因其在工业领域中的重要应用和对钝化合金局部腐蚀行为的典型意义,不锈钢在含Cl-溶液中的点蚀行为已被广泛讨论。对于不锈钢点蚀生长的动力学行为,可以通过测量蚀坑几何参数、金属离子浓度变化、及电化学信号等进行分析与研究[1~3]。其中EN测量实施方便,能较好地体现蚀坑生长的腐蚀电化学特征,获得了广泛的应用。如通过施加恒定极化电位采集电流噪声 (ECN) 信号,可以分析暂态峰形态对应的点蚀机理及影响因素[4],亚稳点蚀生长电流密度、电流增长速率、寿命[5],或稳定点蚀生长的电流密度、电流增长速率等[6]。对ECN信号的统计处理表明其标准偏差可以反映材料的抵抗点蚀能力[7],利用小波分析、递归分析参数等进行人工神经网络分析或机器学习能区分腐蚀类型或局部腐蚀的不同阶段[8,9]。考虑EN测量的本意,即在自然电位下测量电极电位/电流随腐蚀过程的自发波动,则需考虑双电极的对称性。传统的EN测量一般采用名义相同的电极对进行耦合,测量其耦合电位及传递电流的波动,根据双电极阻抗相等的假设处理采集的噪声信号。实际测量中这种假设并不完全可靠,因双电极在初始状态的相同,并不能保证其在浸泡过程中的相同腐蚀动态发展,反映在电流噪声信号中为直流偏移或不对称的电流传递。常用的应对措施是采用一定的信号处理技术如带通滤波或其它时频处理方法消除直流偏移满足平稳时间序列的假设[10]。研究人员[11~13]尝试采用不对称面积或不对称表面状态的电极对进行EN测量,表明可以提高信号的采集效率,即能获得更多小面积电极上阳极事件的有效电流暂态信号,从而使后续的统计分析、小波分析 (WT)、递归分析 (RP) 等信号处理方法获得更好的效果。值得注意的是如果采用不同材料电极进行噪声测量及噪声电阻/阻抗的分析,需考虑到计算参数解释的困难,往往不能得到令人满意的结果[14,15]。
因此,如果想通过不对称电极对测量自然电位下的EN信号并将噪声信号分析结果归为某个电极,合适的设计思路是使用相同材料的电极,避免出现两个电极电位或阻抗的较大差距,其次是需要提供一定的电极表面状态的差异,如面积差异、表面处理方式的差异等,以获得局部腐蚀事件在某个敏感表面的优先发展,从而采集到一定时段内的单向电化学噪声,反映敏感电极的局部腐蚀发展过程。基于此设计思路,本文提出采用相同材料不同表面处理方法的电极配对方式,采集单向电流噪声信号,并根据等效电路模型及测量的电化学动力学参数估计点蚀生长的阳极电流分配系数,从而根据测量的EN信号还原真实的阳极电流,并采用小波分析、交互递归分析方法分析点蚀生长事件形成的电化学暂态动力学特征。通过304不锈钢电极在NaCl溶液中的测量结果验证所提方法及对信号分析结果解释的有效性,有望为其它合金局部腐蚀行为的EN测量及电化学动力学分析提供新的思路。
1 电化学模型
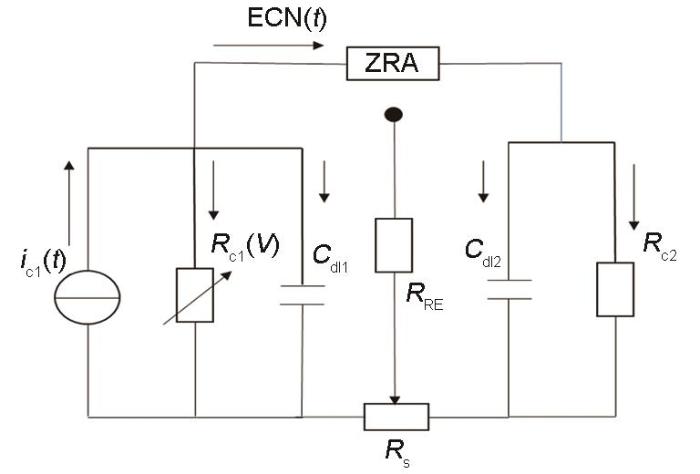
采用不对称表面电极时,由于表面状态的区别,其开路电位 (OCP)、维钝电流密度、点蚀电位等电化学参数也将不同,如304不锈钢经硝酸钝化处理后可提高在静态NaCl溶液中的耐点蚀能力[16]。如果将机械研磨-抛光电极与进一步硝酸钝化处理电极通过零电阻电流计 (ZRA) 联结,由于两者腐蚀电位的不同可能产生一定程度的电偶腐蚀,不过304不锈钢在氯化钠溶液中的腐蚀电位一般处于钝化区间,因此电位区别对两个电极极化的影响更多体现在钝化膜溶解速率的改变及局部腐蚀发生的几率上,即较高电位的钝化电极受阴极极化而减小钝化膜溶解速率,更加难以形成局部腐蚀,而较低电位的抛光电极处于阳极极化状态而更容易诱发局部腐蚀的形成与扩展。因此,对此不对称电极对进行自然腐蚀电位下的EN测量,可能得到近似单侧的电流暂态,反映更易受局部侵蚀的抛光电极的点蚀萌生和扩展动力学特征。对敏感电极1的局部腐蚀事件形成的暂态电流源记为ic1,从阳极事件释放的电子电流的角度考虑其分配特性,根据三电极测量配置可建立电化学等效电路模型,如图1。
图1
图1
单个电极上形成点蚀生长电流暂态的等效电路模型
Fig.1
