双相不锈钢 (DSS) 具有相近比例的奥氏体相和铁素体相显微组织,拥有优异的力学性能和高耐腐蚀性能,被广泛应用于工业设备和海洋结构物中[1~3]。研究表明,相较于标准奥氏体相不锈钢,在中等温度下,双相不锈钢对氯化物具有更高的耐腐蚀性,能提供更高的基体强度[4]。与1.4301奥氏体不锈钢和1.4509铁素体不锈钢相比,1.4062双相不锈钢在海洋条件下显示出优异的耐腐蚀性能,除了经济优势之外,双相不锈钢还具有技术优势,具有更高的机械强度、更低的冷焊倾向、更好的涂层表面性能[5]。然而,在含有氯化物和溶氧量的海洋环境中,双相不锈钢也会不可避免地遭受局部腐蚀,影响船舶设备和海上结构物的安全性和可靠性,对经济和安全造成明显的不利影响[5~8]。
1 实验方法
实验材料为SAF 2304双相不锈钢,浙江青山钢铁公司提供。经直读光谱仪检测,其化学成分为 (质量分数,%) 为:C 0.02, N 0.09, Si 0.37, Mn 1.07, Cr 22.98, Ni 3.65, Cu 0.30, Co 0.12, Mo 0.21, Nb 0.03, S 0.001, P 0.021, Fe余量。
采用D8 ADVANCE型X射线衍射仪 (XRD) 进行物相分析,扫描角度为10°~90°,扫描时间为14 min。试样工作面用水墨砂纸150~2000#砂纸逐级打磨,抛光至镜面且表面无明显划痕,后用50% (体积分数) 氯化铜盐酸酒精腐蚀液在室温下进行侵蚀,约20 s左右。腐蚀完成后用NMM-800RF型金相显微镜 (OM) 和Sirion 200型扫描电镜进行显微组织分析。
电化学测试在AutoLab302电化学工作站上进行,测试材料为SAF2304双相不锈钢,对照材料为碳钢。测试系统采用三相电极体系,其中参比电极 (RE) 是饱和甘汞电极,辅助电极 (CE) 为铂电极,工作电极 (WE) 是待测电极。测试主要包括开路电位、阻抗和极化曲线。将材料通过线切割加工成20 mm×15 mm×3 mm尺寸,一面与铜线连接,采用环氧树脂冷镶封装非工作面。工作面用水磨砂纸150~2000目砂纸逐级打磨,然后抛光成镜面,表面无明显划痕,依次放在丙酮、乙醇溶液中超声清洗10 min,烘干后放在干燥箱中备用。测试溶液为3.5% (质量分数) NaCl溶液。开路电位 (OCP) 数据采集间隔时间为4 h,测试时间6个月。阻抗和极化曲线测试之前先在溶液中浸泡约15 min,待开路电位稳定后开始曲线测量。实验中阻抗频率范围105~10-2 Hz,交流电压幅值为±10 mV,极化曲线扫描速率5 mV/s。为保证实验结果的可靠性,所有电化学实验均重复3次。
近海暴露腐蚀地点为中国东海舟山海域,深度约为10 m,试样为长约30 cm,直径约为1.5 cm的圆柱钢筋。实验之前,将样品吹干并称重,记录质量,样品在海水中分别浸泡1个月、3个月、6个月、12个月后取出干燥后称重,计算增重速率,并在扫描电镜下观察腐蚀产物表面形貌,使用D8 Advance Davinci型X射线衍射 (XRD)、Nicolet 6700型红外光谱仪 (FTIR) 对样品表面的腐蚀产物进行物相分析。然后用20% (体积分数) 硝酸水溶液去除表面腐蚀产物,吹干,称重,计算失重速率,用扫描电镜观察腐蚀形貌。
2 结果与讨论
2.1 显微组织
图1表示SAF 2304双相不锈钢的XRD图谱。从图中可以看出,SAF 2304双相不锈钢主要由奥氏体相和铁素体相组成。
图1
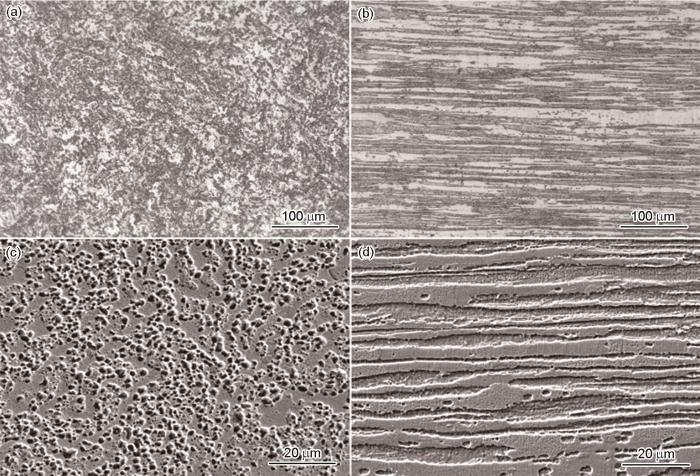
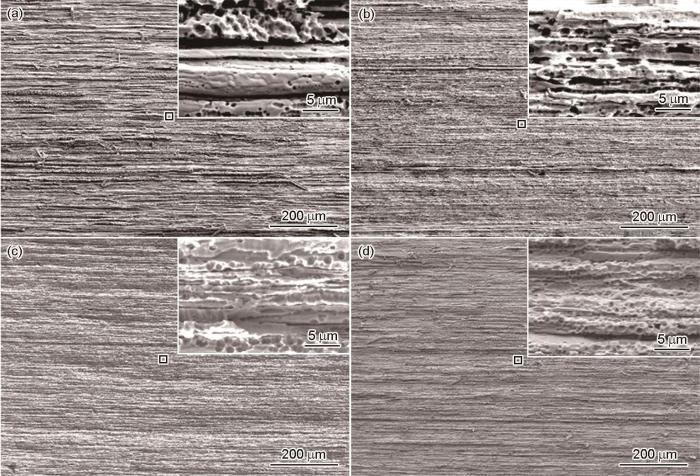
