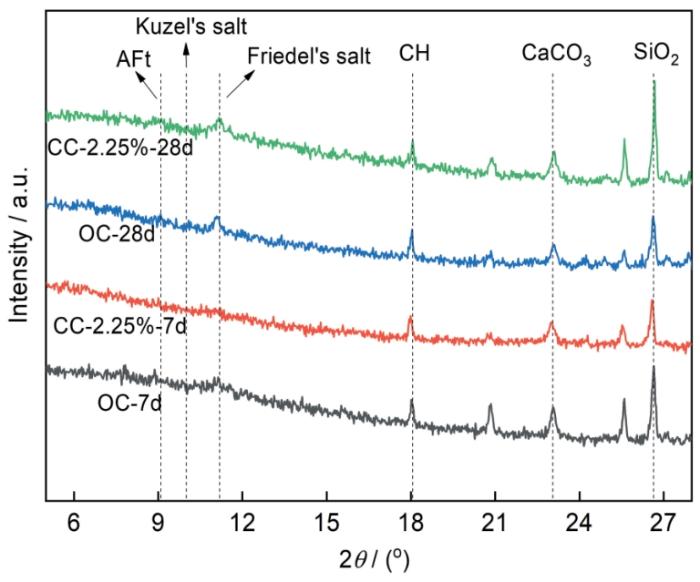
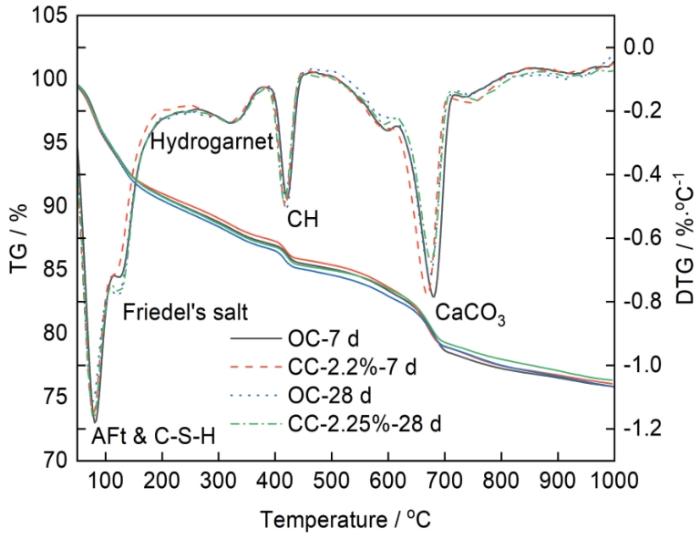
图6为龄期7和28 d时,掺加2.25%C-S-H组净浆和OC组净浆的XRD谱。由图6可知,养护龄期7 d时,掺加C-S-H组水泥净浆XRD谱中无明显Friedel's盐衍射峰,而OC组净浆中可以观察到Friedel's盐衍射峰。龄期28 d时,掺加C-S-H组与OC组中Friedel's盐衍射峰明显提高。由此可见,掺加C-S-H在水泥净浆龄期7 d时不利于Friedel's盐的生成,Cl-化学结合的主要产物为Friedel's盐,C-S-H的加入影响了水泥水化早期Cl-的化学结合。水泥水化产物对Cl-的结合主要为C-S-H对Cl-的物理吸附以及AFm对Cl-的化学结合,AFm相结合Cl-含量占总Cl-结合量的70%,并且AFm相结合Cl-的能力为C-S-H凝胶的5倍[4]。因此,化学结合对总的Cl-结合量至关重要,掺加C-S-H不利于净浆早期水化过程中Cl-的化学结合,从而使得掺加C-S-H组自由Cl-含量在水泥水化龄期为7 d时小于OC组。
图6
图6
水泥净浆各龄期的X射线衍射图谱
Fig.6
X-ray diffraction (XRD) patterns of two cement pastes with different ages
2.3.2 TG-DTG分析
图7为掺加2.25% C-S-H组净浆和OC组净浆7和28 d的热重分析(TG-DTG)曲线。从图可以明显的看出钙矾石&C-S-H(60~80℃)、Friedel's盐(100~200℃)、水榴石(290~310℃)、氢氧化钙(350~550℃)、碳酸钙(600~700℃)的吸热脱水热分解峰。相对于OC组,在水泥净浆中掺加C-S-H并没有产生新的物质。在水泥净浆龄期为7 d时,掺加C-S-H组净浆的DTG曲线上无明显的Friedel's盐分解峰,在OC组中则可以观察到。在龄期为28 d时,掺加C-S-H组以及OC组都出现了较为明显的Friedel's盐的分解峰,此结果与XRD测试结果一致。由此可知,C-S-H的掺加不利于水泥水化龄期7 d时Friedel's盐的生成,减少了Cl-的化学结合,降低了水泥净浆总的Cl-结合量,故水泥水化前期掺加C-S-H组水泥净浆自由Cl-含量高于OC组。
图7
图7
水泥净浆各龄期的热重分析曲线
Fig.7
Derivative thermogravimetric analysis (TG-DTG) curves of two cement pastes with different ages
2.3.3 SEM和EDS分析
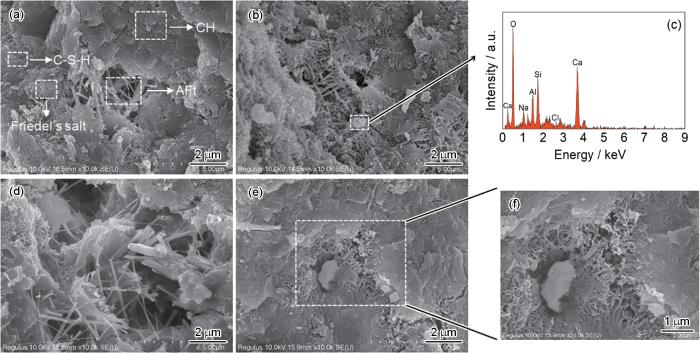
掺加2.25%C-S-H组净浆和OC组净浆7 d以及28 d的扫描电子显微镜(SEM)照片和能谱(EDS)图谱如图8所示。由图可知,OC组和掺加C-S-H组水泥净浆龄期为7 d时,其孔隙形貌存在差异。在图8a可以观察到长棒状钙矾石、片状氢氧化钙、簇拥呈花瓣形的六方板状Friedel's盐以及纤维状C-S-H。然而,在图8b中,除了明显的钙矾石可以观察到,其他孔隙形貌均为纤维状C-S-H凝胶,无Friedel's盐形貌。由此可见,C-S-H的加入促进了水泥净浆C-S-H的生成,但不利于Friedel's盐的生成。由于Cl-化学结合量远大于物理吸附量,故掺加C-S-H组净浆在7 d时自由Cl-含量高于OC组,这也印证了XRD以及TG-DTG测试结果。在图8b可以观察到孔隙中存在颗粒状物体,对其进行EDS分析,其组成元素主要为Ca、Si、O,因此推断其为C-S-H。掺加的C-S-H颗粒在孔隙中为水泥水化生成C-S-H提供了新的成核位点,从而促进了水泥水化的进行[21]。在龄期为28 d时,相较于图8d和e净浆孔隙结构更为致密,孔隙由更多的C-S-H凝胶填充。由此也表明,加入的C-S-H凝胶促进了水泥早期水化进程,促进了C-S-H凝胶的生成,同时生成更多的Ca(OH)2,使得掺加C-S-H组净浆pH在水化早期时高于OC组,这与图5中pH值变化趋势一致。