Equivalent circuit model of pitting growth current transient formed on one electrode, where ic1 represents the electron current transient released due to pitting growth and is shunt into two parts. One part flows through the impedance of the local electrode, and the other part flows to the impedance of the opposite electrode via ZRA. The electrode impedance can be equivalent to the parallel connection of the cathode reaction resistance Rc and the double-layer capacitor Cdl
2 实验方法
采用商用304不锈钢条切断后制作测试电极,其化学成分 (质量分数,%) 为: C 0.063, N 0.069, Cr 18.14, Ni 8.07, Cu 0.52, Mo 0.1, Si 0.367, Mn 1.75, S 0.026, P 0.038,Fe余量。电极尺寸为直径10 mm,厚度10 mm的圆柱,经过硝酸钝化、焊接铜导线后镶嵌在环氧树脂中留出一个直径10 mm的工作平面,暴露面积为0.785 cm2。对工作平面进行机械研磨至2000#砂纸后用2.5 μm抛光膏抛光,经过酒精与去离子水超声清洗后保存在干燥箱48 h形成空气钝化膜。将部分研磨抛光样品浸入30%体积比的硝酸中在水浴槽中恒温30 ℃钝化12 h,形成硝酸氧化钝化膜,提高其耐点蚀性能,然后用去离子水清洗后保存。腐蚀测试溶液采用3.5% (质量分数) NaCl溶液,由分析纯试剂和去离子水配制。
采用Autolab 101电化学工作站进行电化学测试,分别测试相同处理的抛光电极对、硝酸钝化电极对及抛光-钝化电极对进行24 h连续电化学噪声测试,每组测试至少进行3次以保证其可重复性。测试在自然电位下进行,采用饱和甘汞电极测量电位噪声 (EPN),零电阻电流计测量电流噪声。EN测试前后对两个电极分别测量其开路电位 (OCP) 10 min。测量结束后再对两个电极分别测量OCP,获得其电极电位变化的信息,最后采用去离子水清洗表面并观察其表面状态变化。常见的不锈钢亚稳点蚀暂态特征频率在1 Hz左右,因此根据Nyquist采样定律将EN测量的采样频率设为10 Hz应能分辨出单个亚稳点蚀事件,避免混叠效应,电流范围设为自动调节适应电流的波动。阴极动电位极化测试的电位扫描范围设为开路电位到-0.4 V vs. OCP,扫描速率设为-1 mV/s,获得各表面状态的不同阴极动力学参数。
对ECN信号的处理采用Matlab软件的小波分析函数,利用‘db4’型小波分解信号为18个晶胞,再计算每个晶胞局部信号的标准偏差值 (SDPS) 以得到不同时段主要瞬态所在频率范围[18]。根据等效电路模型及分配系数估计方法还原阳极电流的计算过程采用自编的Matlab程序,交互递归定量分析及递归图分析采用Marvan等编制的crp工具箱运行。
3 结果与讨论
3.1 噪声信号时频解析
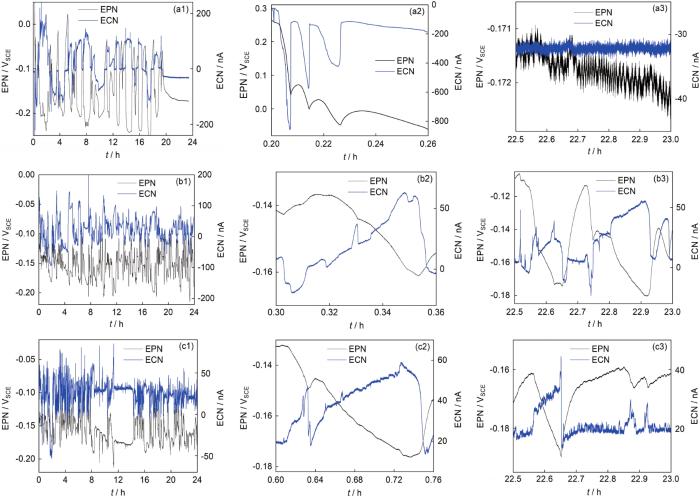
经过多次重复实验,表明按相同工艺处理的304不锈钢试样在EN测量时表现出类似的噪声形态,具体表现为较低频率的暂态及叠加在其上的少量高频暂态。典型的时域噪声信号如图2所示。
图2
图2
304不锈钢钝化-钝化电极对、抛光-抛光电极对、抛光-钝化电极对在3.5%NaCl溶液浸泡24 h的EN时域信号,及其开端和末尾时段的细节
Fig.2
EN time record of 304 stainless steel soaked in 3.5%NaCl solution for 24 h for the passivation-passivation electrode pair (a1), polishing-polishing electrode pair (b1), the polishing-passivation electrode pair (c1), and details of their beginning (a2-c2) and end period (a3-c3)
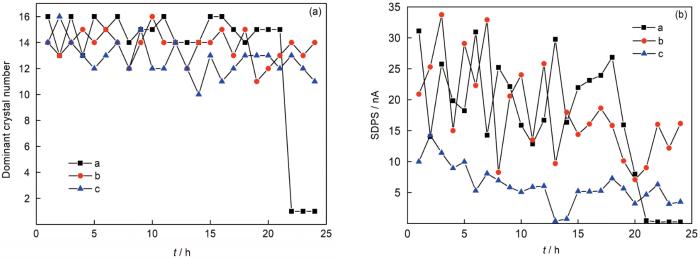
