金属腐蚀是指金属在环境介质作用下产生损耗与破坏的过程,金属腐蚀会导致材料逐渐失去原有性能,不仅会造成经济损失,还会引起严重的安全事故。因此,必须采取一定的防护措施来减缓金属的腐蚀过程。目前,常用的金属防护方法主要有缓蚀剂保护法,电化学保护法和涂层保护法[1]。其中,涂层保护法和缓蚀剂保护法应用最为广泛。
涂层保护是在金属表面涂上防腐涂层,使其与腐蚀介质隔离[10,11]。涂料应具有良好的电气绝缘性能、疏水性能和理想的附着力。在传统的原生涂层固化过程中,伴随着溶剂的蒸发,涂层内部及表面会形成微小缺陷,随着涂层服役时间的延长,这些缺陷会诱使水分子或其他腐蚀介质渗透涂层,进而使涂层失效[12]。因此,研制安全、环保、毒性低、自修复的防腐蚀涂料是十分必要的[13~16]。许多研究人员在涂料中加入纳米材料,从而提高涂层的耐蚀性能。常用的纳米改性材料有纳米钛粉[17]、六方氮化硼粉末及其纳米片 (BNNs)[18,19]等,而这些纳米材料在制备和使用过程中都存在一些缺点。例如,纳米级钛粉表面活性高,倾向于自发团聚,在涂层基体中的分散稳定性以及界面相容性较差[20];六方氮化硼粉末在涂层中难以分散,使其应用受到限制;BNNs的制备工艺以液相剥离为主,生产过程中生成挥发性有机物 (VOC),且剥离效率低,在涂层领域的大规模应用受限。因此,研制在涂层基体中分散性好,环保以及经济的纳米填料是十分必要的。近年来,通过与多种已有的缓蚀剂以及纳米填料的对比研究表明,零维碳纳米材料-碳点 (CDs) 更能满足作为防腐材料使用的要求[21~24]。
1 CDs用作缓蚀剂
1.1 N掺杂CDs
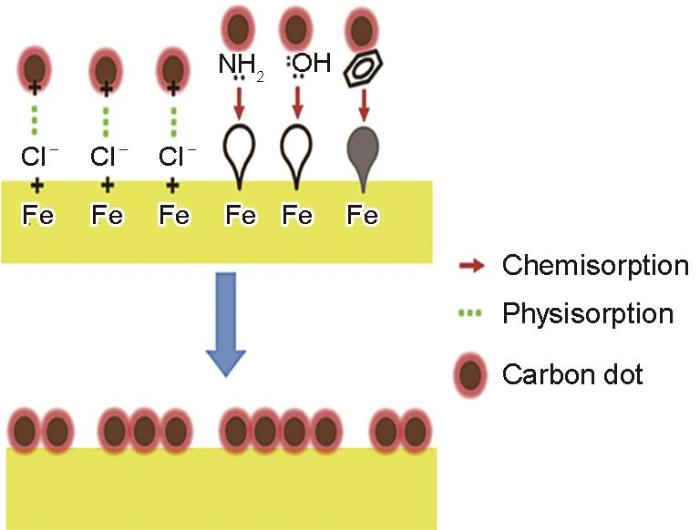
常用的一些酸洗缓蚀剂通常是含有电负性原子 (如:N、O、S、P等)、芳香环和π键的有机物,这些结构使得缓蚀剂与金属之间通过共价键、配位键或者静电作用吸附在金属表面形成一层保护层,从而抑制金属的腐蚀[52~55]。鉴于此,研究者合成N掺杂CDs来研究其对金属的缓蚀效果。Cui等[56]用4-氨基水杨酸制备了N掺杂CDs(N-CDs),并将其用作环保型缓蚀剂,考察其在1 mol/L HCl溶液中对碳钢的缓蚀效果。随着CDs浓度增加,腐蚀电位负移,腐蚀电流密度下降一个数量级。研究提出的腐蚀抑制机制如图1所示。经质子化的CDs以静电吸附作用吸附在金属表面。CDs通过掺杂原子N和O以及自由电子与铁原子之间形成配位键吸附在Q235钢表面,CDs在碳钢表面形成一层具有屏蔽作用的吸附膜,从而达到防腐的效果。
图1
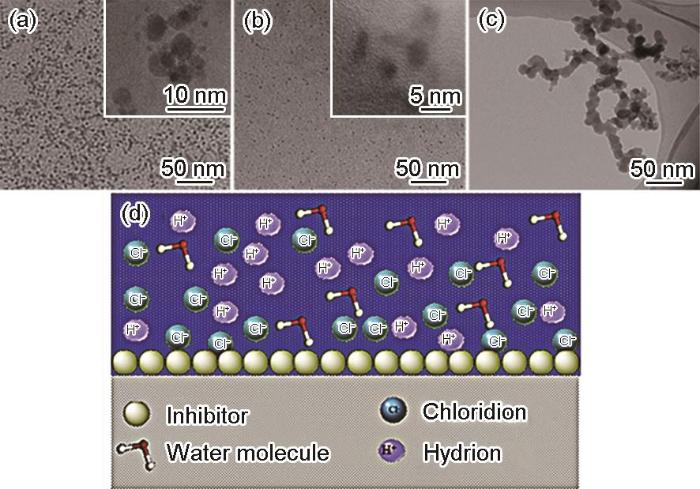
为了进一步了解CDs对金属的缓蚀效果,此方面的报道开始大量涌现。魏佳煜[57]以左旋多巴胺为原料,在碱性条件下用水热法制备N-CDs,在1 mol/L HCl 溶液中,当CDs的浓度为300 mg/L时,对碳钢的缓蚀效率可达97.9%。Ye等[58]以柠檬酸铵为原料,通过控制水热时间从而制备了3种N掺杂CDs(N-CDs1,N-CDs2和N-CDs3)。在1 mol/L HCl 溶液中,N-CDs2的浓度为200 mg/L时,对碳钢的缓蚀效率达到94.7%。N-CDs2优异的缓蚀效率得益于N-CDs2在溶液里具有优异的分散性,使其在碳钢表面的吸附更均匀。从图2a-c所示的TEM测试结果可以看出,相较于N-CDs1和N-CDs3,N-CDs2分布更均匀且无明显的团聚现象。腐蚀抑制机制如图2d所示,CDs中的石墨N原子以物理吸附的方式吸附在金属表面,形成团聚效应;化学吸附是由于CDs中的吡啶/吡咯N原子的孤电子对与金属的空轨道之间形成配位键。该团队以甲基丙烯酸和乙基 (甲基) 胺[59]为前驱体制备N-CDs,研究其在3.5% (质量分数)NaCl溶液中对碳钢的缓蚀效果。N-CDs浓度为200 mg/L时,对碳钢的缓蚀效率为88.96%。Zhu等[60]和Niu等[61]以柠檬酸为碳源,分别以乙二胺和硫脲来为氮源制备不同的N掺杂CDs,研究了N-CDs在酸性溶液中对Q235和X80的缓蚀效果。研究表明,N-CDs均是以物理化学吸附作用吸附在金属基体表面从而起到缓蚀作用。史艳艳等[62]采用柠檬酸和硫脲为原料合成N-CDs,在0.1 mol/L HCl溶液中,N-CDs浓度为100 mg/L时,对X80碳钢的缓蚀效率达到92.98%。肖晗等[63]以聚乙烯吡咯烷酮为原料采用水热法合成N-CDs,用失重法研究了腐蚀温度对缓蚀性能的影响,随着温度的升高,N-CDs在Q235钢表面的覆盖率不断减少并且腐蚀速率加快,N-CDs的缓蚀效率逐渐下降,N-CDs浓度为200 mg/L时,温度从303 K升高到333 K时,缓蚀效率从94.0%下降到了72.4%。Zhang等[64]以氨基酸为原料制备的N-CDs,在0.5 mol/L H2SO4腐蚀介质中对Cu的缓蚀效率高达到98.5%。
图2
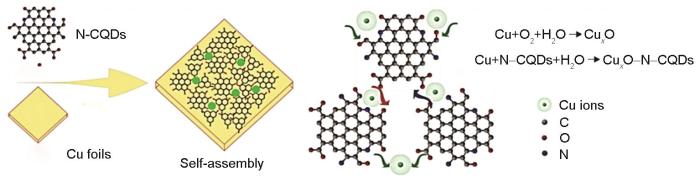