图2是SAF 2304双相不锈钢的横截面和纵截面金相的光学显微组织和SEM图。从图中可以看出铁素体相和奥氏体相各占约50%,其中,在金相组织中较暗的部分为铁素体组织,较亮的部分为奥氏体组织,分别沿着轧制方向呈条状分布。
图2
图2
SAF 2304双相不锈钢横截面和纵截面金相的光学显微组织及表面SEM形貌
Fig.2
Metallographs of cross-section (a) and longitudinal section (b) and SEM images of cross-section (c) and longitudinal section (d) of SAF 2304 duplex stainless steel
2.2 电化学测试
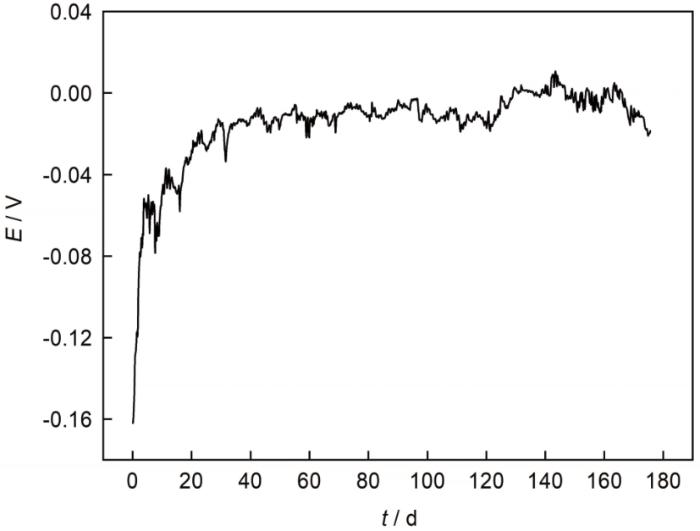
图3为SAF 2304双相不锈钢在常温3.5% NaCl溶液中的开路电位。由图可见,10 d之前,基材表面与溶液发生反应生成钝化膜,钝化膜对基体具有保护作用,使腐蚀电位正移;在10~30 d,钝化膜逐渐破裂,同时又有新的钝化膜生成,腐蚀电位正移速率放缓;30 d之后,腐蚀电位基本不变,钝化膜的生成和破裂处于一个动态平衡中。
图3
图3
SAF 2304双相不锈钢在3.5%NaCl溶液中的开路电位
Fig.3
Open circuit potential of SAF 2304 duplex stainless steel in 3.5%NaCl solution
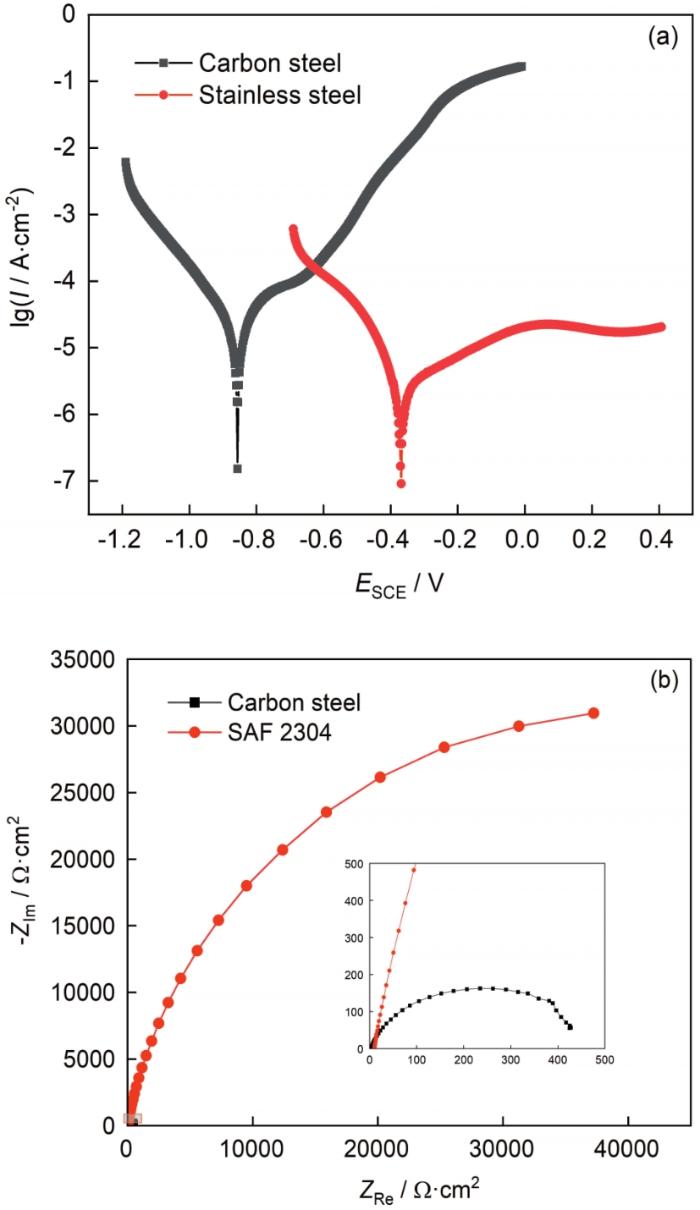
图4a是碳钢和SAF 2304双相不锈钢在3.5% NaCl溶液中动电位极化曲线。碳钢腐蚀电位为-0.857 VSCE,维钝电流密度为87.30 μA∙cm-2,稳定钝化区间较小;SAF 2304双相不锈钢腐蚀电位为-0.369 VSCE,维钝电流密度为18.03 μA‧cm-2,稳定钝化区间大。图4b显示了碳钢和SAF 2304双相不锈钢在3.5% NaCl溶液中的阻抗谱。可以看出,SAF 2304双相不锈钢容抗弧直径远大于碳钢,Nyquist图的弧半径大小反映的是电极表面电子转移过程受到了阻抗,圆弧越大,阻碍作用越大。表明同碳钢相比,SAF 2304双相不锈钢具有较好的钝化能力,生成的钝化膜耐腐蚀性能优异。
图4
图4
碳钢和SAF 2304双相不锈钢在3.5%NaCl溶液中动电位极化曲线及阻抗谱
Fig.4
Polarization curves (a) and EIS results (b) of carbon steel and SAF 2304 duplex stainless steel in 3.5%NaCl solution
2.3 近海暴露实验
图5
图5
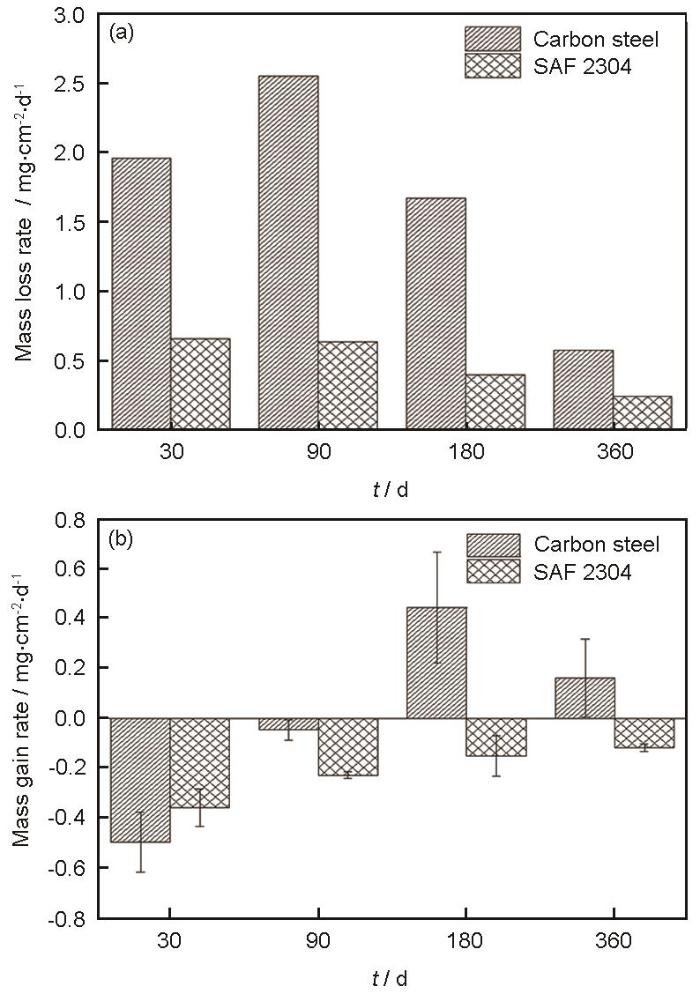
碳钢和SAF 2304双相不锈钢在近海浸泡不同时间后的失重和增重速率
Fig.5
Mass loss rate (a) and mass gain rate (b) of carbon steel and SAF 2304 duplex stainless steel after immersion in Zhoushan offshore
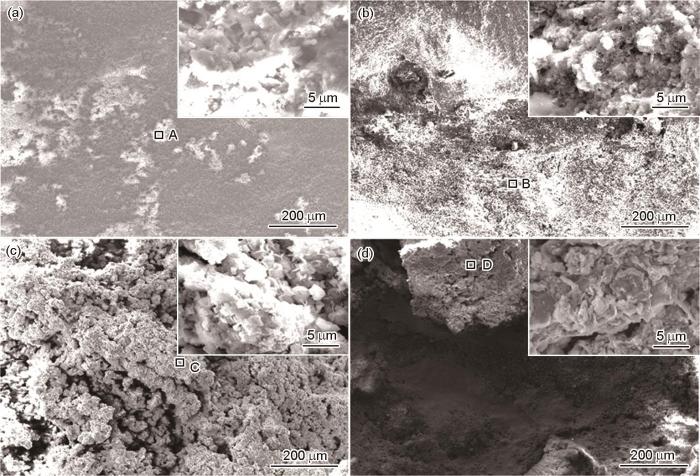
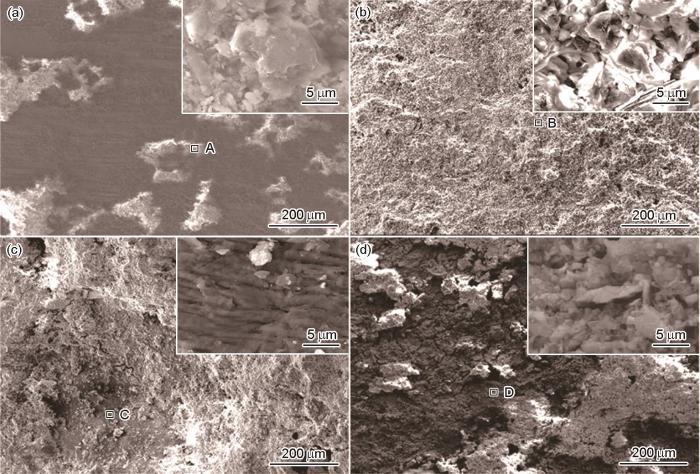
