研究[10~12]表明,超疏水涂层因其优异的拒水性,有利于阻隔腐蚀介质与材料表面的接触,从而广泛的应用于防水、防污、自清洁和流体减阻等领域。表面结构和表面能是影响材料表面润湿性的主要因素 [13]。目前,主要通过表面结构调控和低表面能物质改性制备超疏水涂层 [14, 15],电沉积是构建粗糙表面结构的有效途径之一 [16]。以电沉积过程产生的氢气泡作为模板,可得到具有多层次结构的镍镀层 [17]。该方法在燃料电池和电催化领域受到广泛关注,但采用此方法制备超疏水涂层的报道相对较少 [18~20]。另外,研究 [21~23]表明,通过向涂层中添加纳米粒子也有助于涂层表面形成突出,从而构建多层次微纳结构。
前期研究 [24]表明,采用氢气泡模板法电沉积三维结构的多孔镍层经硬脂酸改性后可使涂层获得超疏水表面。为进一步提高涂层的疏水性,我们将在本工作中把ZnO纳米粒子添加到电沉积多孔镍镀液中,考察纳米粒子对复合涂层的润湿性能和腐蚀防护能力的影响。
1 实验方法
实验选用尺寸为30 mm×20 mm×2 mm的AZ31镁合金为基底。基材成分除了Mg外,还包括 (质量分数,%):Al 3.0、Zn 1.0和Mn 0.2。电沉积时阳极材料为高纯镍片 (≥99.99%)。化学镀镍溶液组成为:六水硫酸镍 (NiSO4‧6H2O,≥98.5%),柠檬酸 (C6H8O7‧6H2O,≥99.5%),次亚磷酸钠 (NaH2PO2‧H2O,≥99.0%),氟化氢铵 (NH4HF2,≥98.0%),硫脲 (CH4N2S,≥99.0%)。电沉积镍溶液组成为:氯化铵 (NH4Cl,≥99.5%),六水氯化镍 (NiCl2‧6H2O,≥98.0%),ZnO纳米粒子 (30±10 nm,≥99.9%),十二烷基硫酸钠 (SDS,≥98.0%)。所有化学试剂,包括乙醇 (C2H5OH,≥99.7%),氨水 (NH3‧H2O,25~28%),磷酸 (≥85.0%,400 mL·L-1) 氢氟酸 (HF,≥40%) 和硬脂酸 (≥99%)。
将镁合金用240、400和1200#的砂纸依次打磨抛光,以去除表面氧化物。用去离子水将镁合金冲洗干净后,浸入丙酮中超声除油10 min。使用含有50.0 g‧L-1 NaOH和10.0 g‧L-1 Na3PO4的碱性溶液在65 °C下脱脂10 min,并用去离子水冲洗后分别置于H3PO4 (85.0%,400 cm3‧L-1) 和HF (≥40%,350 mL‧L-1) 溶液中酸洗和活化。最后,将前处理好的镁合金置于化学镀镍溶液中进行化学沉积。化学镀镍溶液组成 (g‧L-1) 为:C6H8O7‧H2O 5.0、NiSO4‧6H2O 25.0、NaH2PO2‧H2O 25.0、NH4HF2 10.0和CH4N2S 1.0。溶液pH值为5.4~5.8,沉积温度为87 °C,沉积时间为2.0 h。将得到的样品用热风吹干,标记为ENP。
超疏水镍基涂层的制备:以高纯镍片作为阳极,化学镀镍镁合金为阴极,在NiCl2‧6H2O和NH4Cl为主盐,SDS为分散剂的电沉积液 (pH 6.4~7.0) 中进行电沉积,电沉积时电流密度为1.0 A cm-2,沉积时间为60 s。纳米粒子ZnO的浓度为2.0~10.0 g‧L-1。电沉积得到的样品吹干后标记为ENP/NZ-x (x=1, 2, 3, 4, 5)。将新制备的ENP/NZ-x样品在35 °C下置于硬脂酸的乙醇溶液中浸渍24 h,然后用乙醇和蒸馏水依次冲洗干净并吹干,标记为ENP/NZSA-x (x=1, 2, 3, 4, 5)。为了对比,同时制备了未添加ZnO纳米粒子的对照镀层,并将电沉积层和硬脂酸改性后的镀层分别表示为ENP/NZ-0和ENP/NZSA-0。