针对CDs的合成方法,Cui等[65]以不同摩尔比的一水柠檬酸和乙醇胺为原料,采用微波法合成N-CDs,缩短了N-CDs的合成时间。当一水柠檬酸和乙醇胺的摩尔比为1∶10时,所得N-CDs缓蚀效果最优,在N-CDs浓度为500 mg/L的0.1 mol/L HCl溶液中浸泡1 h后,对碳钢的缓蚀效率约为89%。研究员[66, 67]采用电解柠檬酸铵制备N掺杂CDs,并研究其在1 mol/L HCl溶液中对碳钢的缓蚀作用。Xu等[68]采用水热法合成N-CDs,研究了在0.5 mol/L H2SO4溶液中,N-CDs对Cu的缓蚀作用。N-CDs的浓度越高,对Cu的缓蚀效果越好,N-CDs浓度为100 mg/L时,对Cu的缓蚀效率达96.1%。Zhou等[69]采用电化学方法制备出平均尺寸为25.65 nm、N含量达30.18%的N-CDs,研究了在3.5%NaCl溶液中N-CDs对Cu的缓蚀效果。N-CDs在Cu的表面通过物理化学吸附作用形成一层吸附膜来阻止腐蚀的发生,抑制机制如图3所示,N原子不仅能和Cu基底形成配位键,加强CDs与Cu之间的相互作用,而且能够和水之间形成氢键。Lorite等[70]研究证明纳米颗粒可以通过量子限制效应、分子间作用力以及静电相互作用趋向团聚。N-CDs在溶液中通过扩散作用和静电作用吸附在Cu表面,并团聚成大颗粒沉积在Cu表面。N-CDs在局部区域具有较强的氧化能力[71],使Cu的表面容易生成Cu x O。
图3
针对合成CDs的原料,陈佳起等[72]以桂圆壳为原材料,通过煅烧法合成CDs,同时采用水热法合成N-CDs,比较了两种CDs在1 mol/L HCl溶液中对碳钢的缓蚀效率。结果表明,N-CDs具有更好的缓蚀效果。在1 mol/L HCl溶液中,桂圆壳CDs浓度为100 mg/L时,缓蚀效率为91.61%,而N-CDs浓度为20 mg/L时,缓蚀效率已达93.84%。
目前,N-CDs的缓蚀机理可归纳为:(1) N-CDs通过在金属基底表面形成一层吸附膜起到防腐作用;(2) N-CDs在金属基底的吸附类型为物理化学吸附;(3) N原子含有孤对电子,能与Fe、Cu的空轨道结合形成配位键,吸附在金属基底表面;(4) N-CDs由于团聚效应聚集到金属基底表面。
1.2 Ce、N共掺杂CDs
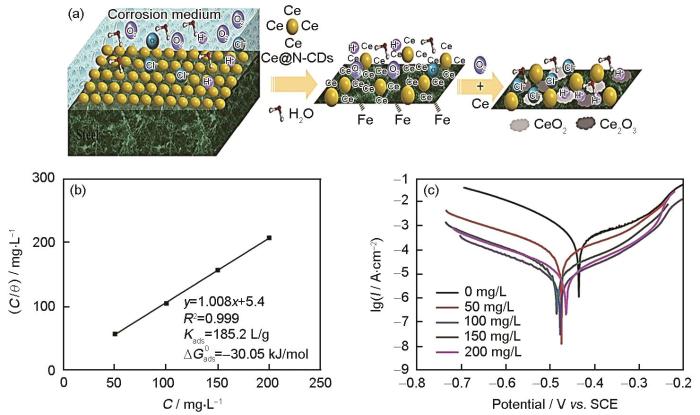
我国是世界上稀土矿资源最丰富的国家,1984年,稀土金属元素开始被应用于金属材料的缓蚀剂[73]。研究者们考虑将稀土元素与其他缓蚀剂复配来研究二者之间的缓蚀协同效应。Liu等[74]以六水硝酸铈和柠檬酸为原料,采用水热法合成了平均粒径为3~6 nm的Ce和N共掺杂CDs (Ce@N-CDs)。Ce@N-CDs通过静电作用吸附在Q235钢表面,形成一层吸附膜,抑制腐蚀性离子与金属直接接触,这属于物理吸附;Ce@N-CDs与Q235钢形成化学键,加强了缓蚀剂与金属界面的结合,属于化学吸附;特别的,合成的Ce@N-CDs中的Ce与N、O配体有很强的配位能力。因此,在腐蚀过程中可以形成含Ce络合物,保护Q235钢不被腐蚀,抑制机制如图4a所示。Langmuir吸附拟合同样表明Ce@N-CDs在Q235钢表面的吸附属于物理化学吸附,如图4b所示。从图4c中的极化曲线可以看出,Ce@N-CDs的浓度从0增加到200 mg/L时,腐蚀电流密度减少了近30倍,此时的缓蚀效率为96.4%。相比其它CDs,Ce@N-CDs对金属表现出更优异的缓蚀效果,见表1[75~82],这可归因于Ce与N-CDs在腐蚀溶液中对金属的协同保护作用。
图4
表1 CDs的缓蚀效率
Table 1
| Raw materials | Concentration mg·L-1 | Corrosion medium | Inhibition efficiency | Reference |
|---|---|---|---|---|
| EDTA, PA, urea | 100 | 15% HCl | 90% | [75] |
| p-phenylenediamine | 200 | 1 mol/L HCl | 88% | [76] |
| Tryptophan | 200 | 1 mol/L HCl | 96% | [77] |
| Citric acid and L-histidine | 200 | 1 mol/L HCl | 96% | [78] |
| Citric acid and imidazole | 200 | 1 mol/L HCl | 93% | [79] |
| Citric acid and urea | 30 | 0.5 mol/L H2SO4 | 95% | [80] |
| Ammonium citrate | 200 | 1 mol/L HCl | 94% | [81] |
| Methacrylic acid and n-butylamine | 200 | 1 mol/L HCl | 95% | [82] |
在1 mol/L HCl溶液中,Ce@N-CDs对碳钢具有优异的缓蚀能力。目前,稀土元素掺杂CDs应用于金属防腐方面的报道较少,可探索不同的稀土元素与CDs之间缓蚀效果的协同作用,同时研究对其它金属的缓蚀效果。
1.3 N、S共掺杂CDs
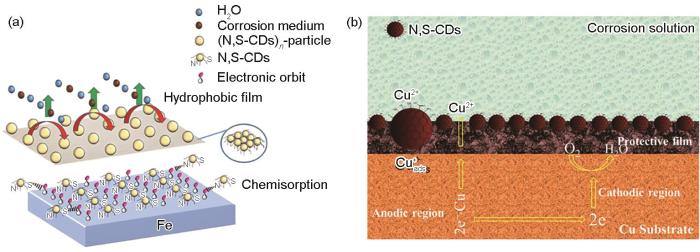