从图2可见经硝酸钝化处理后的304不锈钢电极在3.5%NaCl溶液中浸泡前期仍可能遭受局部攻击,其强度从ECN幅值来看甚至高于抛光处理电极。经约20 h浸泡后,钝化电极表面钝化膜经过溶解-重新形成,逐渐变得完整,表现在EN暂态随浸泡时间的减少[19],如图2a1所示;而抛光电极经过24 h浸泡后其局部攻击强度变化不大,表现在图2b2和b3的类似暂态峰形态上;与之对比可见抛光-钝化电极对产生的电流暂态基本出现在正值方向,除了开始阶段少数几个负向的暂态 (图2c1),表明此电极配对将主要诱发钝化膜保护性差的抛光电极的局部攻击。进一步考察暂态形式发现这3种情况下的暂态持续周期都较长,可达数百秒,远超一般的亚稳点蚀暂态几秒的周期[5],表明在本试样中可能存在较多钝化膜缺陷引起暂态叠加。通过对ECN时域分段信号进行小波分析,可揭示主要暂态所对应的晶胞频率区间,如图3所示。从图3a可见3种电极配对产生的暂态都以低频波动为主,集中在d10~d16晶胞,对应的暂态周期为51.2~6553.6 s,考虑暂态叠加的影响,d10晶胞对应的50~100 s周期可能为该腐蚀体系单元点蚀事件的特征周期。抛光-钝化电极对ECN信号的主导晶胞周期及波动强度都低于相同电极对的局部攻击信号,表明信号特点更接近单个电极的局部攻击周期及强度,而受对电极的叠加干扰较少。以上分析表明采用不对称表面处理电极采集EN信号具有对称电极不具备的优点,能采集到主要来自于钝化膜保护性质更弱的敏感电极表面的局部腐蚀形成、扩展电化学信号,并基本免受电偶腐蚀效应的影响;采集的信号单向性好,能保持更多局部腐蚀攻击的单元信息免受过多的叠加干扰。
图3
图3
图2所示ECN信号经小波分析得到的部分信号标准偏差(SDPS)最大值对应的晶体序号,及相应的SDPS值
Fig.3
Crystal number corresponding to the max SDPS value (a), and the calculated max SDPS value (b) in each hour by the wavelet analysis on the ECN signals in Fig.2, where three lines a, b and c represent different values of passivation-passivation, polishing-polishing and polishing-passivation electrode pair respectively
3.2 噪声信号暂态解析
考虑图1所示等值电路,如电极1发生局部腐蚀事件,产生的暂态电流将分配到本电极的电容与阴极反应电阻及对电极的电容及阴极反应电阻,具体分配比例则由电阻及容抗模相对大小决定。304不锈钢电极表面双层电容Cdl大小约为10~20 μF/cm2,钝化处理后电容值可能稍小也在此量级[20]。取抛光-钝化电极对采集的ECN信号SDPS最大的晶胞对应的0.02~0.0007 Hz(d10-d14)作为ECN信号的主要特征频率范围,则双层电容的容抗模约为0.5×106~15×106 Ω·cm2。在抛光处理电极上根据阴极极化曲线测量的极化电阻Rp (近似为腐蚀电位处的阴极反应电阻Rc) 约为0.1×106 Ω cm2,远小于容抗模值,因此可认为暂态电流在两个电极上的分配主要受两者阴极反应电阻Rc的相对大小控制,而界面电容更多的起平衡两个电极电位的作用。抛光及钝化表面的典型阴极极化曲线如图4所示。
图4
图4
304不锈钢抛光/钝化处理电极在3.5%NaCl溶液中的动电位阴极极化曲线
Fig.4
Potentiodynamic cathodic polarization curves of 304 stainless steel with polishing/passivation elec-trode in 3.5%NaCl solution
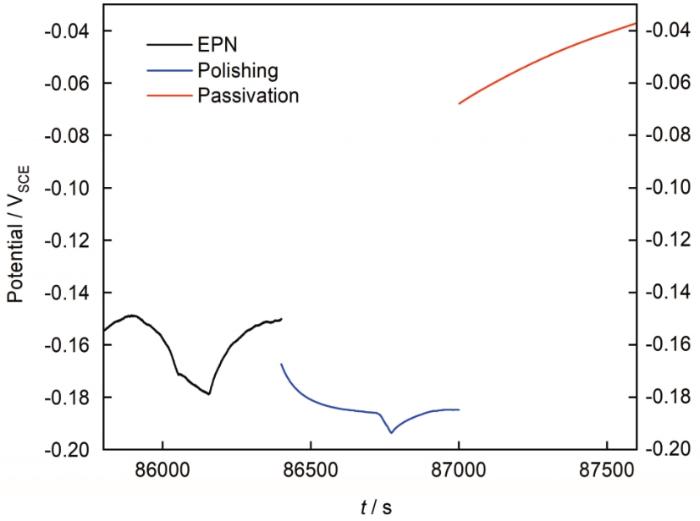
值得注意的是,电极表面的Rc将随电极电位发生变化,即局部腐蚀事件释放的电子将首先对本电极表面电容放电并降低电极电位,产生明显可测的电位降低EPN暂态,使得阴极反应电阻Rc降低,加速电子消耗;根据两个电极Rc的相对值分配暂态电流,通过ZRA逐渐对对电极的界面电容进行放电,拉平两电极之间的电位差,而对电极的阴极反应电阻较大,反应速度受到电子电流的影响较小,可视为不变的Rc2。两电极经ZRA耦合表现出的相同电位可能是由于两个电极双层电容充放电的表观形态,并不代表两者的相同稳态电位,如图5所示。
图5
图5