使用带有能谱仪 (EDS) 的Gemini 500型扫描电子显微镜 (SEM) 观察样品的表面形貌和截面厚度。采用DX-2700BH型X射线衍射仪 (XRD) 对样品进行晶相结构分析。XRD的详细参数如下:Cu靶,扫描速率5º/min,扫描角度范围10°~90°。利用Nicolet 6700型Fourier红外光谱仪 (FTIR) 在波数为400~4000 cm-1范围内对样品表面的化学基团进行表征。利用250Xi型X射线光电子能谱仪 (XPS) 分析样品表面的元素组成、价态和化学键。利用SL200KS光学接触角测量仪测试涂层的静态接触角 (CA)。室温下,将体积为5 μL的水滴滴在水平放置的样品上,使用仪器自带的软件对结果进行拟合。
使用Gamry电化学工作站和常规的三电极体系进行电化学阻抗谱 (EIS) 和Tafel曲线测试。电化学测试在室温下、3.5% (质量分数) NaCl溶液中进行。三电极体系,饱和甘汞电极为参比电极,铂电极为辅助电极,测试样品为工作电极 (暴露面积为1.0 cm2)。测试前,先测量至少1200 s的开路电位-时间 (OCP-t) 曲线,以获得相对稳定的OCP值。EIS测试频率范围为105~10-2 Hz,施加振幅为10 mV的扰动电位。Tafel曲线的电位测试范围为-0.5~0.5 V (vs. OCP),扫描速率为1 mV·s-1。EIS和Tafel测试数据使用Gamry Analyst软件进行分析。每种样品均重复测试3次以上,以确保测试结果的可靠性及可重复性。
2 结果与讨论
2.1 复合涂层的微观形貌、结构和组成
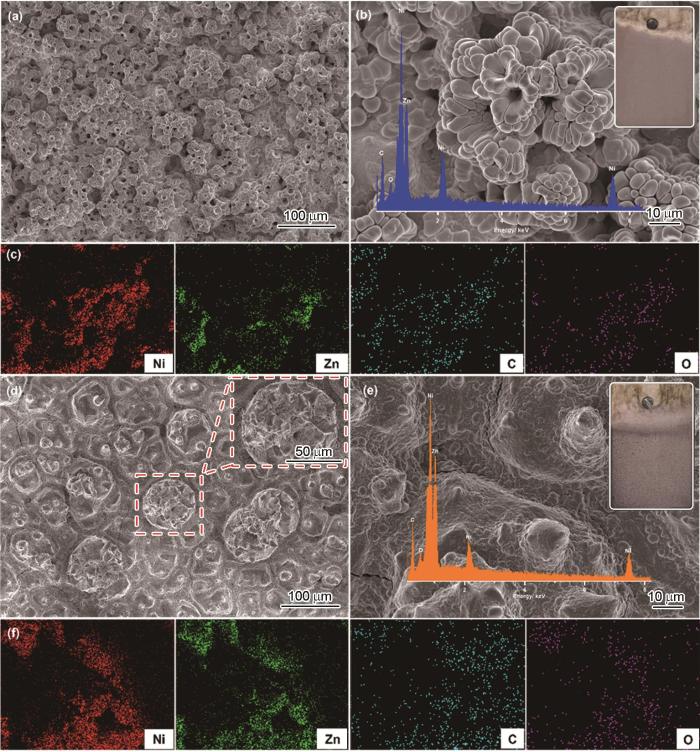
当电沉积镀液中ZnO纳米粒子的浓度增加到10.0 g‧L-1时,镀层表面出现大量粗糙颗粒,且结合力差,极易脱落。因此,本文主要探讨ZnO纳米粒子添加浓度为2.0和5.0 g‧L-1时所得镀层经硬脂酸改性后复合涂层的结构和性能。两种复合涂层的SEM和相应的元素分布显示 (图1),纳米粒子的添加对涂层表面形貌有很大影响。纳米ZnO含量的增加获得了更加粗糙的表面结构。当添加2.0 g‧L-1 ZnO纳米颗粒时,镍层表面呈现无数“喇叭花”状凸起结构 (图1a)。根据高倍数SEM图,凸起的“喇叭花”由胞状镍团簇构成,花中间有许多微米级孔隙 (图 1b)。凸起结构将会增大涂层表面粗糙度,而孔隙则可以截留空气,形成“空气”气垫,有利于提升涂层疏水性。当纳米颗粒增加至5.0 g‧L-1时,低倍SEM图像显示 (图1d),镍层表面有许多大小不一的球状突起,直径最大达到76.2 μm。将直径较大的球状突起放大后看到,其表面还有许多尺寸更小的圆锥形凸起 (图1d插图和图1e),说明镀层表面形成了 (亚) 微米级的层次结构。由镀层的EDS谱图 (图1b和e的插图以及图1c和f) 可知,镀层表面主要均匀分布Ni、Zn、C和O。其中,Ni和Zn来自于电沉积溶液和ZnO纳米粒子,说明ZnO纳米粒子成功均匀分散于镀层之中,或者Zn与Ni均匀形成合金;C和O来自于长链硬脂酸,表明电沉积镍层表面形成了均匀的有机膜层。两种镀层的EDS分析 (表1) 显示,当电沉积镍溶液中纳米粒子浓度增加时,镀层中Zn含量显著增加。但是,当电沉积溶液中ZnO纳米粒子浓度过高时,镀层表面的突出容易掉落,不利于镀层应用。
图1
图1
ENP/NZSA-2和ENP/NZSA-5复合镀层的SEM形貌和相应的EDS面扫描图
Fig.1
SEM images (a, b, d, e) and corresponding EDS elemental mappings (c, f) for ENP/NZSA-2 (a-c) and ENP/NZSA-5 (d-f) composite coatings. The insets in Fig.1b and e show the photographs and EDS spectra of corresponding coatings
表 1 两种镀层表面不同元素的含量 (mass fraction / %)
Table 1
| Sample | Ni | Zn | C | O |
|---|---|---|---|---|