N、S均是电负性原子,研究者们研究N、S共掺杂CDs (N,S-CDs) 对不同金属的缓蚀效果。针对最外层有空轨道的Fe、Cu,研究者们以不同的前驱体合成N,S-CDs,来研究其在不同腐蚀介质中对金属的缓蚀效果。Cen等[83]以4-氨基水杨酸和硫脲为原料,采用水热法合成N,S-CDs,研究N,S-CDs在饱和CO2的NaCl溶液中对碳钢的缓蚀性能,N,S-CDs浓度为50 mg/L时,N,S-CDs对碳钢的缓蚀效率达到了93.0%,抑制机制如图5a所示。Luo等[84]通过控制原料蛋氨酸与柠檬酸铵的配比来合成不同N、S配比的N,S-CDs,在1 mol/L HCl溶液中,N,S-CDs的浓度为200 mg/L时,对碳钢的缓蚀效率达到98%。Zhang等[85]以邻苯二胺和硫脲为前驱体,合成了平均粒径为10~20 nm,N,S含量分别高达17%和19%的N,S-CDs。在0.5 mol/L H2SO4溶液中,N,S-CDs主要是通过化学吸附作用吸附在Cu的表面形成保护层,浓度为50 mg/L时,对Cu的缓蚀效率可达99.88%,抑制机制如图5b所示。
图5
对于最外层没有空轨道的Al、Mg,研究者合成不同的N,S-CDs,来研究其在不同腐蚀介质中对金属的缓蚀效果。Cen等[48]以4-氨基水杨酸和硫脲为原料,采用水热法合成N,S-CDs,在1 mol/L HCl溶液中,当N,S-CDs浓度为5 mg/L时,N,S-CDs对Al的缓蚀效率达到了85.9%,腐蚀抑制机理如图6a所示。刘甜甜[86]用枇杷合成N,S-CDs,在0.6 mol/L NaCl水溶液中,随着腐蚀时间的延长,N,S-CDs对Al的缓蚀效果越来越显著。Liang等[87]以4-氨基水杨酸和硫脲为原料合成N,S-CDs,研究了在3.5%NaCl溶液中N,S-CDs对Mg的缓蚀能力。当N,S-CDs浓度为50 mg/L时,缓蚀效率为86.0%,缓蚀效率处于中上水平,腐蚀抑制机理如图6b所示。
图6
2 CDs用作涂层填料
涂层高温固化时,随着溶剂不断地蒸发,涂层表面及内部会产生非常多微孔,局部的微孔会连通在一起,形成中大孔,这对涂层的耐腐蚀性能和硬度会产生致命影响。CDs由于尺寸小可以填充这些微孔,达到改善涂层的防腐效果,而且CDs作为缓蚀剂,能够呈现良好的缓蚀效果。因此,将CDs作为填料填充到涂层中可以制得防腐效果优异的涂层。Zhu等[93]将CDs引入到聚合物涂层中 (聚甲基丙烯酸酯、聚氨酯)。结果表明,经CDs掺杂的复合涂层表现出良好的自修复功能和防腐性能。该团队认为在聚合物中掺入CDs能够实现复合材料的自修复能力可归因为:(1) CDs和聚合物之间可形成-C=O-NH共价键,在聚合物断裂处发生强相互作用;(2) 相邻CDs之间通过范德华力相互连接,引发局部裂纹自修复。
2.1 CDs用作溶剂型涂料填料
CDs表面官能团丰富,在水中的分散性良好,但在有机溶剂中的分散性欠佳。若想将CDs加入到溶剂型涂层中来改善溶剂型涂料的防腐性能,需要解决CDs在溶剂型涂料中的分散性问题。
杜非凡[94]以一水合柠檬酸和乙二胺为原料合成N-CDs,将其作为纳米粒子填料添加到环氧树脂涂层中,制备复合涂层。N-CDs表面的酰胺基参与环氧树脂的固化过程,形成共价键,增强了其与环氧树脂的结合力;N-CDs中的羧基、氨基和酰胺基相互之间也可以形成氢键和共价键,使N-CDs之间相互结合形成一个整体来阻止腐蚀介质对环氧树脂的渗透,延迟环氧树脂的降解失效,提高环氧树脂涂层的防腐能力。Pourhashem等[95]利用硅烷偶联剂对CDs进行改性,提高了CDs在涂层中的分散性,改善了溶剂型涂料的防腐性能。在3.5%NaCl溶液中,纯环氧树脂涂料对碳钢的防腐效率为33.7%,添加硅烷功能化CDs复合涂层的防腐效率达到92.2%。Pourhashem等[96]以炭黑为原料,采用“自上而下”的方法合成CDs,将其作为填料加入环氧涂层中,制备的纳米复合涂层更加致密,缺陷减少,耐蚀性能提高。Ramezanzadeh等[97]热解柠檬酸合成的CDs亲水性很强,在环氧树脂基体中的分散性较差。该团队利用聚苯胺修饰CDs,聚苯胺功能化的CDs表面沉积了一层聚苯胺膜,在环氧树脂基体中的分散性变好,制备的复合涂层致密性提高,缺陷减少。
2.2 CDs用作水性涂料填料
溶剂型涂料在固化过程中会挥发大量的有机物,污染环境,危害人类身体健康。水性涂料由于其环保无污染等特性,成为防腐领域的研究热点。但水性涂料含有大量亲水性基团,会严重影响其耐蚀性能。为了提升水性涂料的耐蚀性能,研究人员做了大量工作。
Ren等[98]以对苯二胺为原料,采用溶剂热法合成N-CDs,并将其引入水性环氧树脂涂层中,制备复合涂层。复合涂层的阻抗模量高于纯环氧树脂涂层的阻抗模量,含2%CDs/环氧复合涂层在3.5%NaCl溶液中浸泡33 d后,其防腐性能是纯环氧涂层的364倍,表明CDs可以作为一种纳米填料改善水性环氧涂层的防腐性能。Ren等[98]对此现象做出的解释为:(1) CDs表面的官能团将CDs与环氧树脂连接起来,抑制了孔隙的增大;(2) CDs捕获涂层中的氧气,并将其还原为水,抑制了金属基体与氧气的接触,起到防腐效果。Wang等[99]以四氨基水杨酸为前驱体,利用溶剂热法合成了具有高乙醇溶解性的N-CDs,并以此作为水性环氧树脂涂层纳米填料制备复合涂层。相比纯环氧涂层,0.5%N-CDs-EP和1.0%N-CDs-EP复合涂层能够明显改善涂层的耐蚀性能,而加入过量N-CDs之后,复合涂层的耐蚀性能有所下降,但仍优于纯环氧涂层。Wan等[100]将FCDs/BNNs复合材料用于增强水性环氧涂料的耐蚀性。该团队以氨基功能化CDs (FCDs) 为插层剂对BN进行剥离和改性,制备FCDs/BNNs复合材料,并将其分散在环氧涂料中,制备复合涂层。研究表明,Cu在3.5%NaCl溶液中腐蚀40 d,相较于纯环氧涂层,复合涂层的阻抗模量提高了一个数量级。一方面,BNNs作为物理屏障,形成迷宫效应,延长了腐蚀性离子到达基底的路径;另一方面,FCDs作为插层剂,增强了BNNs在水性环氧涂层中的分散性,FCDs作为有效的缓蚀剂吸附在裸露的或者被腐蚀的Cu表面,阻碍腐蚀的进一步发生,FCDs的自修复能力与BNNs的物理屏障效应的协同作用使得FCDs/BNNs复合材料具有优异的防腐效果。