304不锈钢抛光-钝化电极对在3.5%NaCl中浸泡24 h末尾10 min EPN信号、及随后分别测量的抛光、钝化电极开路电位变化
Fig.5
EPN signal of 10 minutes at the end of 24 h immersion of 304 stainless steel in 3.5%NaCl and the open-circuit potential of the polished and passivated electrodes measured afterwards respectively
从图5可见耦合EPN信号主要反映的是遭受局部攻击的抛光电极的较低电位,而经过10 min电容再充电后,钝化电极电位已经恢复到接近其自然电位状态,远高于抛光电极电位。由此可知EPN信号表现的相同耦合电位是一种不稳定的表观现象,并不代表钝化电极的真实表面状态,因此钝化电极上的阴极反应电阻受电位降低的影响应该很小,而抛光电极上的自然电位则非常接近 (略低于) 测量的EPN曲线。根据以上分析,假设局部腐蚀事件产生的阳极电流主要由两个电极的阴极反应电阻Rc的相对大小决定,且测量的EPN信号主要反映遭受局部攻击电极的电位波动状态,而对电极的Rc受电位波动很小。假设自然电位下遭受局部攻击电极的Rc值随电位的变化规律与阴极极化曲线的阴极动力学特征类似,则可根据同步测量的ECN和EPN信号估计电流分配比例并还原真实的阳极暂态电流,其步骤如下:
(1) 通过拟合阴极极化曲线获得304不锈钢抛光电极动力学参数,及钝化电极的腐蚀电流值;
(2) 根据EPN记录的电位波动值估计抛光电极上阴极电流i1c(t)随电位下降的增长值,并与钝化电极的腐蚀电流i20相比较,获得该时刻的电流分配系数
(3) 根据真实阳极电流的分配公式
估计局部腐蚀事件形成的真实阳极电流i1a。根据图4对钝化电极的Tafel区域进行半对数线性拟合曲线与腐蚀电位交点得到的腐蚀电流值为97.4 nA;而对抛光电极的i1c采用分段拟合的方式:
图6
图6
304不锈钢抛光-钝化电极对在3.5%NaCl溶液中浸泡不同时段真实阳极电流暂态ia的估计值、ECN测量值及表观阴极电流值ic的形态
Fig.6
Estimated real anodic current transient ia (a) the measured ECN and the calculated apparent cathodic current ic (b) of 304 stainless steel polishing-passivation electrode pair soaked in 3.5%NaCl solution at different time periods
3.3 自发点蚀生长电化学动力学
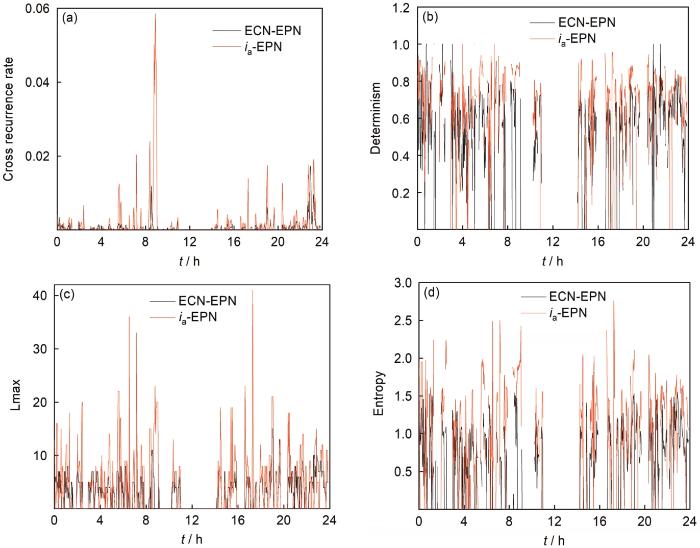
自然电位下的点蚀生长释放的电子将降低发生点蚀事件的电极电位,从而抑制点蚀的进一步加速生长,其动力学行为可能与控制电位下的点蚀生长有所不同。控制电位下的点蚀生长一般呈现持续增长的电流形态,而自然电位通常低于诱发点蚀的控制阳极电位,产生的点蚀生长电流密度较小,达到稳定点蚀的几率也更低。因此,根据阳极电流增长-电位降低耦合暂态信号分析自然电位下的点蚀生长电化学动力学特征,能够揭示与实际情况更接近的局部腐蚀机制。如果直接采用自发波动产生的ECN和EPN信号进行电化学动力学分析,会受到阴极反应的干扰无法表现出真实的阳极电流增长与电位降低的关系。若采用估计的阳极电流及记录的EPN信号 (近似为局部腐蚀电极的电位波动),可能得到具有更清晰物理意义的分析结果。对304不锈钢电极抛光-钝化电极对的估计真实阳极电流ia与造成的电位降 (EPN) 进行交互递归定量分析,可获得两个相量之间互相耦合的程度,以交互递归比 (RR)、确定性比 (DET)、最长对角线长度 (Lmax) 和对角线熵 (ENT) 等参数反映出来,如图7所示。
图7
图7
304不锈钢抛光-钝化电极采集的ECN-EPN信号及估计阳极电流ia-EPN的交互递归定量参数随时间的演变
Fig.7