图6是碳钢近海浸泡不同时间后的腐蚀形貌。经过在海水中浸泡后,碳钢试样表面形成了疏松的锈层。随着浸泡实验时间的推移,碳钢表面被黄褐色腐蚀产物所覆盖,基底腐蚀产物为黑褐色,与基体结合较疏松,浸泡一定时间后出现了脱落现象。图7表示SAF 2304双相不锈钢在舟山海域中浸泡不同时间后的腐蚀形貌。同碳钢一样,在舟山海域中浸泡一个月后,表面只覆盖了一部分腐蚀产物,尚有部分基体未被腐蚀。同时注意到,浸泡一个月后未被侵蚀的部分其轧制条纹清晰可见,仍保留原来的形貌,表明腐蚀未深入。随着浸泡时间的延长,表面逐渐完全被腐蚀产物所覆盖,腐蚀产物呈灰白色,附着在SAF 2304双相不锈钢表面,与基体结合较为牢固。碳钢和SAF 2304双相不锈钢表面腐蚀产物膜对应位置的EDS分析结果见表1和2。可知,碳钢和SAF 2304双相不锈钢腐蚀产物主要由Fe、Al、Mg、Si、Ca组成。由表1可知,碳钢浸泡前中期,腐蚀产物中Fe变化较大,表明碳钢钝化膜保护性较差,基体受到侵蚀。通过表2可知,不锈钢暴露6个月内的腐蚀产物主要元素及其对应的含量差别不大;在经过长达12个月的暴露后,不锈钢部分外层腐蚀产物出现剥落,内层腐蚀产物的主要成分为铁氧化物。
图6
图6
碳钢近海浸泡不同时间后的腐蚀形貌
Fig.6
Corrosion morphologies of carbon steel after immersion in Zhoushan offshore with 30 d (a), 90 d (b), 180 d (c) and 360 d (d) exposure time
图7
图7
SAF 2304双相不锈钢近海浸泡不同时间后的腐蚀形貌
Fig.7
Corrosion morphologies of SAF 2304 duplex stainless steel after immersion in Zhoushan offshore with 30 d (a), 90 d (b), 180 d (c) and 360 d (d) exposure time
表1 图6对应位置的表面EDS元素成分分析
Table 1
| Position | C | O | Fe | Si | Na | Cl | Al | Mg | Ca | K | I | S | Cr | Ti |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 11.51 | 65.27 | 2.53 | 4.40 | 0.40 | 0.21 | 1.45 | 2.72 | 10.88 | 0.34 | 0.30 | - | - | - |
| B | 33.52 | 49.17 | 2.84 | 6.26 | 0.19 | - | 2.87 | 1.25 | 2.89 | 0.77 | - | - | 0.13 | 0.11 |
| C | 6.48 | 66.77 | 21.31 | 1.02 | 1.01 | 0.90 | 0.31 | 0.60 | 0.25 | 0.51 | ||||
| D | 46.03 | 27.34 | 14.46 | 3.96 | - | - | 2.35 | 0.45 | - | 1.08 | - | 4.33 | - | - |
表2 图7对应位置的表面EDS元素成分分析
Table 2
| Position | C | O | Fe | Si | Na | Al | Mg | Ca | K | I | Cl | Cr | Sn | Sb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 8.03 | 64.73 | 1.57 | 14.47 | 0.41 | 5.73 | 2.09 | 1.57 | 1.40 | - | - | - | - | |