无论是溶剂型涂料还是水性涂料,CDs制备的复合涂层的防腐效果都得到了明显的改善,将CDs加入到涂层中需要解决的是CDs在涂层基底中的分散性问题。
3 结语与展望
本文综述了CDs在防腐领域的两个应用场景:缓蚀剂和涂层填料。总结了近年来CDs在防腐领域的研究成果,基于此,在未来的研究中,研究者们可致力于以下几方面的研究:
(1) 深入研究N、O、S、P等电负性原子以及不同种类稀土元素掺杂CDs的协同效应;在单原子,双原子掺杂的基础上,研发多原子掺杂的CDs,并探索其在防腐领域的应用研究。
(2) 目前报道的有CDs对碳钢、铜、镁、铝的防腐效果,金属种类较少,后续研究可探索CDs对不同金属的防腐能力。
(3) CDs还无法实现量产,今后的研究中可集中在CDs的大规模、低成本制备方面。
(4) 用于防腐领域的CDs前驱体大多是昂贵的化学试剂以及来源受限的生物质,我国拥有储量丰富的煤炭资源,可探索煤基CDs的制备及其在防腐领域的应用,为煤炭的清洁利用提供新思路。
(5) CDs在树脂体系中的分散性和稳定性是制约CDs复合涂层防腐效果的主要因素,因此,CDs作为涂层纳米填料的研究中,仍将集中在CDs在涂层中的分散性及稳定性方面。
(6) 实际涂料中除了成膜物质,还包含各种填料和助剂,它们之间的相容性也是影响涂料防腐性能的主要因素。因此,CDs和涂料中其他填料和助剂的相容性值得深入研究。
CDs在防腐领域的研究还处于起步阶段,CDs复杂的制备工艺以及高昂的纯化成本,使其工业应用受到极大的限制,但其独特的结构和优异的防腐性能使其在防腐领域具有良好的发展前景和应用潜力,值得科研工作者进行广泛而深入的研究。
参考文献
Corrosion and protection analysis of metal materials
[J].
金属材料的腐蚀与防护分析
[J].
Research progress of waterborne epoxy resin anticorrosion coatings
[J].
水性环氧防腐涂料的研究进展
[J].
Corrosion inhibition of mild steel in acidic solution by Tagetes erecta (Marigold flower) extract as a green inhibitor
[J].
Preparation and corrosion inhibition of super hydrophobic adsorption film of lotus leaf extract on mild steel
[J].
Q235钢表面的超疏水吸附层形成与缓蚀研究
[J].用新鲜荷叶作为研究对象,经过简便的乙醇回流萃取取得提取物。室温条件下,荷叶提取物能够在THF/HCl水溶液的混合溶液 (体积比为1/1,1.0 mol/L HCl溶液) 中产生聚集。傅立叶变换红外光谱以及X射线光电子能谱的结果证明了荷叶提取物在Q235钢样品表面发生化学作用,能够形成超疏水的吸附层。电化学结果表明荷叶提取物对碳钢在HCl溶液中具备良好的缓蚀性能,在0.4 g/L浓度下,最大缓蚀效率达到93.14%。
Synergistic inhibition effect of walnut green husk extract complex inhibitors on steel in phosphoric acid
[J].
磷酸中核桃青皮复配缓蚀剂对冷轧钢的缓蚀协同效应
[J].采用失重法、电化学法及表面分析测试研究了农林废弃物核桃青皮提取物 (WGHE) 与阴离子表面活性剂十二烷基磺酸钠 (SLS) 对冷轧钢在2.0 mol/L H<sub>3</sub>PO<sub>4</sub>介质中的缓蚀协同效应,并对WGHE中的缓蚀有效成分进行了探究。结果表明:单独的WGHE、SLS具有中等程度的缓蚀性能,50 ℃时100 mg/L的缓蚀率仅为50%左右;WGHE/SLS复配后缓蚀率不断上升,最高缓蚀率可达95.3%,两者之间存在显著的缓蚀协同效应,缓蚀协同效应系数随温度的升高而增大。WGHE/SLS复配缓蚀剂更能同时有效抑制阴极和阳极反应;Nyquist图谱呈现单一弥散容抗弧,电荷转移电阻排序为:WGHE/SLS>WGHE>SLS。WGHE中主成分芦丁、槲皮素、1-甲基萘醌与SLS之间存在缓蚀协同作用,但协同性能低于WGHE/SLS复配缓蚀剂。
Corrosion inhibition of aluminum in HCl solution by flos sophorae immaturus extract [J] J
槐米提取物对Al在HCl溶液中的缓蚀作用
[J].