| ENP/NZSA-2 | 81.98 | 13.40 | 4.15 | 0.46 |
| ENP/NZSA-5 | 65.73 | 28.87 | 4.99 | 0.40 |
为了确定Zn在镀层中的存在形式,测定了不同镀层的XRD谱图 (图2a)。镁合金表面化学沉积的镍磷合金为非晶态结构,在XRD谱中表现为宽泛的衍射峰 (2θ=44.51°)。在32.31°和36.75°处观察到两个尖锐的衍射峰,源自于镁合金基底。当在镍磷合金表面电沉积多孔镍层后,ENP/NZ-0镀层XRD谱图中显示出多个尖锐的衍射峰,表明电沉积镍层为晶态结构。镀层中3个明显的衍射峰,位于衍射角 (2θ) 为44.51°、52.81°和76.34°处,分别对应于Ni的 (111)、(200) 和 (220) 晶面 (JCPDS No:04-0850)[25]。当电沉积溶液中添加ZnO纳米粒子后,上述归属于Ni不同晶面的峰位置没有发生变化,但峰宽增加,说明结晶性受到一定影响。相较于ENP/NZ-0和ENP/NZ-2镀层,ENP/NZ-5镀层在80.29°处出现明显归属于Ni5Zn21合金的衍射峰;在62.97°处观察到微弱的属于ZnO(103) 晶面的衍射峰 (JCPDS No:36-1451) [26]。XRD测试结果表明,当电沉积溶液中纳米粒子增加到一定浓度后,Zn以镍锌合金和ZnO纳米粒子的形式同时存在于电沉积镀层中。不过,由于含量较低,在图谱中的衍射峰强度很弱,在SEM中也很难分辨完整的纳米粒子。尤其是在ENP/NZSA-2镀层的XRD谱中,难以观察到镍锌合金或者ZnO纳米粒子的衍射峰。复合涂层的FTIR如图2b-e所示,位于3418 cm-1的强宽吸收峰源于样品表面吸附H2O分子中羟基 (-OH) 的伸缩振动。两种涂层的FTIR谱均在2850~2980 cm-1范围内出现弱的吸收峰,这主要源于-CH3和-CH2的对称和不对称伸缩振动 [27]。由于金属Ni和Zn的影响,C=O的吸收峰发生偏移。C=O和C-O键的对称和不对称伸缩振动导致的吸收峰分别出现在波数为1639和1454 cm-1处。波数位于1385 cm-1的吸收峰对应于C-H的弯曲振动 [28]。FTIR谱结果进一步证实,硬脂酸层成功附着于电沉积镍层表面。
图2
图2
ENP,ENP/NZ-0,ENP/NZ-2和ENP/NZ-5复合涂层的XRD谱及ENP/NZSA-2和ENP/NZSA-5复合镀层的FT-IR光谱
Fig.2
XRD patterns of ENP, ENP/NZ-0, ENP/NZ-2 and ENP/NZ-5 composite coatings (a), FT-IR spectra of ENP/NZSA-2 and ENP/NZSA-5 composite coatings (b) and their enlarged views in different wavenumber ranges (c-e)
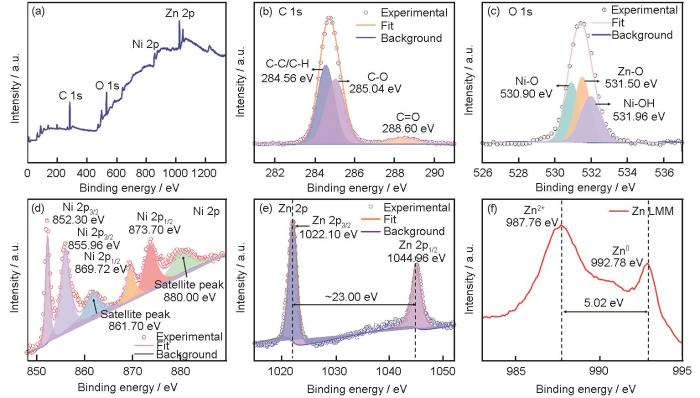
利用高分辨率XPS谱对ENP/NZSA-5镀层进行表征,以进一步分析复合涂层表面元素的组成和化学键信息。XPS全谱 (图3a) 显示复合涂层包含C,O,Ni和Zn。图3b~e分别列出了C,O,Ni和Zn 4种元素的高分辨率XPS谱。C 1s的高分辨率谱 (图3b) 可拟合为3个峰,分别归属于C-C/C-H键 (284.56 eV)、C-O键 (285.05 eV) 和C=O键 (288.60 eV) 的特征峰[29],再次证明硬脂酸分子成功附着于电沉积镍层表面。O 1s的XPS谱 (图3c) 也可以拟合为3个峰,包括结合能为530.90 eV处的Ni-O键的峰,531.96 eV处源于Ni (OH)2的峰和531.50 eV处源于Zn-O键的峰 [30, 31]。Ni的高分辨率XPS谱 (图3d) 拟合为6个峰,包括结合能为852.30和855.96 eV的Ni 2p3/2特征峰;结合能为869.72和873.70 eV的Ni 2p1/2特征峰;以及Ni 2p3/2 sate和Ni 2p1/2 sate对应的卫星峰,结合能分别为861.70和880.00 eV [31, 32]。