Evolution of the recurrence quantitative parameters (a) RR, (b) DET, (c) Lmax, (d) ENT calculated with the ECN-EPN signal, or the ia-EPN signal, collected by the 304 stainless steel polishing-passivation electrode, the parameters are set as time delay τ=1, embedding dimension m=4, phasor distance as Euclidean distance, time window w=500 s, moving step Δt=100 s, threshold ε=0.02
从图7中可见,当采用估计的真实阳极电流与EPN信号进行交互递归分析时,所有的参数值在整个测量时段内都整体高于ECN-EPN信号的交互递归参数值。如递归比RR决定了两个相量距离相近的点所占比例,表征了电流与电位信号的相关程度,确定性比DET则反映了两个相量轨迹的相似运行趋势点所占比例,这两个参数通常是局部腐蚀形成及扩展的显著标志,较高的值表明发生局部腐蚀事件的频率越高;Lmax和ENT表明了两个相量按相似趋势演化的最长时间与对角线长度分布的复杂程度,这两个参数则表明局部腐蚀扩展的持续时间及其分布特征,值越高意味着更长时间的生长与更丰富的细节组成。总之,通过这4个参数可以指示局部腐蚀发生的时间范围、持续周期、细节分布等重要信息,显然根据估计的真实阳极电流与EPN信号进行交互递归分析能获得更显著的指示作用,这是因为估计的阳极电流基本剔除了同时发生的阴极反应的干扰,能更明确地揭示局部腐蚀事件对应的阳极电流生长-电位降低的电化学暂态行为。
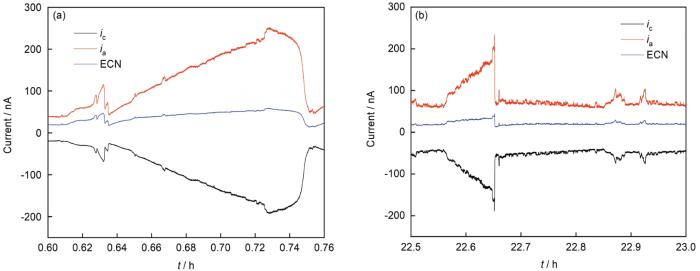
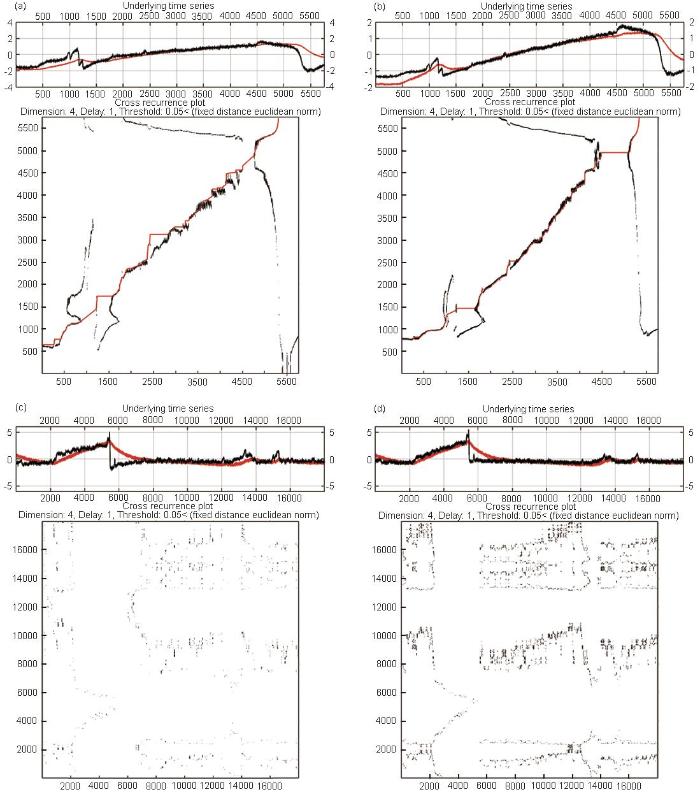
特定时段的交互递归图能具体地反映阳极电流增长与电极电位降低之间的相互关系,如图8所示。图8a、b分别是304不锈钢抛光-钝化电极对采集的前期ECN-EPN信号与估计的阳极电流ia-EPN信号交互递归图,比较两者的同步线可发现后者斜率更高,体现了电流随电压降的加速上升趋势,更符合实际的活化动力学特点;前者则表现出较小且不变的斜率,由于ECN被阴极电流抑制而无法体现真实的阳极电流生长趋势。对测量末尾阶段较长时段的交互递归图(图8c、d)则体现了用ia-EPN进行分析对局部腐蚀事件探测的灵敏度明显高于用ECN-EPN信号,前者出现的递归点明显更多,从中可以发现暂态出现时交互递归点构成的局部纹理形态;而后者形成的递归点数量过少,很难判断其中的具体纹理结构,因而也难以对相应的点蚀事件动态行为进行分析。
图8
图8
304不锈钢抛光-钝化电极对在不同时段的ECN-EPN信号、估计阳极电流ia-EPN信号的交互递归图
Fig.8
Cross recurrence plot of ECN-EPN signal (a), and ia-EPN signal (b) of 304 stainless steel polishing-passivation electrode at 0.6-0.76 h, where the red line is the fitted synchronous line representing their synchronous evolution trend; the cross recurrence plot of ECN-EPN signal (c) and ia-EPN signal (d) at 22.5-23 h, the negative value of EPN signal is used to make the current potential grow in the same direction and the threshold is ε=0.05, other parameters are set the same as in Fig.7
总之,根据不对称表面处理方式构成的不锈钢电极对可以采集单向性好的电流噪声信号,从而在自然电位下获得主要来自敏感电极的点蚀事件电化学信息。根据提出的点蚀生长阳极电流分配模型可估计真实的阳极电流暂态。虽然动电位极化获得的电化学参数与自然腐蚀状态下的电化学参数有所差别,但其差值同时作用在两个电极上可一定程度上相互抵消,使得分配系数的估计误差大大降低。根据所得估计阳极电流能够还原表观阴极电流波形,并进一步分析真实的阳极电流增长-电位降的动力学关系。