| B | 16.64 | 59.63 | 0.60 | 4.82 | - | 1.90 | 1.54 | 11.18 | 1.07 | 0.46 | - | 0.57 | 1.58 | |
| C | 32.59 | 55.61 | - | - | 0.40 | 0.21 | - | 10.97 | - | - | - | - | - | |
| D | - | 60.37 | 19.07 | 6.66 | - | 4.19 | 3.41 | 2.69 | 0.77 | - | 2.08 | 0.76 | - | - |
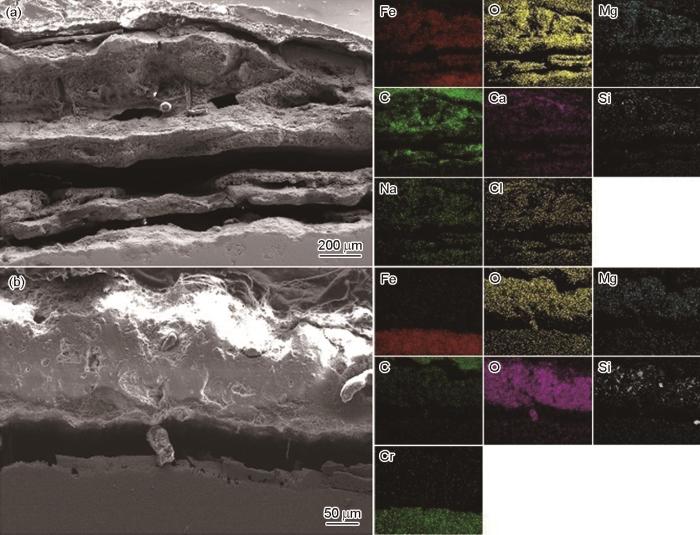
横截面形貌可以更好地揭示腐蚀产物的层状结构。图8为碳钢和SAF 2304双相不锈钢在舟山近海中浸泡12个月后的横截面腐蚀形貌和EDS能谱图。经过长达12个月的海水暴露后,碳钢锈层厚度长达500 μm左右,锈层中存在许多空隙和裂纹,与基体结合较为疏松;SAF 2304的腐蚀产物厚度约为100 μm,腐蚀产物较为紧密。结合EDS能谱面扫分析可知,碳钢腐蚀层中和基体界面检测到Cl-、Na+、Ca2+等离子,表明碳钢锈层无法有效隔离海水。与碳钢不同的是,SAF 2304双相不锈钢腐蚀层中Fe的含量较少,没有检测到Cl-,基体腐蚀氧化程度较低,这可能是由于暴露前期不锈钢表面形成了稳定且保护性较好的金属钝化膜,随着暴露时间的延长,SiO2、Ca2+、Mg2+等沉积在不锈钢表面形成了致密的腐蚀层,有利于阻隔Cl-侵蚀,减缓基体阴极去极化反应,也起到了保护作用。
图8
图8
碳钢和SAF 2304双相不锈钢在舟山近海中浸泡12个月后的横截面腐蚀形貌和EDS能谱图
Fig.8
Cross-section morphologies and EDS of corrosion morphologies formed on carbon steel (a) and SAF 2304 duplex stainless steel (b) after immersion in Zhoushan offshore for 12 months
图9
图9
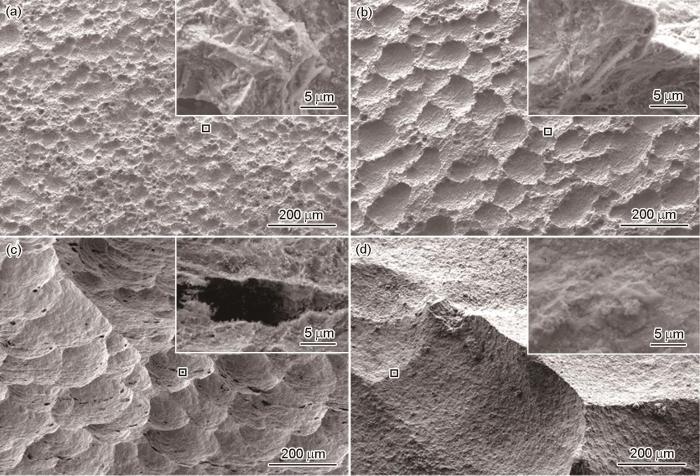
碳钢近海浸泡不同时间后去除腐蚀产物后的表面形貌
Fig.9
Surface morphologies of carbon steel after removing corrosion products after immersion in Zhoushan offshore with 30 d (a), 90 d (b), 180 d (c) and 360 d (d) exposure time
图10
图10
SAF 2304双相不锈钢近海浸泡不同时间后去除腐蚀产物后的表面形貌
Fig.10
Surface morphologies of SAF 2304 duplex stainless steel after removing corrosion products after immersion in Zhoushan offshore with 30 d (a), 90 d (b), 180 d (c) and 360 d (d) exposure time
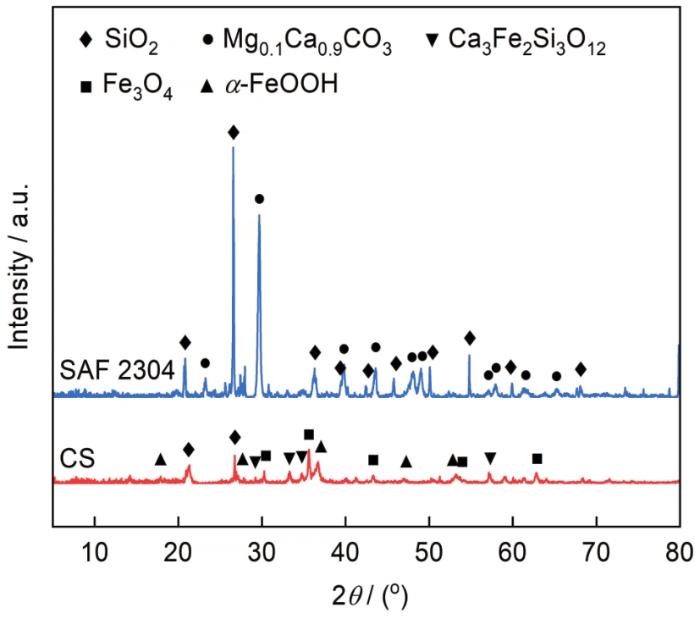
图11是碳钢和SAF 2304双相不锈钢浸泡12个月后的腐蚀产物粉末X射线衍射图。结果表明,碳钢腐蚀产物包括α-FeOOH、Fe3O4、Ca3Fe2Si3O12、SiO2。SAF 2304双相不锈钢主要由SiO2、Mg0.1Ca0.9CO3沉积物组成。
图11
图11
碳钢和SAF 2304双相不锈钢浸泡12个月后的腐蚀产物粉末XRD谱