Zn 2p的高分辨率XPS谱 (图3f) 有两个峰,结合能为1022.10和1044.96 eV,对应于Zn 2p3/2和Zn 2p1/2,两个峰结合能相差约23.00 eV,说明镍层中存在二价Zn [33, 34]。通过俄歇电子能谱可以进一步确认镍层中同时存在金属态和二价Zn (如图3f)。Zn LMM俄歇电子能谱图中动能为992.78 eV的峰归因于金属态Zn[35]。同时,在动能为987.76 eV处存在另一Auger峰,与金属态Zn的动能差值为5.02 eV,归因于二价Zn(Zn2+)[35, 36]。XPS结果再次证明,Zn在电沉积层中以锌镍合金和ZnO纳米粒子两种存在形式。
图 3
图 3
ENP/NZSA-5涂层的高分辨XPS谱
Fig. 3
High resolution XPS spectra of ENP/NZSA-5 coating: (a) survey spectrum, and fine peaks of (b) C 1s, (c) O 1s, (d) Ni 2p, (e) Zn 2p and (f) Zn LMM
复合涂层横截面SEM (图4a) 表明,涂层外侧横截面呈山峰状起伏,这源于电沉积镍层的多孔结构,导致表面形成许多凹凸起伏的沟壑,极大地增大了涂层表面的粗糙度。根据不同元素的EDS面扫谱图,可以计算化学沉积镍层和电沉积镍层的厚度。由图4a-c可知,复合涂层总厚度为 (76.2±2.0) μm;根据线扫描结果 (图4b) 和P的EDS面扫描谱图 (图4e),化学沉积镍基合金膜层的厚度约为 (60.9±2.0) μm。因为化学沉积镍基合金膜层中含有P,而电沉积层中没有P,所以电沉积层的厚度为涂层总厚度与化学沉积镍层的差值,约为 (15.3±2.0) μm。因Zn同时存在于电沉积层和基底镁合金,因而图 4g的上侧和下侧均能看到明显的富Zn区域。同时,图4g上侧Zn的均匀分布也证实,Zn以合金和纳米粒子形式均匀分散于电沉积层。图4d-f中所示的Mg和C分别来源于镁合金基底和树脂层,与图4a所示复合镀层的形状相匹配。由于树脂中本身含O,而制样过程中镁合金基底和镍基镀层均暴露于水和空气中,所以图4h显示O均匀分布于复合镀层的整个截面。因为硬脂酸膜层很薄,无论是通过线扫描还是面扫描,都难以在EDS谱图中体现。
图4
图4
ENP/NZSA-5样品的SEM截面形貌以及EDS线扫和面扫结果
Fig.4
Cross-sectional morphology (a), line scanning profiles along the direction of the arrow in Fig.4a (b), and corresponding EDS elemental mappings of Ni (c), Mg (d), P (e), C (f), Zn (g) and O (h) for ENP/NZSA-5 coating
2.2 复合涂层的疏水性
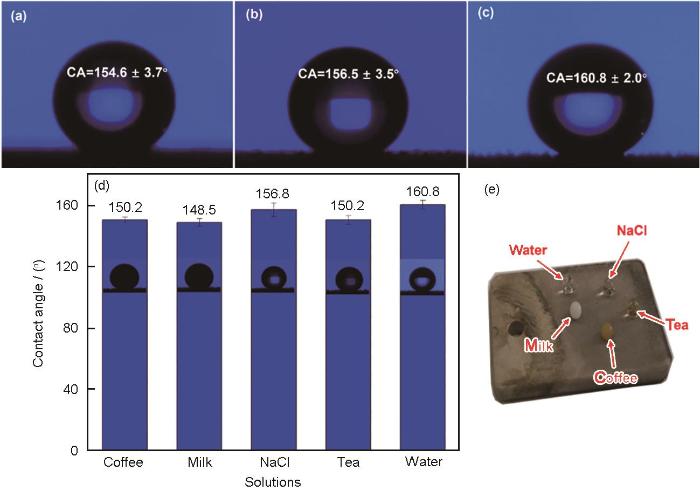
镀层的润湿性测试结果显示 (图5),未掺杂ZnO纳米粒子获得的多孔镍镀层经硬脂酸改性后 (ENP/NZSA-0),其表面静态水接触角为154.6°±3.7°。相较于ENP/NZSA-0涂层,当向电沉积溶液中添加纳米粒子后,复合涂层的疏水性均明显增加。当添加2.0 g‧L-1纳米ZnO时,ENP/NZSA-2涂层接触角为156.5°±3.5°;当纳米粒子浓度提升至5.0 g‧L-1时,ENP/NZSA-5涂层的接触角增加至160.8°±2.0°。由SEM可知,引入纳米粒子后,涂层表面微观结构发生明显变化,这可能是接触角发生显著变化的主要原因。与ENP/NZSA-2涂层相比,ENP/NZSA-5涂层具有更为突出的 (亚) 微米级层级结构。根据Cassie-Baxter润湿模型 [37],当水滴接触涂层表面时,微米级圆锥之间的孔隙会形成空气“气穴”,提高涂层的拒水性。一般地,超疏水涂层对日常污染物均具有一定的排斥能力。生活中几种常见溶液 (咖啡、牛奶、3.5% NaCl、去离子水和茶) 在ENP/NZSA-5涂层表面的接触角如图5d和e所示。可以看到,除了牛奶,其它几种溶液在ENP/NZSA-5复合涂层表面的接触角都超过150°,不同液滴在涂层表面都近似球体,说明涂层具有良好的拒液能力。