4 结论
不对称电极对采集电化学噪声信号的优点正在逐渐显现,由于噪声电阻对于腐蚀过程的稳定性要求,对不对称电极采集的噪声信号不太适用于噪声电阻的分析,而更适合采用小波分析、递归分析等不要求过程稳定性的信号分析方法。这是由于不对称电极的局部腐蚀事件的形成、发展很可能在一定时段内优先发生在敏感电极上,从而偏离稳定性条件。本文提出了不同表面处理的同种不锈钢材料构成不对称电极对的EN信号采集模式,采集的信号相对于同种处理工艺的电极对具有更好的单向性,主要反映更敏感电极的点蚀形成与生长过程产生的EN暂态特征。对ECN的小波分析结果表明不对称电极对采集的噪声较少受对电极干扰,体现在主导晶胞向高频方向的转移,更好地反映基元点蚀事件的频率特征。根据等值电路模型及电流分配方法估计的真实阳极电流则进一步反映了点蚀生长过程的阳极电流增长与相应的电极电位降低之间的动力学关系,通过交互递归定量分析与交互递归图能得到清晰的体现,即估计的阳极电流比记录的ECN信号能更灵敏地指示局部腐蚀的形成和发展,并揭示在自然电位下的点蚀生长电化学动力学特征。由于等效电路模型及电流分配模型的较少限制性条件,如果能正确的估计不同表面的阴极反应动力学参数,本文提出的模型与方法有望在其它的钝性合金局部腐蚀体系中得到进一步的应用与完善。
参考文献
Quantitative 3D characterization for kinetics of corrosion initiation and propagation in additively manufactured austenitic stainless steel
[J].
Corrosion product concentration in a single three-dimensional pit and the associated pitting dynamics
[J].
Electrochemical noise analysis of pitting corrosion of type 304L stainless steel
[J].Electrochemical current and potential noise were simultaneously acquired from Type 304L stainless steel (UNS S30403) in 0.05 M ferric chloride (FeCl3) using a three-electrode configuration. Power spectral, statistical, and wavelet analyses have been used to know the uniqueness of the parameters proposed for the identification of various types of corrosion processes. Roll-off slopes derived from power spectral analysis and statistical parameters such as standard deviation, localization index, and kurtosis corroborated with pitting as the corrosion mechanism. Energy distribution plots (EDP) obtained from wavelet analysis of current noise was found to be useful to derive mechanistic information on the progress of corrosion. Discrete wavelet transform was used to decompose the signals into a D1, D2, D3…D8, S8 set of coefficients. The EDP showed that the contribution from the medium time scale crystal, D5, prevailed over the smaller time scale crystals and larger time scale crystals