Fig.11
X-ray diffraction patterns of corrosion products of carbon steel and SAF 2304 duplex stainless steel after immersion for 12 months
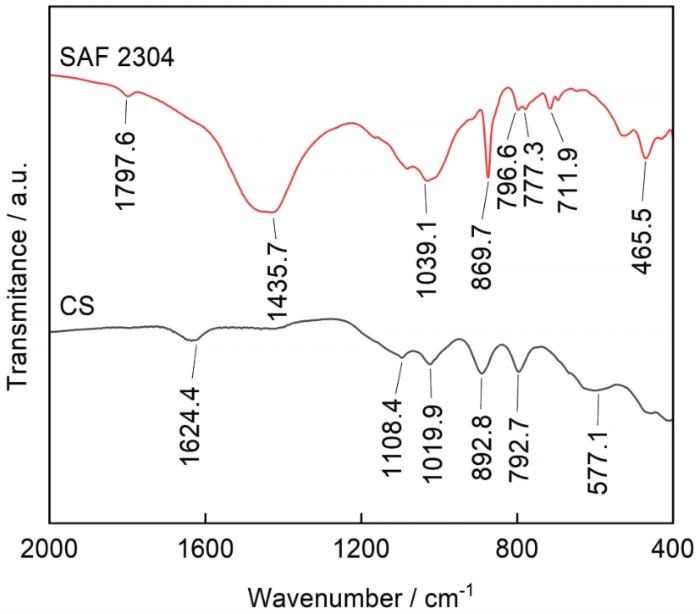
图12显示了碳钢和不锈钢形成的腐蚀产物粉末的FTIR,以进一步确定腐蚀产物的成分。红外光谱的结果与参考光谱进行了比较[26~31]。在碳钢腐蚀产物红外谱图中,577 cm-1处的峰属于磁铁矿Fe3O4,α-FeOOH在892.8和792.7 cm-1处显示了两个较强的谱带,其中792.7 cm-1处的吸收峰对应于α-FeOOH的O-H弯曲。β-FeOOH中Fe-O键的振动导致了1624.4 cm-1附近的吸收带。1019.9和1108.4 cm-1处分别是由γ-FeOOH和δ-FeOOH的O-H弯曲形成。在SAF 2304腐蚀产物红外谱图上,465.5 cm-1处为SiO2 Si-O-Si对称伸缩振动吸收峰,796.6和777.3 cm-1处的特征双峰源自Si-O-Si弯曲振动,双峰表示环中的不同键角。1039.1 cm-1处归属于SiO2 Si-O-Si的不对称伸缩振动吸收峰。711.9 cm-1频率处的吸收峰归属于碳酸根C-O面外弯曲振动,869.7 cm-1频率处的吸收峰归属于碳酸根C-O面内变形振动,1435.7 cm-1频率处吸收峰归属于碳酸根C-O反对称伸缩振动,1797.6 cm-1频率处的吸收峰归属于碳酸根C=O伸缩振动。
图12
图12
碳钢和SAF 2304双相不锈钢浸泡12个月后的腐蚀产物粉末FTIR谱
Fig.12
FTIR spectra of corrosion products of carbon steel and SAF 2304 duplex stainless steel after immersion for 12 months
3 结论
(1) 碳钢的自腐蚀电位为-0.857 VSCE,维钝电流密度为87.30 μA‧cm-2,钝化区间较小,容抗弧半径较小,耐腐蚀性较差。SAF 2304双相不锈钢在3.5%NaCl溶液中的开路电位前期正移,随着浸泡时间的延长逐渐趋于稳定,形成了稳定的钝化膜,自腐蚀电位-0.369 VSCE,维钝电流密度为18.03 μA‧cm-2,钝化区间大,容抗弧半径较大,阻抗较高,耐蚀性能优异。
(2) 近海浸泡试验中,普通碳钢的失重速率远大于SAF 2304双相不锈钢,碳钢腐蚀层中存在空隙和裂纹,没有保护作用。SAF 2304双相不锈钢表面在近海暴露前期形成了致密的金属氧化膜,保护了基体;随着暴露时间的增加,不锈钢氧化膜表面附着了一层较为致密的钙镁沉积层和SiO2,与不锈钢结合紧密,具有一定保护作用。SAF 2304双相不锈钢在近海中的腐蚀形貌表现为均匀腐蚀,具有很好的耐蚀性能。
(3) 通过XRD和IR分析可知,碳钢腐蚀产物中的物质主要为Fe3O4和α-FeOOH以及SiO2,SAF 2304双相不锈钢腐蚀产物主要为海洋污损钙镁沉积层和SiO2。
参考文献
Super duplex stainless steels
[J].
Characteristics and manufacturability of duplex stainless steel: A review
[J].
Duplex stainless steels—Alloys for the 21st century