图5
图5
ENP/NZSA-0,ENP/NZSA-2和ENP/NZSA-5的静态水接触角和ENP/NZSA-5复合镀层表面咖啡、牛奶、3.5% NaCl溶液、茶和去离子水的静态接触角以及相应形貌
Fig.5
Static water contact angles of ENP/NZSA-0 (a), ENP/NZSA-2 (b) and ENP/NZSA-5 (c) coatings, static contact angles (d) and corresponding photographs (e) of ENP/NZSA-5 coating for coffee, milk, 3.5% NaCl, tea and water
图6
图6
NP/NZSA-2和ENP/NZSA-5复合涂层在去离子水中浸入和取出过程的数码照片
Fig.6
Digital photos of ENP/NZSA-2 (a-e) and ENP/NZSA-5 (f-j) composite coatings during immersing into deionized water and taking out
2.3 复合涂层的耐腐蚀性能
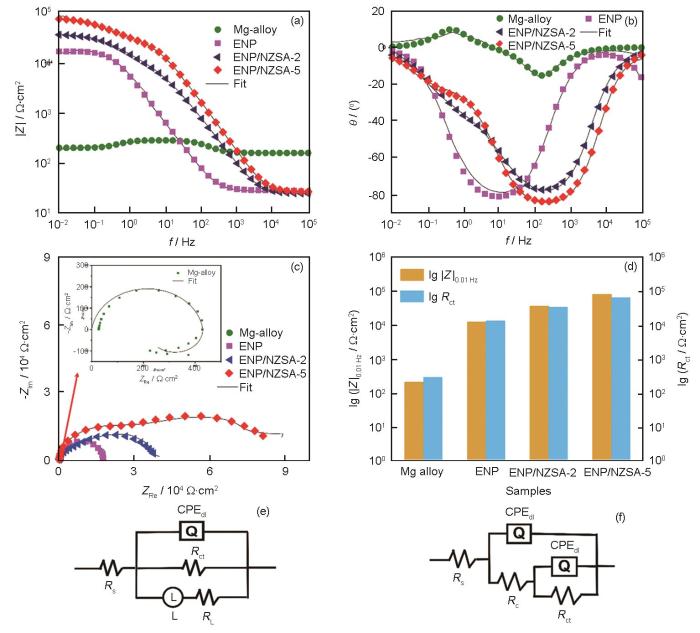
EIS和Tafel曲线是评估材料耐腐蚀性能的有效手段 [40]。通过EIS测试可以获得体系的电荷转移和双电层电容等动力学信息。图7为裸镁合金和不同涂层在3.5% NaCl溶液中的EIS谱及相应的等效电路图 (EC)。EIS谱的高频区、中频区和低频区对应样品/溶液界面不同区域的性质。其中,Bode幅频图的低频区、Bode相频图的中高频区和Nyquist图容抗弧的直径均可以反映涂层的耐蚀性能。一般地,涂层的低频阻抗模值越高,容抗弧直径越大,涂层的耐蚀性越好[41]。由图7a可知,相较于镁合金,不同涂层,尤其是ENP/NZSA-5涂层在中低频区域的阻抗值都显著提升。在0.01 Hz处,镁合金的模值仅为0.218 kΩ·cm2,而ENP/NZSA-5复合涂层的模值增加到了89.38 kΩ·cm2,提升了两个数量级。不同涂层的Bode相频图 (图7b) 中,ENP/NZSA-5涂层的最大相位角对应频率最高,表明其具有最优的防腐能力。Nyquist图 (图7c) 同样证实,所有涂层的容抗弧相较于裸镁合金都明显提升,说明几种涂层均具有良好的耐蚀能力。其中,ENP/NZSA-5复合涂层的容抗弧最大,说明其能为镁合金提供最好的防护。
图7
图7
AZ31镁合金以及ENP、ENP/NZSA-2与ENP/NZSA-5涂层在3.5%NaCl溶液中的电化学阻抗谱及其等效电路图
Fig.7
Impedance module (a), phase angle (b), Nyquist (c) plots and corresponding logRct and log|Z| f=0.01 Hz (d) for bare magnesium alloy, ENP, ENP/NZSA-2 and ENP/NZSA-5 coatings in 3.5% NaCl solution, and equivalent circuit diagrams for AZ31 Mg-alloy (e) and various coatings (f)
表2 AZ31镁合金以及ENP、ENP/NZSA-2与ENP/NZSA-5涂层的EIS拟合值