during the initial stages of immersion. With an increase in the time of immersion, the energy deposition on the larger time scale crystals increased and the maximum energy was concentrated on the D8 crystals, indicating that the dominant process occurring on the specimen surface was stable pitting. The results of the investigation are detailed in the paper.
Environmental factors affecting pitting corrosion of type 304 stainless steel investigated by electrochemical noise measurements under potentiostatic control
[J].
Metastable pitting corrosion of 304 stainless steel in 3.5% NaCl solution
[J].
On the propagation of open and covered pit in 316l stainless steel
[J].
Novel strategies for assessing the pitting corrosion resistance of stainless steel surfaces
[J].
Determination of SS321 pitting stage in FeCl3 solution based on electrochemical noise measurement data using artificial neural network
[J].Artificial neural network (ANN) was used successfully to determine the pitting stage of stainless steel 321 (SS321) based on electrochemical noise (ECN) data. The ANN inputs were the parameters derived from the analysis of ECN data. The target values were the pitting stage of SS321 in FeCl3 solution, which were determined using galvanostatic electrochemical impedance spectroscopy (GEIS) tests. The ANN was validated by using a large number of available experimental data. Four approaches have been used for ANN development. The approaches differ in the number and type of inputs. The results showed that the best performance of ANN was achieved when all ECN analysis parameters were used as ANN inputs. These ECN analysis parameters were derived from the time domain, the frequency domain and the time-frequency domain analysis methods. Based on the results of this study, ANN has a very good performance in determining the pitting stage based on ECN analysis results.