[J].Duplex stainless steels were first manufactured early in the 20th century, but it was the introduction in the 1970s of the argon-oxygen decarburisation (AOD) steel making process and the addition of nitrogen to these steels, that made the alloys stronger, more weldable and more corrosion resistant. Today, duplex stainless steels can be categorised into four main groups, i.e., “lean”, “standard”, “super”, and “hyper” duplex types. These groups cover a range of compositions and properties, but they all have in common a microstructure consisting of roughly equal proportions of austenite and ferrite, high strength, good toughness and good corrosion resistance, especially to stress corrosion cracking (SCC) compared with similar austenitic stainless steels. Moreover, the development of a duplex stainless-steel microstructure requires lower levels of nickel in the composition than for a corresponding austenitic stainless steel with comparable pitting and crevice corrosion resistance, hence they cost less. This makes duplex stainless steels a very versatile and attractive group of alloys both commercially and technically. There are applications where duplex grades can be used as lower cost through-life options, in preference to coated carbon steels, a range of other stainless steels, and in some cases nickel alloys. This cost benefit is further emphasised if the design engineer can use the higher strength of duplex grades to construct vessels and pipework of lower wall thickness than would be the case if an austenitic grade or nickel alloy was being used. Hence, we find duplex stainless steels are widely used in many industries. In this paper their use in three industrial applications is reviewed, namely marine, heat exchangers, and the chemical and process industries. The corrosion resistance in the relevant fluids is discussed and some case histories highlight both successes and potential problems with duplex alloys in these industries. The paper shows how duplex stainless steels can provide cost-effective solutions in corrosive environments, and why they will be a standard corrosion resistant alloy (CRA) for many industries through the 21st century.
Technical and economical stainless steel alternatives for civil engineering applications
[J].
Long-term corrosion behaviour of stainless steels in marine atmosphere
[J].
Erosion—corrosion of duplex stainless steel under Kuwait marine condition
[J].
Erosion-corrosion behaviour of lean duplex stainless steels in 3.5% NaCl solution
[J].
Effect of hydrostatic pressure on corrosion behavior of X70 steel in simulated sea water
[J].
静水压力对X70钢在海洋环境中腐蚀行为影响研究
[J].利用高温高压反应釜,采用失重、电化学实验和慢应变拉伸方法,结合X射线衍射 (XRD) 、扫描电子显微镜 (SEM) 和能量散射X射线谱 (EDS) 等手段研究了0~3 MPa静水压力对X70钢在模拟海洋环境中的腐蚀行为的影响。结果表明:静水压力在0~2 MPa范围内,X70钢的腐蚀形态表现为局部腐蚀,腐蚀产物主要成分为FeOOH。静水压力为3 MPa时,腐蚀形态倾向于均匀腐蚀,腐蚀产物除FeOOH外,还出现少量的Fe<sub>3</sub>O<sub>4</sub>。随着静水压力的增加,X70钢的腐蚀速率先增加后减小,在2 MPa时达到最大。静水压力在0~2 MPa范围内,X70钢SCC敏感性随着压力增加而增加;继续增加到3 MPa时,SCC敏感性有降低的趋势。X70钢在模拟海洋环境溶液中应力腐蚀开裂敏感性取决于金属表面点蚀的状况,而不一定正相关于静水压力。随静水压力的增加,X70钢表面的阳极溶解被促进,同时也促进更多的氢原子进入钢中,其应力腐蚀开裂机制是由阳极溶解和氢致开裂共同控制的混合机制。
Research progress of corrosion protection of carbon steel in spray zone
[J].