Cucumber (Cucumis sativus L.) leaf extract as a green corrosion inhibitor for carbon steel in acidic solution: electrochemical, Functional and Molecular Analysis
[J].An extract of cucumber leaves (ECSL) was prepared as a green corrosion inhibitor for carbon steel. Its carbon steel corrosion inhibition performance against 0.5 mol L−1 H2SO4 was investigated using electrochemical methods and scanning electron microscopy (SEM). Its composition was analyzed by gas chromatography and mass spectroscopy (GC−MS). Quantum chemical calculations and molecular dynamics simulations (MDS) were conducted to elucidate the adsorption mechanism of the inhibitor molecules on the carbon steel surface. The results indicated that the inhibition efficiency increases with its increasing concentration. The extract acted as a mixed type corrosion inhibitor, and its inhibition properties were ascribed to the geometric coverage effect induced by its adsorption on the metal surface in accordance with Langmuir’s law. The active components in the extract were identified as mainly organic compounds with functional groups such as aromatic moieties and heteroatoms. The inhibition activities of ECSL are delivered through the ability of the active components to adsorb on the metal surface through their functional groups to form a protective layer which hinders the contact of aggressive substances with carbon steel and thus suppresses its corrosion. This research provides an important reference for the design of green corrosion inhibitors based on plant waste materials.
Chitosan as a green inhibitor for copper corrosion in acidic medium
[J].The behavior of copper in 0.5 M HCl acid containing different concentrations of chitosan has been studied by weight loss, potentiodynamic polarization, electrochemical impedance spectroscopy (EIS) and electrochemical frequency modulation (EFM) measurements. Potentiodynamic polarization measurements show that the chitosan acts essentially as a mixed-type inhibitor. EFM can be used as a rapid and non destructive technique for corrosion rate measurements without prior knowledge of Tafel constants. The results of EIS indicate that the value of CPEs tends to decrease and both charge transfer resistance and inhibition efficiency tend to increase by increasing the inhibitor concentration. The investigated inhibitor has shown good inhibition efficiency in 0.5 M HCl. The adsorption of inhibitor on the copper surface obeys Langmuir's isotherm. Metal surface characterization was performed using scanning electron microscope (SEM) and Fourier transform infrared spectroscopy (FT-IR). Also, the relationship between quantum chemical calculations and experimental inhibition efficiency of the inhibitor was discussed.Copyright © 2013 Elsevier B.V. All rights reserved.
Understanding the adsorption and anticorrosive mechanism of DNA inhibitor for copper in sulfuric acid
[J].
Preparation and anticorrosion performance of M-phenylenediamine-graphene oxide/organic coating
[J].
间苯二胺-氧化石墨烯/有机涂层的制备及防腐性能研究
[J].
Anti-corrosion behaviors of epoxy composite coatings enhanced via graphene oxide with different aspect ratios
[J].
Exploring corrosion protection properties of solvent based epoxy-graphene oxide nanocomposite coatings on mild steel
[J].
Biofriendly vegetable oil healing agents used for developing self-healing coatings: a review
[J].
Development and performance of phosphate-based protective insulation coating for non-oriented electrical steel
[J].
无取向电工钢用磷酸盐系绝缘环保涂层的研制及性能研究
[J].以磷酸盐为基础成膜剂,添加稀土钝化剂与硅烷偶联剂,研制了一种无取向电工钢用绝缘环保涂料,对添加不同助剂的涂层的显微形貌、电化学性能、成分组成和盐雾性能进行了表征和测试。结果表明,在成膜剂中添加稀土钝化剂可有效填补涂层中的孔洞,进一步添加硅烷偶联剂可分散稀土盐沉淀,使复合涂层表面更均匀且无明显缺陷。电化学测试结果显示复合涂层的腐蚀电流密度最小,极化电阻值最大,结合盐雾测试结果表明其具有出色的耐腐蚀性能。此外,复合涂层的层间电阻、附着力及铅笔硬度等性能均优于工业标准。
Preparation and characterization of microcapsules based self-healing coatings containing epoxy ester as healing agent
[J].
Preparation and performance of epoxy resin coating with benzotriazole inhibitor charged nano-halloysite tubes
[J].
纳米埃洛石装载苯并三氮唑自修复涂层研究
[J].研究了高温微波加热改性后的埃洛石纳米管在装载金属缓蚀剂苯并三氮唑后,利用超声波振荡法分散,用差热分析测定样品的装载量,采用扫描电镜对涂层的表面形貌进行观察,采用电化学阻抗方法对涂层腐蚀性能进行测试。结果表明,改性之后纳米埃洛石的表面形貌呈现破碎的管状结构的聚合体,超声震荡8 h具有最好的担载效果,苯并三氮唑负载在纳米埃洛石中形成的环氧涂层具有良好的保护金属材料的作用。
A state-of-the-art review on passivation and biofouling of Ti and its alloys in marine environments
[J].High strength-to-weight ratio, commendable biocompatibility and excellent corrosion resistance make Ti alloys widely applicable in aerospace, medical and marine industries. However, these alloys suffer from serious biofouling, and may become vulnerable to corrosion attack under some extreme marine conditions. The passivating and biofouling performance of Ti alloys can be attributed to their compact, stable and protective films. This paper comprehensively reviews the passivating and biofouling behavior, as well as their mechanisms, for typical Ti alloys in various marine environments. This review aims to help extend applications of Ti alloys in extremely harsh marine conditions.
Corrosion resistance of silane film modified by hexagonal boron nitride
[J].
六方氮化硼改性硅烷膜耐蚀性能研究
[J].采用多巴胺对氮化硼进行非共价改性,通过SEM、红外光谱和TGA对粉末进行了表征。使用浸泡法在40Cr合金钢表面制备了掺杂改性氮化硼 (mBN) 的硅烷复合膜,运用SEM、红外光谱、润湿性测试以及动电位极化曲线研究了硅烷复合膜的耐蚀性能。结果表明,聚多巴胺成功附着在氮化硼表面,掺杂mBN的硅烷膜厚度增大至1.812 μm,mBN/BTESPT硅烷复合膜的表面接触角达到91.97°,动电位腐蚀电流密度为9.187×10<sup>-8</sup> A/cm<sup>2</sup>,耐蚀性能相比单一硅烷膜提高了约30倍,在中性盐雾测试中表现出较好的耐蚀性。mBN通过对硅烷膜的物理填充与化学键结合,阻碍了腐蚀介质的扩散,显著增强了金属的耐蚀性能。
Assessing corrosion resistance of two-dimensional nanomaterial-based coatings on stainless steel substrates
[J].
The preparation and modification of Ti particles and its influence on the property of the epoxy coating
[D].
纳米钛粉制备与改性及其对环氧涂层性能影响
[D].
Corrosion inhibition behavior of electrochemically synthesized carbon dots on Q235 carbon steel
[J].
Ionic liquid-assisted preparation of N, S-rich carbon dots as efficient corrosion inhibitors
[J].
Synthesis and application of CCQDs as a novel type of environmentally friendly scale inhibitor
[J].
Corrosion protection investigations of carbon dots and polydopamine composite coating on magnesium alloy
[J].
Water-soluble fluorescent carbon quantum dots and photocatalyst design
[J].
Facile access to versatile fluorescent carbon dots toward light-emitting diodes
[J].