Table 2
| Sample | |Z|ƒ=0.01 HzkΩ·cm2 | RsΩ·cm2 | CPEc10-6 S·s n ·cm-2 | RckΩ·cm2 | CPEdl10-7 S·s n ·cm-2 | RctkΩ·cm2 | χ210-3 |
|---|---|---|---|---|---|---|---|
| Mg-alloy | 0. 218 | 32.04 | / | / | 102.80 | 0.32 | 1.53 |
| ENP | 13.54 | 26.26 | 36.22 | 9.65 | 78.41 | 14.85 | 1.85 |
| ENP/NZSA-2 | 39.81 | 25.39 | 3.43 | 14.77 | 76.40 | 35.05 | 3.87 |
| ENP/NZSA-5 | 89.38 | 26.40 | 1.02 | 24.56 | 67.31 | 72.79 | 4.90 |
图8为常温下镁合金和不同涂层在3.5% NaCl溶液中的Tafel曲线以及相应的Ecorr和Icorr值。由图8a可知,与裸镁合金相比,沉积涂层后样品的Ecorr明显正移,Icorr显著降低。根据拟合得到的Tafel参数 (表3),镁合金的Ecorr为-1.55 V,Icorr为7.37×10-5 A·cm-2。化学镀镍后Ecorr正移至-0.61 V,Icorr降低至7.32×10-6 A·cm-2。相较于单一ENP镀镍层,ENP/NZSA-2和ENP/NZSA-5的Ecorr进一步正移至-0.32 V,Icorr降低至8.41×10-7 A·cm-2。Tafel曲线结果同样证实,复合涂层明显提高了对镁合金的保护能力。图8b更直观反映了不同样品间Ecorr和Icorr的差异。显然,不同涂层的腐蚀防护能力按照以下顺序依次降低:ENP/NZSA-5>ENP/NZSA-2>ENP涂层>裸镁合金,与EIS结果一致。
图8
图8
AZ31镁合金和不同涂层在3.5%NaCl溶液中的Tafel曲线及相应的Ecorr和Icorr值
Fig.8
Tafel curves (a) and corresponding Ecorr and Icorr (b) of AZ31 Mg-alloy and various coatings in 3.5% NaCl solution
表3 AZ31镁合金和不同涂层的Tafel参数拟合值
Table 3
| Sample | βa / mV·dec-1 | -βc / mV·dec-1 | Ecorr / V | Icorr / A·cm-2 |
|---|---|---|---|---|
| Mg-alloy | / | 186.30 | -1.55 | 7.37×10-5 |
| ENP | 410.90 | 137.30 | -0.61 | 7.32×10-6 |
| ENP/NZSA-2 | 320.10 | 154.60 | -0.49 | 2.04×10-6 |
| ENP/NZSA-5 | / | 149.00 | -0.32 | 8.41×10-7 |
3 结论
(1) 结合化学沉积、电沉积和动态氢气泡模板法,并利用硬脂酸改性,可以在镁合金表面获得超疏水耐腐蚀的镍基复合涂层。
(2) 电沉积镍电解质中添加ZnO纳米粒子后,Zn会以镍锌合金和纳米颗粒两种形式存在于多孔镍层中。
(3) 当电沉积溶液中ZnO纳米粒子浓度为5.0 g/L时,具有最佳的耐蚀性能和拒液能力。
(4) 与裸镁合金相比,最优复合涂层的腐蚀电位明显正移,腐蚀电流密度和电荷转移电阻分别显著降低和提升。同时,复合涂层对生活中几种常见的溶液均表现出优异的排斥能力,具有良好的超疏水性能。
参考文献
Fabrication of super-hydrophobic surface on AM60 Mg-alloy and its corrosion resistance
[J].