低碳钢在浪花飞溅区的腐蚀防护研究进展
[J].
Effect of Tungsten on the pitting and crevice corrosion resistance of type 25Cr super duplex stainless steels
[J].
Corrosion and passive behaviour of duplex stainless steel 2205 at different cooling rates in a simulated marine-environment solution
[J].
Study on the correlation between passive film and AC corrosion behavior of 2507 super duplex stainless steel in simulated marine environment
[J].
Effect of aging time on the microstructure and corrosion behavior of 2507 super duplex stainless steel in simulated marine environment
[J].
Effect of pH regulation by sulfate-reducing bacteria on corrosion behaviour of duplex stainless steel 2205 in acidic artificial seawater
[J].
Research on initial corrosion behavior of X60 pipeline steel in simulated tidal zone
[J].
X60管线钢在模拟潮差区初期腐蚀行为研究
[J].
2012 Frank newman speller award: cathodic protection of offshore structures—history and current status
[J].
Research on the corrosion behavior of cast duplex stainless steel in seawater environment
[J].
海水环境中铸造双相不锈钢的腐蚀行为研究
[J].
Investigation of microorganisms in corrosion product scales on Q235 carbon steel exposed to tidal-and full immersion zone at Qindao-and Sanya-sea waters
[J].
不同海域、不同腐蚀区带Q235碳钢实海挂片腐蚀产物层内微生物调查
[J].
Early corrosion behavior of EH36 ship plate steel in tropical marine atmosphere
[J].
热带海洋大气环境中EH36船板钢早期腐蚀行为研究
[J].在高湿、高热、高盐度和强辐照的湛江海洋大气腐蚀试验站对EH36船板钢进行了15、30、90、180和360 d的暴露实验。通过腐蚀失重计算了不同暴露周期的腐蚀速率,采用SEM观察了锈层表面和截面的微观形貌,采用X射线衍射仪分析了锈层的组成成分,采用EDS分析了锈层中的元素分布,同时对暴露后的试样进行了极化曲线测试。结果表明:EH36船板钢的腐蚀速率先增大、后减小;暴露360 d后,Cr、Ni和Si扩散到锈层中,分布较为均匀,提高了钢的耐腐蚀性能;暴露180和360 d的锈层中均含有γ-FeOOH、β-FeOOH、Fe<sub>3</sub>O<sub>4</sub>和α-FeOOH,暴露360 d的锈层中α-FeOOH较多,β-FeOOH较少,锈层中α/γ=0.615,尚未形成稳定的保护性锈层。
In situ marine exposure study on corrosion behaviors of five alloys in coastal waters of western Pacific Ocean
[J].
Long term corrosion characteristics of metallic materials in marine environments
[J].
Fracture toughness of welded commercial lean duplex stainless steels
[J].
The weldability of duplex stainless-steel in structural components to withstand corrosive marine environments
[J].There is still a considerable gap in the definition of the weldability of Duplex Stainless Steel (DSS). A lack of clarity that is explained by the standard specification of the maximum content of equivalent carbon that defines a “weldable” steel coupled with the fact that the alloying elements of DSS exceed this defined limit of weldability. In this paper, welding quality in an inert environment and in presence of chlorides is analyzed with the aim of defining optimum welding conditions of 2001, 2304, and 2205 DSS. The same procedure is followed for a hybrid weld between DSS 2205 and a low carbon mild steel, S275JR. As main output, this study defined the optimal welding conditions with tungsten inert gas without filler for each type of DSS weld that showed excellent anti-corrosion performance, with the exception of the DSS 2205-S275JR weld where widespread corrosion was observed. Additionally, this study established a relationship between the thermal input during welding and the content of alloying elements in defect-free joints. Furthermore, it demonstrated that an increase in ferrite content did not lead to a worse corrosion resistance, as expected after passivation.
Use of 2304 stainless steel reinforcement in Hong Kong-Zhuhai-Macau bridge—corrosion behaviors of 2304 stainless steel reinforcement
[J].
2304不锈钢钢筋在港珠澳大桥的应用—钢筋耐蚀性能研究
[J].
Corrosion behavior and regularities of two stainless steels in seawater environment of different harbors
[J].
两种不锈钢在港口海水环境中的腐蚀行为和规律研究
[J].
Identification of silicooxygen rings in SiO2 based on IR spectra
[J].
Rust evolution and electrochemical properties of field-exposed carbon steel in a tropical marine environment
[J].
Study on characteristics of two-dimensional diffuse reflectance infrared spectrum of silicon dioxide
[J].
二氧化硅二维漫反射红外光谱特征研究
[J].
Influence of seawater on the carbon steel initial corrosion behavior
[J].
Study on third-step infrared spectroscopy of calcium carbonate
[J].
碳酸钙三级红外光谱研究
[J].