Graphene quantum dots, graphene oxide, carbon quantum dots and graphite nanocrystals in coals
[J].Six coal samples of different ranks have been used to prepare single-layer graphene quantum dots (S-GQDs). After chemical oxidation and a series of centrifugation separation, every coal could be treated into two fractions, namely, CoalA and CoalB. According to the characterization results of TEM, AFM, XRD, Raman and FTIR, CoalA was revealed to be mainly composed of S-GQDs, which have an average height of about 0.5 nm and an average plane dimension of about 10 nm. The obtained S-GQDs showed excitation-dependent fluorescence and excellent electrochemiluminescence. CoalB was found to be some other carbon-based nanomaterials (CNMs), including agglomerated GQDs, graphene oxide, carbon quantum dots and agglomerated carbon nanocrystals. Generally, low-ranked coals might be more suitable for the preparation of S-GQDs. The production yield of S-GQDs from the six investigated coals decreased from 56.30% to 14.66% when the coal rank increased gradually. In contrast, high-ranked coals had high production yield of CoalB and might be more suitable for preparing other CNMs that were contained in CoalB, although those CNMs were difficult to separate from each other in our experiment.
Mass production of tunable multicolor graphene quantum dots from an energy resource of coke by a one-step electrochemical exfoliation
[J].
Graphene oxide quantum dots derived from coal for bioimaging: facile and green approach
[J].Graphene oxide quantum dots (GOQDs) are usually prepared using expensive carbon precursors such as carbon nanotubes (CNT) or graphene under the strong acidic condition, which requires an additional purifying process. Here, we first develop a facile pulsed laser ablation in liquid (PLAL) technique for preparing GOQDs using earth-abundant and low-cost coal as a precursor. Only ethanol and coal are used to produce GOQDs with excellent optical properties. The prepared GOQDs exhibit excellent optoelectronic properties which can be successfully utilized in bioimaging applications.
Coal-derived graphene quantum dots produced by ultrasonic physical tailoring and their capacity for Cu(II) detection
[J].
Photo-Fenton reaction of graphene oxide: a new strategy to prepare graphene quantum dots for DNA cleavage
[J].Graphene quantum dots (GQDs) are great promising in various applications owing to the quantum confinement and edge effects in addition to their intrinsic properties of graphene, but the preparation of the GQDs in bulk scale is challenging. We demonstrated in this work that the micrometer sized graphene oxide (GO) sheets could react with Fenton reagent (Fe(2+)/Fe(3+)/H(2)O(2)) efficiently under an UV irradiation, and, as a result, the GQDs with periphery carboxylic groups could be generated with mass scale production. Through a variety of techniques including atomic force microscopy, X-ray photoelectron spectroscopy, gas chromatography, ultraperformance liquid chromatography-mass spectrometry, and total organic carbon measurement, the mechanism of the photo-Fenton reaction of GO was elucidated. The photo-Fenton reaction of GO was initiated at the carbon atoms connected with the oxygen containing groups, and C-C bonds were broken subsequently, therefore, the reaction rate depends strongly on the oxidization extent of the GO. Given the simple and efficient nature of the photo-Fenton reaction of GO, this method should provide a new strategy to prepare GQDs in mass scale. As a proof-of-concept experiment, the novel DNA cleavage system using as-generated GQDs was constructed.
Microwave synthesis of fluorescent carbon nanoparticles with electrochemiluminescence properties
[J].
Facile preparation of low cytotoxicity fluorescent carbon nanocrystals by electrooxidation of graphite
[J].
Versatility with carbon dots-from overcooked BBQ to brightly fluorescent agents and photocatalysts
[J].
Carbon dots as nontoxic and high-performance fluorescence imaging agents
[J].
Dopamine-induced reduction and functionalization of graphene oxide nanosheets
[J].
Study on the preparation and application of carbon nano-dots from Chinese cabbage
[D].
大白菜制备碳纳米点及其应用研究
[D].
Preparation of kiwifruit biomass carbon dots by microwave method and application of Fe3+ detection in honeysuckle
[J].
微波法制备猕猴桃生物质碳点及应用于金银花中Fe3+的检测
[J].
Facile synthesis of carbon dots from wheat straw for colorimetric and fluorescent detection of fluoride and cellular imaging
[J].
A facile and green method towards coal-based fluorescent carbon dots with photocatalytic activity
[J].
Luminescent polymer composite films containing coal-derived graphene quantum dots
[J].
Fluorescent carbon nano dots from lignite: unveiling the impeccable evidence for quantum confinement
[J].Synthesizing nano carbon from its bulk precursors is of recent research interest. In this report, luminescent carbon nanoparticles (CNPs) with tunable particle size and surface functionality are fabricated from lignite using ethylenediamine as the reactive solvent and surface passivating agent via different experimental methods. From the steady-state and time-resolved photophysical studies of these differently sized CNPs, it is unveiled that the energy of the excitons generated after photoexcitation is quantum confined, and it influences the observed photophysical behaviour significantly only when the particle size is less than 10 nm. A larger size of the CNPs and less surface functionalization lead to aggregation, and quenching of the fluorescence. But by dispersing smaller size CNPs in sodium sulfate matrix exhibits fluorescence in the solid state with an absolute fluorescence quantum yield of ∼34%. The prospective application of this hybrid material in sensing and removal of moisture in the atmosphere is illustrated.
Facile conversion of coal tar to orange fluorescent carbon quantum dots and their composite encapsulated by liposomes for bioimaging
[J].
Intrinsic and extrinsic defects in a family of coal-derived graphene quantum dots
[J].In this letter, we report on the high frequency (239.2 and 336 GHz) electron spin resonance (ESR) studies performed on graphene quantum dots (GQDs), prepared through a wet chemistry route from three types of coal: (a) bituminous, (b) anthracite, and (c) coke; and from non-coal derived GQDs. The microwave frequency-, power-, and temperature-dependent ESR spectra coupled with computer-aided simulations reveal four distinct magnetic defect centers. In bituminous- and anthracite-derived GQDs, we have identified two of them as intrinsic carbon-centered magnetic defect centers (a broad signal of peak to peak width = 697 (10−4 T), g = 2.0023; and a narrow signal of peak to peak width = 60 (10−4 T), g = 2.003). The third defect center is Mn2+ (6S5/2, 3d5) (signal width = 61 (10−4 T), g = 2.0023, Aiso = 93(10−4 T)), and the fourth defect is identified as Cu2+ (2D5/2, 3d9) (g⊥ = 2.048 and g‖ = 2.279), previously undetected. Coke-derived and non-coal derived GQDs show Mn2+ and two-carbon related signals, and no Cu2+ signal. The extrinsic impurities most likely originate from the starting coal. Furthermore, Raman, photoluminescence, and ESR measurements detected no noticeable changes in the properties of the bituminous GQDs after one year. This study highlights the importance of employing high frequency ESR spectroscopy in identifying the (magnetic) defects, which are roadblocks for spin relaxation times of graphene-based materials. These defects would not have been possible to probe by other spin transport measurements.
Carbon quantum dots: comprehensively understanding of the internal quenching mechanism and application for catechol detection
[J].
Synthesis of carbon dots and their applications in biosensing and pollutant detection
[D].
碳点的合成及其在生物传感和污染物检测中的应用
[D].
Fabrication of silica-decorated graphene oxide nanohybrids and the properties of composite epoxy coatings research
[J].