AM60镁合金超疏水表面制备及防腐蚀性能的研究
[J].通过化学刻蚀,以硬脂酸为修饰剂,成功实现AM60镁合金表面的超疏水改性,并采用扫描电镜、接触角仪、电化学工作站等对处理前后的AM60镁合金表面的微观形貌、疏水性能和耐腐蚀性能进行分析。结果表明:AM60镁合金仅经盐酸刻蚀处理后,表现为超亲水性,再经硬脂酸浸泡后才达到疏水的效果;随着硬脂酸浸泡时间的增加,该合金的表面接触角呈现先增加后减小的趋势,在浸泡12 h时,接触角最大为150.18°,滚动角小于10°,此时合金表面具有超疏水性能;同时,相比于未处理的AM60镁合金而言,超疏水改性后样品的腐蚀电流密度降低了88.19%,腐蚀电压提高了19.72%,耐腐蚀性能得到明显改善;而且,超疏水改性还可提高合金对粉尘和水溶液的自清洁性能。
One-pot scalable in situ growth of highly corrosion-resistant MgAl-LDH/MBT composite coating on magnesium alloy under mild conditions
[J].Current corrosion-resistant layered double hydroxide (LDH) coating on Mg alloy is usually in situ grown in autoclave by hydrothermal methods under high temperature and high-pressure conditions, which is unfavorable for industrial application. We report that an inhibitor (2-mercaptobenzothiazole, MBT) incorporated composite (MgAl-LDH/MBT) coating can be in situ deposited on bare AZ31 Mg alloy surface with the assistance of a chelating agent (ethylenediaminetetraacetic acid) under a relatively low temperature (95 °C) and ambient pressure by a one-pot method. The successful formation of LDH/MBT composite coating is confirmed by a series of characterizations, such as X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), and energy dispersive spectroscopy (EDS). The corrosion resistance of the composite coating is evaluated by means of hydrogen evolution measurement, electrochemical impedance spectroscopy (EIS), Tafel polarization curves, and neutral salt spray test. The tests show that the LDH/MBT composite coating has a very low corrosion current density (1.73 10-8 A cm-2), an extremely high charge transfer resistance (2.336 MΩ cm2), and does not show any corrosion pits even after 15 d of exposure to a NaCl solution or 7 d of exposure to salt fog environment, manifesting the good and robust corrosion protection. Lastly, the deposition and corrosion protection mechanisms of the MgAl-LDH/MBT composite coating are also discussed and proposed based on the EDS characterization of the coating after long-time exposure.
Electrochemical deposition and characterization of layered double hydroxide film on magnesium alloys
[J].
镁合金表面层状双氢氧化物的电化学沉积和表征
[J].报道了利用电化学沉积直接在AZ31镁合金基底上制备镁铝层状双氢氧化物 (MgAl-LDH) 的方法。通过XRD,FT-IR,SEM,EIS和Tafel极化曲线表征涂层的表面形貌和耐腐蚀性能。结果表明,与镁合金基体相比,优化工艺下制得的涂层在3.5% (质量分数) NaCl溶液中具有良好的耐腐蚀性,可以有效地保护镁合金免受腐蚀。涂层的阻抗模值相比于镁合金基体增加了2个数量级,自腐蚀电位提高了0.96 V,腐蚀电流密度降低了3个数量级。结果显示通过简单的电化学沉积可以在镁合金表面形成具有良好耐腐蚀性能的MgAl-LDH涂层。
Nickel interlayer enables indirect corrosion protection of magnesium alloy by photoelectrochemical cathodic protection
[J].
Self-cleaning and self-healing protective coating on magnesium alloy
[J].
镁合金表面自清洁、自修复防护膜研究
[J].
simple and non-toxic oxalate pretreatment and its corrosion protection
[J].
Pretreatment-free Ni-P plating on magnesium alloy at low temperatures
[J].
Electroless Ni-Cu-P/Ni-P composite coatings on magnesium alloys and anti-corrosion mechanisms
[J].
镁合金化学镀Ni-Cu-P/Ni-P复合镀层及腐蚀防护机理研究
[J].
A nickel-underlayer/LDH-midlayer/siloxane-toplayer composite coating for inhibiting galvanic corrosion between Ni layer and Mg alloy
[J].
Superhydrophobic coatings for corrosion protection of magnesium alloys
[J].Magnesium (Mg) alloys show great potential to be extensively applied in practice owing to their superior properties, while the poor corrosion resistance does undoubtedly restrict their applications. Superhydrophobic coatings with good repellency to corrosive solutions can significantly decrease the interaction between the corrosive species and the substrate, so that they are receiving a lot of attention to improve the corrosion resistance of Mg alloys. Various strategies have been introduced to develop a superhydrophobic coating on Mg alloys, which were reviewed to elucidate the current research status to provide a clue or thinking for beginning researchers. Further, the existing issues of superhydrophobic coating were discussed, especially for their real applications in practice, mainly owing to their poor mechanical stability. Based on the existing issues, the future study was discussed to improve the stability of hierarchical structures and entrapped air pockets, impart the superhydrophobic coating self-healing property to repair the damaged area during service, provide double protection by incorporation of corrosion inhibitors, or even introduce slippery liquid-infused porous surfaces with lubricant layer to provide better corrosion protection for Mg alloys.
Icephobic/anti-icing properties of superhydrophobic surfaces
[J].
Simple and rapid fabrication and properties of superhydrophobic coatings on the surface
[J].
简易快速的表面超疏水涂层制备及其性能
[J].
Corrosion-resistant and superhydrophobic nickel-phosphorus/nickel/PFDTMS triple-layer coating on magnesium alloy
[J].
Superhydrophobic paraloid B72
[J].
Enhanced corrosion resistance and self-cleaning properties of superhydrophobic nickel coating fabricated by one-step electrodeposition
[J].
Hydrogen bubble-templated electrodeposition of superhydrophobic Zn-Ni films
[J].The superhydrophobic Zn–Ni films without modification prepared using a bubble dynamic template exhibited excellent self-cleaning properties, corrosion resistance, and against water jet impact, which would expand the application range of Mg alloys.