图8
图8
水泥净浆各龄期的SEM像与EDS图谱
Fig.8
SEM images of the OC (a, d) and CC-2.25% (b, e) cement pastes with the ages of 7 d (a, b) and 28 d (d, e), EDS spectrum of the marked particle in Fig.8b (c) and enlarged view of the marked area in Fig.8e (f)
3 结论
(1) 在龄期7~35 d时,净浆试件中自由Cl-含量随龄期的增加皆呈先下降后上升的趋势。
(2) 在净浆中掺加合成的C-S-H为水泥水化产物生成提供新的成核位点,降低了水泥水化反应的成核势垒,促进水泥水化,提高水泥水化早期的pH值,且pH值随C-S-H掺量的增加而增加。
(3) 掺加C-S-H后反而不利于水泥净浆对Cl-的结合,这是因为掺加的合成C-S-H粒径相对较大,比表面积相对较小,使得净浆中C-S-H总比表面积降低,降低了C-S-H的Cl-结合量;其次,掺加C-S-H水泥净浆pH值相对未掺加C-S-H净浆较高,这也不利于C-S-H对Cl-的吸附;再次,掺加C-S-H净浆早期水化产物中无Friedel's盐生成,从而使得掺加C-S-H净浆化学结合Cl-能力相对较弱。
(4) 在龄期28 d时掺加C-S-H组水泥净浆,其自由Cl-含量与未掺加C-S-H的水泥净浆趋于一致。在龄期28 d之后,由于掺加C-S-H组净浆中较多SiO2的存在,易发生火山灰反应,使得35 d时自由Cl-含量上升明显。
参考文献
Durability deterioration of concrete under marine environment from material to structure: a critical review
[J].
Modern concrete materials and structure service characteristics and the development
[J].
现代混凝土材料与结构服役特性的研究进展
[J].
Corrosion characteristics of carbon steel in simulated marine atmospheres
[J].
基于海洋大气环境因素影响下的碳钢腐蚀特征研究
[J].
Chloride binding and its effects on microstructure of cement-based materials
[J].
Cl-结合及其对水泥基材料微观结构的影响
[J].
Durability evaluation of concrete structure based on reinforcement corrosion
[J].
基于钢筋锈蚀的混凝土结构耐久性评定
[J].
Effect of Cl- and HSO
Cl-与HSO
Adsorption of chloride ion by calcium silicate hydrate
[J].
水化硅酸钙对Cl-的吸附
[J].