Carbon dots as effective corrosion inhibitor for 5052 aluminium alloy in 0.1 M HCl solution
[J].
Adsorption and anticorrosion mechanism of glucose-based functionalized carbon dots for copper in neutral solution
[J].
Enhanced anticorrosion performance of copper by novel N-doped carbon dots
[J].
Insights into the newly synthesized N-doped carbon dots for Q235 steel corrosion retardation in acidizing media: a detailed multidimensional study
[J].
Corrosion inhibition of mild steel by two new S-heterocyclic compounds in 1 M HCl: experimental and computational study
[J].
Effect of nitrogen doping on corrosion inhibition performance of carbon nanoparticles
[J].
氮掺杂对碳纳米颗粒缓蚀性能的影响
[J].
Weight loss, electrochemical, quantum chemical calculation, and molecular dynamics simulation studies on 2-(benzylthio)-1, 4, 5-triphenyl-1H-imidazole as an inhibitor for carbon steel corrosion in hydrochloric acid
[J].
Electrochemical investigation on the corrosion inhibition of mild steel by Quinazoline Schiff base compounds in hydrochloric acid solution
[J].
Carbon dots as new eco-friendly and effective corrosion inhibitor
[J].
Study on the corrosion inhibition performance of levodopa derivatives
[D].
左旋多巴衍生物缓蚀性能研究
[D].
A high-efficiency corrosion inhibitor of N-doped citric acid-based carbon dots for mild steel in hydrochloric acid environment
[J].
Evaluation of the inhibition behavior of carbon dots on carbon steel in HCl and NaCl solutions
[J].An eco-friendly and effective corrosion inhibitor (N-CDs) was acquired by hydrothermal method in methacrylic acid and ethyl(methyl)amine precursors. Afterwards, the weight loss and electrochemistry measurement were chosen to appraise the corrosion inhibition behavior of as-prepared N-CDs for Q235 steel in Cl- contained solutions. The change rules of EIS and Tafel data displayed that the as-prepared N-CDs revealed a high-efficiency protection for steel in all test environments. Meanwhile, the inhibition efficiency of steel reached up to 93.93 % (1 M HCl) and 88.96 % (3.5 wt% NaCl) at 200 mg/L of N-CDs. Furthermore, the N-CDs could form the adsorption film on steel surface to avoid the strong attack of Cl-. By analysis, the adsorption mechanism of as-prepared N-CDs on steel surface was physicochemical interaction, which strictly complied with the Langmuir adsorption model in both solutions.
Corrosion inhibition of eco-friendly nitrogen-doped carbon dots for carbon steel in acidic media: performance and mechanism investigation
[J].
Inhibition behavior of nitrogen-doped carbon dots on X80 carbon steel in acidic solution
[J].
Inhibition behavior of nitrogen-doped carbon dots on X80 carbon steel in acidic solution
[A].
在酸性溶液中氮掺杂碳点对X80碳钢缓蚀行为的实验研究
[A].
Corrosion inhibition performance and mechanism of N-doped carbon dots to Q235 steel
[J].An environmentally friendly and highly efficient N-CDs is synthesized by hydrothermal method with polyvinylpyrrolidone as raw materials. Its structure is characterized by FTIR. The corrosion inhibition performance and corrosion inhibition mechanism of N-CDs on Q235 steel in 1 mol/L HCl solution are studied by electrochemical method, weight loss method and scanning electron microscope (SEM). The results show that when the dosage of N-CDs is 200 mg/L, the corrosion inhibition efficiency could be as high as 95.6%. The electrochemical tests show that N-CDs is a mixed corrosion inhibitor which mainly inhibits the anode reaction. The inhibition mechanism is that N-CDs forms a stable adsorption film on the surface of Q235 steel under the combined action of physical adsorption and chemical adsorption, which effectively inhibits the corrosion of carbon steel by 1 mol/L HCl solution, and follows with Langmiur isothermal model. In addition, SEM clearly observes that the corrosion degree of Q235 steel is obviously improved after adding N-CDs.
氮掺杂碳点对Q235钢的缓蚀性能及机理研究
[J].
Solvothermal synthesis of functionalized carbon dots from amino acid as an eco-friendly corrosion inhibitor for copper in sulfuric acid solution
[J].
Microwave synthesis of eco-friendly nitrogen doped carbon dots for the corrosion inhibition of Q235 carbon steel in 0.1 M HCl
[J].
Inhibition behavior of graphene quantum dots for carbon steel in HCl solution
[J].
盐酸溶液中石墨烯量子点对碳钢的缓蚀性能
[J].
Preparation and corrosion inhibition of graphene oxide quantum dots
[D].
氧化石墨烯量子点的制备及缓蚀性能研究
[D].
Understanding the adsorption and inhibitive properties of Nitrogen-Doped Carbon Dots for copper in 0.5 M H2SO4 solution
[J].
Large-scale electrochemical fabrication of nitrogen-doped carbon quantum dots and their application as corrosion inhibitor for copper
[J].
Influence of the nanoparticles agglomeration state in the quantum-confinement effects: experimental evidences
[J].
Free-radical-assisted rapid synthesis of graphene quantum dots and their oxidizability studies
[J].This work reports a modified electrochemical method for rapid and large-scale preparing graphene quantum dots (GQDs) by introduction of active free radicals, which were produced by hydrogen peroxide or ultraviolet radiation. These free radicals can deepen the oxidized or reduced level of working electrode in electrochemical process and thus lead to GQDs with high concentration and small size, but different surface oxidized degree. The improved oxidation and reduction mechanism were analyzed in this work. Meanwhile, the optical properties and oxidizability of GQDs with different surface oxidized degree were investigated. It is found that these GQDs can be used as an oxidizing agent and their oxidizability is related to the degree being oxidized.
Corrosion inhibition on carbon steel in acidic solution by carbon dots prepared from waste longan shells
[J].
酸性介质中桂圆壳碳点对碳钢的缓蚀性能研究
[J].为开发环境友好、高缓蚀效率的新型缓蚀剂,以桂圆壳生物质为碳源,通过煅烧法和水热法分别合成桂圆壳碳点 (longan shell-CDs,ls-CDs) 和氮掺杂桂圆壳碳点 (N-lsCDs)。在此基础上,本文通过FT-IR、XPS、TEM、电化学方法、荧光光谱分析 (FL) 和静态失重法等手段对其光学性质、结构组成和缓蚀性能进行了测定分析。结果表明:在1 mol·L<sup>-1</sup> HCl体系中,当ls-CDs和N-lsCDs的浓度为100和20 mg·L<sup>-1</sup>时,对Q235钢的缓蚀效率分别达到89.49%和92.41%。尤其是N-lsCDs,具有投加量低、原料废物利用、缓蚀性能优异的特点。极化曲线测试表明N-lsCDs为混合型抑制剂,并且N-lsCDs在碳钢表面的吸附符合Langmuir吸附等温式,同时存在物理吸附与化学吸附。利用生物质为原料制备环保新型缓蚀剂能够变废为宝,具有诱人的潜在应用前景。
Status of development of rare earth industry in China
[J].