Fabricating a slippery porous nickel coating based on the hydrogen bubble template method for corrosion protection with self-healing property
[J].
Porous oxide electrocatalysts for oxygen evolution reaction prepared through a combination of hydrogen bubble templated deposition, oxidation and galvanic displacement steps
[J].
Three-dimensional dendritic Cu-Co-P electrode by one-step electrodeposition on a hydrogen bubble template for hydrogen evolution reaction
[J].
Electrochemical behavior of Ni-Cu foams fabricated by dynamic hydrogen bubble template electrodeposition used for energy applications
[J].
Research for electrodeposited superhydrophobic Ni-W-WS2 coating and its anticorrosion and wear resistance
[J].
Superhydrophobic and corrosion resistant properties of electrodeposited Ni-TiO2/TMPSi nanocomposite coating
[J].
Superhydrophobic surface by SiO2 particle modified SiO2-Ni/a-C: H film deposition and superior corrosion protection
[J].
Stearic acid modified porous nickel-based coating on magnesium alloy AZ31 for high superhydrophobicity and corrosion resistance
[J].
Effect of choline chloride on electrodeposited superhydrophobic nickel film and the corrosion protection application
[J].
Synthesis of flower-like micro/nano ZnO superhydrophobic surfaces: additive effect optimization via designed experiments
[J].
Stearic acid modified zinc nano-coatings with superhydrophobicity and enhanced antifouling performance
[J].
Fabrication of eco-friendly graphene-based superhydrophobic coating on steel substrate and its corrosion resistance, chemical and mechanical stability
[J].Superhydrophobic coatings were successfully fabricated on steel substrates using potentiostatic electrodeposition of Ni and Ni-graphene, Ni-G, coatings followed by immersion in an ethanolic solution of stearic acid, SA. Rice straw, an environmentally friendly biomass resource, was used to synthesize high-quality graphene. The Raman spectra proved the high quality of the produced graphene. The Fourier transform infrared spectroscopy, FTIR, results showed that the Ni coating grafted with stearic acid, Ni-SA, and the Ni-G composite grafted with stearic acid, Ni-G-SA, were successfully deposited on the steel substrate. The scanning electron microscope, SEM, results showed that the prepared superhydrophobic coatings exhibit micro-nano structures. The wettability results revealed that the values of contact angles, CAs, for Ni-SA and Ni-G-SA coatings are 155.7° and 161.4°, while the values of sliding angles, SAs, for both coatings are 4.0° and 1.0°, respectively. The corrosion resistance, chemical stability, and mechanical abrasion resistance of the Ni-G-SA coating were found to be greater than those of the Ni-SA coating.© 2022. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Calcium stearate nanoparticles as building blocks for mechanically durable superhydrophobic coatings
[J].
Effect of additives on the corrosion mechanism of nanocrystalline zinc-nickel alloys in an alkaline bath
[J].
Silica nanoparticles enhanced polysiloxane-modified nickel-based coatings on Mg alloy for robust superhydrophobicity and high corrosion resistance
[J].
Superhydrophobic surface and lubricant-infused surface: implementing two extremes on electrodeposited Ni-TiO2 surface to drive optimal wettability regimes for droplets' multifunctional behaviors
[J].
Quantification of zinc atoms in a surface alloy on copper in an industrial-type methanol synthesis catalyst
[J].
Template-free electrodeposition of ultra-high adhesive superhydrophobic Zn/Zn stearate coating with ordered hierarchical structure from deep eutectic solvent
[J].
Re-oxidation of ZnO clusters grown on HOPG
[J].This article studies the chemical interaction between ZnO and highly oriented pyrolytic graphite for as grown and thermally treated samples. In-situ X-ray photoelectron spectroscopy and ex-situ Raman spectroscopy confirm that graphite is affected by these processes, becoming oxidized and defective only in the presence of ZnO clusters that become recrystallized upon thermal re-oxidation processes performed at 400 °C. By comparing these results with other identical experiments performed with ZnO clusters grown on graphene and even with CoO clusters grown on graphite, the present results show how the interaction of the ZnO clusters with graphitic substrates depend on two factors—firstly, the mode of growth and corresponding morphology, and secondly, the reactivity of the graphitic substrates, either graphene or graphite. The results presented here will help us understand the fundamental interactions in ZnO/graphitic heterostructures and to define their operating limits.
XPS study of the surface chemistry of conventional hot-dip galvanised pure Zn, galvanneal and Zn-Al alloy coatings on steel
[J].
Synthesis of superhydrophobic surfaces with Wenzel and Cassie-Baxter state: experimental evidence and theoretical insight
[J].
Facile fabrication of epoxy/polybenzoxazine based superhydrophobic coating with enhanced corrosion resistance and high thermal stability
[J].
Super-hydrophobic metal-complex film fabricated electrochemically on copper as a barrier to corrosive medium
[J].
Development of a thiophene derivative modified LDH coating for Mg alloy corrosion protection
[J].
Fluorinated epoxy based superhydrophobic coating with robust self-healing and anticorrosive performances
[J].