Identification of corrosive substances and types of corrosion through electrochemical noise using signal processing and machine learning
[J].
Methods of trend removal in electrochemical noise data–Overview
[J].In this paper we shall review popular methods of trend removal from electrochemical noise time records. The basic principles of operation of the six most popular methods are explained. The proposed methods are: high-pass filtering, Moving Average Removal, polynomial detrending, wavelet detrending, Empirical Mode Decomposition and Variational Mode Decomposition. Estimation of trend removal quality is evaluated using statistical measures like a histogram of noise voltage, power spectral density, the correlation coefficient and signal power. The advantages, disadvantages, limitations and applications of all of the methods mentioned are presented. Two examples of electrochemical noise data with a different nature of generation are used for assessing the efficiency of the presented methods. The first set of measurement data concerning electrochemical noise with a thermal drift were observed during uniform corrosion. The second one refers to noise superimposed on a curve of the discharging current of a supercapacitor. This additive noise component is generated by charge redistribution or redox reactions within porous carbon electrodes. A comparison of these methods and an indication of the most suitable one for removing the drift component from the acquired electrochemical data is summarized in this paper. (C) 2018 Elsevier Ltd.
Using of asymmetric cell to monitor corrosion performance of 304 austenitic stainless steel by electrochemical noise method
[J].
A study on synergistic effect of chloride and sulfate ions on copper corrosion by using electrochemical noise in asymmetric cells
[J].The current study includes a systematic examination of copper corrosion initially in each of NaCl and NaSO solutions separately and then in the mixture solution of Cl and SO ions as aggressive ions. Electrochemical current noise (ECN) signals resulting from asymmetric (Asy) as well as symmetric (Sym) cells have been interpreted using wavelet transform (WT) along with statistical procedures. The signals have been detrended and the decomposition of every signal has been carried out into 8 crystals. Then the standard deviation of every crystal has been illustrated with the standard deviation of partial signal (SDPS) plots. The Asy electrodes increased the pitting detection on copper compared with the Sym ones, indicating higher efficiency of the Asy electrodes. Asymmetric-copper electrodes have been studied using SDPS plots at different temperatures (40, 60, as well as 80 °C). Finally, in order to partly understand the effect of Cl and SO ions on the corrosion of copper, the stabilization of Cu cations by Cl and SO ions in aqueous solutions have been modeled by DFT calculations. The derived results are in accordance with the experimental data.© 2022. The Author(s).
Advantages of asymmetrical over symmetrical cells for detection of under deposit CO2 corrosion using electrochemical noise
[J].Electrochemical noise (EN) measurements were done on symmetrical and asymmetrical cells after immersion in 3.5% NaCl solutions saturated with CO2 and containing different concentrations of sodium bicarbonate to get a better interpretation of the under-deposit corrosion of mild steel. The symmetrical cell was constructed from two sand-deposited electrodes and the asymmetrical cell was prepared from one sand-deposited electrode and one bare electrode. The combination of the standard deviation of partial signal (SDPS) plots arising from wavelet analysis and recurrence plots for analyzing the EN signals obtained from symmetrical and asymmetrical cells confirmed the higher detective ability of the asymmetrical cells in comparison with the symmetrical cells for measuring the under-deposit corrosion of mild steel. Both the SDPS and recurrence plots arising from symmetrical cells showed no significant change in the corrosion severity of the steel alloy with adding sodium bicarbonate. In contrast, the SDPS and recurrence plots of the asymmetrical cells proved that the corrosion severity decreased initially with adding the bicarbonate and thereafter remained constant with increasing the bicarbonate concentration. The optical microscopy images of the sand-covered electrode surface confirmed the latter result.
Noise resistance applied to corrosion measurements: V. Influence of electrode asymmetry
[J].
The significance of electrochemical noise measurements on asymmetric electrodes
[J].
Effects of surface treatments on the corrosion and erosion-corrosion of 304 stainless steel in 3.5% NaCl solution
[J].
The effect of passive layer stability on electrochemical noise signals arising from pitting corrosion of stainless steels
[J].
Comparison between ED and SDPS plots as the results of wavelet transform for analyzing electrochemical noise data
[J].
Assessment of stress corrosion crack initiation and propagation in AISI type 316 stainless steel by electrochemical noise technique
[J].
A correlation study of corrosion resistance and semiconductor properties for the electrochemically modified passive film of stainless Steel
[J].
不锈钢钝化膜耐蚀性与半导体特性的关联研究
[J].
Pit volume estimation on the stainless steel according to single current transient
[J].