中国稀土产业的发展现状
[J].
Novel Ce@N-CDs as green corrosion inhibitor for metal in acidic environment
[J].
Carbon dots as green corrosion inhibitor for mild steel in HCl solution
[J].
Novel nitrogen doped carbon dots for corrosion inhibition of carbon steel in 1 M HCl solution
[J].
Effect of reaction parameters on the corrosion inhibition behavior of N-doped carbon dots for metal in 1 M HCl solution
[J].
A feasible method to improve the protection ability of metal by functionalized carbon dots as environment-friendly corrosion inhibitor
[J].
Functionalization of citric acid-based carbon dots by imidazole toward novel green corrosion inhibitor for carbon steel
[J].
Nitrogen-doped carbon dots as high-effective inhibitors for carbon steel in acidic medium
[J].
Corrosion inhibition behavior and mechanism of N-doped carbon dots for metal in acid environment
[J].
An effective corrosion inhibitor of N doped carbon dots for Q235 steel in 1 M HCl solution
[J].
N, S co-doped carbon dots as effective corrosion inhibitor for carbon steel in CO2-saturated 3.5% NaCl solution
[J].
The effect of N and S ratios in N, S co-doped carbon dot inhibitor on metal protection in 1 M HCl solution
[J].
Synthesized carbon dots with high N and S content as excellent corrosion inhibitors for copper in sulfuric acid solution
[J].
Systhesis and application of heteroatom doped carbon dots based on Chinese herbal medicines
[D].
基于中草药材合成杂原子掺杂碳点及其应用
[D].
Nitrogen/sulfur co‐doped carbon dots for enhancing anti‐corrosion performance of Mg alloy in NaCl solution
[J].
Synergism between cerium nitrate and sodium dodecylbenzenesulfonate on corrosion of AA5052 aluminium alloy in 3 wt.% NaCl solution
[J].
Corrosion protection of aluminium alloy 2024-T3 in 0.05 M NaCl by cerium cinnamate
[J].
Corrosion inhibition of aluminum in hydrochloric acid by pyrazinamide derivatives
[J].
Statistical investigation of lead removal with various functionalized carboxylate ferroxane nanoparticles
[J].Four new types of carboxylate-ferroxane nanoparticles, namely; maleate ferroxane (MF), fumarateferroxane (FF), para-amino benzoate ferroxane (PABF) and para-hydroxy benzoate ferroxane (PHBF) were synthesized, characterized and used for lead removal from aqueous solutions. Lepidocrocite nanoparticles were also synthesized and characterized asa precursorforcarboxylate-ferroxanes. FTIR, SEM and DLS analysis characterized the synthesized samplesand final Pb(II) concentration were analysed using inductively coupled plasma atomic emission spectrometer. Performance evaluation of the nanoparticlesin adsorption process was achieved using Taguchi experimental design. Variables in adsorption process were initial pH, contact time, adsorbent dose, adsorbent typeand initial concentration of Pb(2+) ions. The initial Pb(II) concentration was the most influential factor in the adsorption process among the five factors. Adsorption of lead was performed through two possible mechanisms; ion exchange and complex formation. Maleate ferroxane performed the best lead removal efficiency among the four types of ferroxane nanostructures studied. The adsorption kinetic data described well with a pseudo-second-order model and the equilibrium data fitted well to the Frendlich isotherm. Copyright © 2014 Elsevier B.V. All rights reserved.
Study on a hydrophobic nano-TiO2 coating and its properties for corrosion protection of metals
[J].
Carbon dots as fillers inducing healing/self‐healing and anticorrosion properties in polymers
[J].
Anti-corrosion performance of novel epoxy composite coatings filled with N-doped carbon nanodots
[D].
新型氮掺杂碳纳米点环氧复合涂层防腐性能研究
[D].
Corrosion protection properties of novel epoxy nanocomposite coatings containing silane functionalized graphene quantum dots
[J].
Comparing the corrosion protection performance of graphene nanosheets and graphene quantum dots as nanofiller in epoxy coatings
[J].Purpose In this research, the effect of graphene nanosheets and graphene quantum dots (GQDs) as carbon-based nanofillers on corrosion protection performance of epoxy coatings is considered. Design/methodology/approach Graphene nanosheets are synthesized via chemical vapor deposition method, and GQDs are synthesized by a simple and gram scale procedure from carbon black. The prepared nanofillers are characterized by X-ray diffraction technique, Fourier transform infrared spectroscopy and transmission electron microscopy. Further, solvent-based epoxy coatings containing 0.1 Wt.% graphene nanosheets and GQDs are prepared, and the corrosion resistance of nanocomposite coatings is considered by electrochemical impedance spectroscopy. Findings The results indicate that both epoxy/graphene nanosheets and epoxy/GQDs samples have significantly higher corrosion resistance than pure epoxy coating. Meanwhile, GQDs can more effectively enhance the corrosion protection performance of epoxy coatings compared to graphene sheets, which can be attributed to the presence of functional groups on GQDs and improving the dispersion quality in polymer matrice. Originality/value In this research, for the first time, the graphene quantum dots (GQDs) prepared by a "top-down" method from carbon black are used as nanofiller in epoxy coatings, and the potential application of graphene nanosheets and GQDs as anti-corrosion nanofiller in epoxy coatings is investigated.
Synthesis and characterization of polyaniline tailored graphene oxide quantum dot as an advance and highly crystalline carbon-based luminescent nanomaterial for fabrication of an effective anti-corrosion epoxy system on mild steel
[J].
Effect of nitrogen-doped carbon dots on the anticorrosion properties of waterborne epoxy coatings
[J].
Novel nitrogen doped carbon dots enhancing the anticorrosive performance of waterborne epoxy coatings
[J].There are lots of research studies reporting the excellent performances of waterborne epoxy resin coatings to reduce environmental VOC levels. However, it has also been manifested that waterborne epoxy resin coatings do not have high corrosion resistance because of being hydrophilic. Herein, we utilized a kind of N doped carbon dot (N-CD) which has high ethanol solubility and low cytotoxicity to enhance the corrosion resistance of waterborne epoxy resin coatings as a nanofiller. The N-CDs were obtained through a solvothermal method by using 4-aminosalicylic acid (ASA) as a precursor. The diameter and height of N-CDs confirmed by scanning probe microscopy and transmission electron microscopy are 3-5 nm. Corrosion resistance performance of the coatings without and with N-CDs is investigated by electrochemical impedance spectroscopy by immersing them in 3.5 wt% NaCl (aq) for 70 days. The results indicate that the composite coatings with 0.5 wt% N-CDs show superior anticorrosive performance due to bond interactions between N-CDs and polymer chains, the defect repairing effect of N-CDs and the formation of compact FeO and FeO passivation layers.This journal is © The Royal Society of Chemistry.
Functionalization of h-BN by the exfoliation and modification of carbon dots for enhancing corrosion resistance of waterborne epoxy coating
[J].