Experimental and molecular dynamics studies on the transport and adsorption of chloride ions in the nano-pores of calcium silicate phase: the influence of calcium to silicate ratios
[J].
Influence of pH on chloride binding isotherms for cement paste and its components
[J].
Effects of the C-S-H powder on the hydration process and mechanism of cement
[J].
水化硅酸钙粉体对水泥水化反应过程及机理的影响
[J].
New calcium silicate hydrate network
[J].The seeding effect of finely dispersed calcium silicate hydrate (C-S-H) particles on tricalcium silicate hydration and on cement was investigated. An extremely small amount of C-S-H nuclei (0.3%) significantly increases the degree of hydration of both by promoting the growth of C-S-H clusters into the pore solution instead of around the tricalcium silicate or cement grains. Indeed, it was clearly shown that growth takes place around nuclei delivered by seeding materials and therefore in pore spaces. Nevertheless, some of the nuclei persist onto cement grains. The efficiency of seed suspensions for accelerating hydration is qualitatively related to the cluster size of nuclei formed during the synthesis and to the polymer type used to stabilize the smallest particles. Although early growth occurs in the pore solution, the mechanical properties of the cement pastes are preserved, and use of seeding consequently accelerates the strength development of mortars.
Corrosion behavior of steel in simulated concrete pore solutions treated with calcium silicate hydrates
[D].
水化硅酸钙对混凝土模拟孔隙液中钢筋腐蚀行为的影响
[D].
A model for the microstructure of calcium silicate hydrate in cement paste
[J].
Mutual interaction between hydration of portland cement andstructure and stoichiometry of hydrated calsium silicate
[J].
Effects of chloride introduced way and metakaolin on chloride binding capacity of mortar
[J].The same amount of chloride ions was introduced to mortars by simulated sea sand and mixed water respectively. The effects of chloride introduced way and metakaolin on chloride binding capacity were analyzed. And the corresponding test methods were used to analyze the different aspects: energy disperse spectroscopy (EDS) was conducted to analyze the distribution of chloride content; the change of hydration product was analyzed by X-ray diffraction (XRD) and derivative thermogravimetric method (DTG); the change of pore structure was evaluated by mercury intrusion porosimetry (MIP). The results show that there is a diffusion process of chloride ions from surface of sea sand to cementitious materials, and that chloride ions introduced by mixed water distribute evenly in mortar. The chloride binding capacity in mortar at different ages was discussed. The chloride binding capacity in mortar made with sea sand is lower than that made with mixed water at 1 d age, but these two chloride binding capacities tend to be same at 28 d age. The acceleration of metakaolin on early cement hydration reaction promotes the binding process of chloride ions introduced by mixed water. The content of Friedel’s salt and Ca(OH)<sub>2</sub> decreases with the increase of metakaolin content. The chloride ions introduced by mixed water has a better refinement effect on pore structure at 20% and 30% (mass fraction) metakaolin content.
Cl-掺入方式及偏高岭土对砂浆Cl-结合性能的影响
[J].
Effect of mineral admixtures on sulfate attack resistance of cement-based materials
[J].
矿物掺合料改善水泥基材料抗硫酸盐侵蚀性能的微观分析
[J].
Chloride binding behavior of cement paste influenced by metakaolin dosage and chloride concentration
[J].
Chloride binding of alkali-activated slag/fly ash cements
[J].In this study, the effects of slag/fly ash ratio, Na2O concentration, silicate modulus of activators and water/binder ratio on chloride binding of alkali-activated slag/fly ash cements (AACs) are studied, using the binding isotherms. The Langmuir isotherm suits better for chloride adsorption of most of AACs, and the amount of bound chloride is predominantly influenced by the chloride concentration. The Friedel's salt only presents in alkali-activated slag samples after adsorption equilibrium. An appropriate slag/fly ash ratio could improve the chloride binding capacity due to the physical adsorption of N-A-S-H. The chloride binding of AACs decreases with the increased Na2O concentration but increases with the increased water/binder ratio, which is believed to be related to the OH- concentration in pore solutions. (C) 2019 Elsevier Ltd.
Improved chloride binding capacity and corrosion protection of cement-based materials by incorporating alumina nano particles
[J].
Influence of Nano C-S-H on cement hydration, pore structure of hardened cement pastes and strength of concrete
[J].
纳米C-S-H对水泥水化、硬化浆体孔结构及混凝土强度的影响
[J].
Influence of nucleation seeding on the hydration mechanisms of tricalcium silicate and cement
[J].
Volcanic ash effect of fly ash in fly ash concrete review
[J].
粉煤灰混凝土中粉煤灰的火山灰效应综述
[J].
Influence of fly ash on early hydration and pore structure of cement pastes
[J].
粉煤灰对水泥浆体早期水化和孔结构的影响
[J].
Chloride binding behaviors of metakaolin-lime hydrated blends: Influence of gypsum and atmospheric carbonation
[J].
Influence of carbonation on chloride binding of mortars made with simulated marine sand
[J].