传统渗铝温度一般为850~1050 ℃,王正等[7]在950 ℃采用料浆渗铝法在304不锈钢表面进行渗铝,并获得质量较好的渗铝层。由向群和于春兰[8]以20、T10钢为基体,采用料浆渗铝法在950 ℃下制备得到连续、均匀的渗铝层。低Cr马氏体钢回火温度约为760 ℃,高于回火温度渗铝会导致材料力学性能降低。因此,低温渗铝对低Cr马氏体钢高温防护有重要意义。目前已有文献报道这类钢的低温渗铝工艺及渗层抗高温氧化性能。董猛等[9]以G115和T92钢为基体,采用粉末包埋法在650 ℃下制备了FeAl渗层。Agüero等[10]以P92钢为基体,采用料浆渗铝法在700 ℃下制备了致密、连续的渗铝层。但上述研究主要聚焦于渗铝工艺和渗铝层抗氧化性能研究,很少从热力学和动力学角度对低温料浆渗层中各相分布及分层进行机理探讨。
本实验以T92钢作为基体,采用料浆渗铝法在低于T92回火温度的700 ℃下进行了渗铝。对渗铝层截面形貌、成分进行了表征,并以动力学、热力学为基础,从理论上探讨了渗铝层的扩散及生长过程机理。
1 实验方法
基材T92钢的化学成分 (质量分数,%) 为:Cr 10.17,W 1.07,Fe余量。经线切割成11.5 mm×8.5 mm×3.5 mm块状试样,表面经SiC砂纸从80#逐级打磨至400#,在酒精中超声清洗除油。将Al粉 (20%,质量分数),FeAl粉 (18%),Al(OH)3 (30%),NH4Cl (2%),H3PO4 (30%) 混合均匀并加入去离子水配制成具有一定粘稠度的料浆。采用空气喷涂方法将料浆均匀喷涂于待渗试样表面,厚度约80 μm。将喷涂好料浆的试样在室温干燥后置于烘箱内80~90 ℃烘干15 min,再在马弗炉内300 ℃固化0.5 h。将固化好的试样置于管式炉内,在Ar气保护下以5 ℃/min加热至700 ℃后保温至设定时间后随炉冷却即完成渗铝。
采用Nova Nano SEM450型扫描电镜 (SEM) 与自带的能谱仪 (EDS) 观察和分析渗铝层截面形貌和成分,采用D8ADVANCE型X射线衍射仪 (XRD) 分析试样表面渗层物相组成,靶材选用Cu靶,工作电压40 kV,工作电流40 mA,扫描角度20°~80°。
2 结果与讨论
2.1 保温时间对渗铝层形貌与厚度的影响
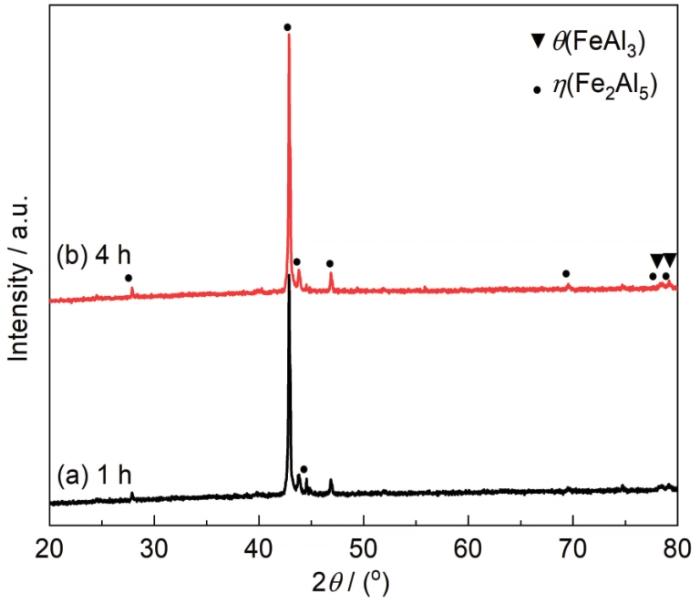
图1为700 ℃不同保温时间渗铝样品的XRD谱。分析可知,渗铝样品表面主要由Fe2Al5 (η) 及少量的FeAl3 (θ) 相组成。
图1
图1
T92钢样品在700 ℃下1和4 h渗铝后的XRD谱
Fig.1
XRD patterns of T92 steel after aluminizing at 700 ℃ for 1 and 4 h
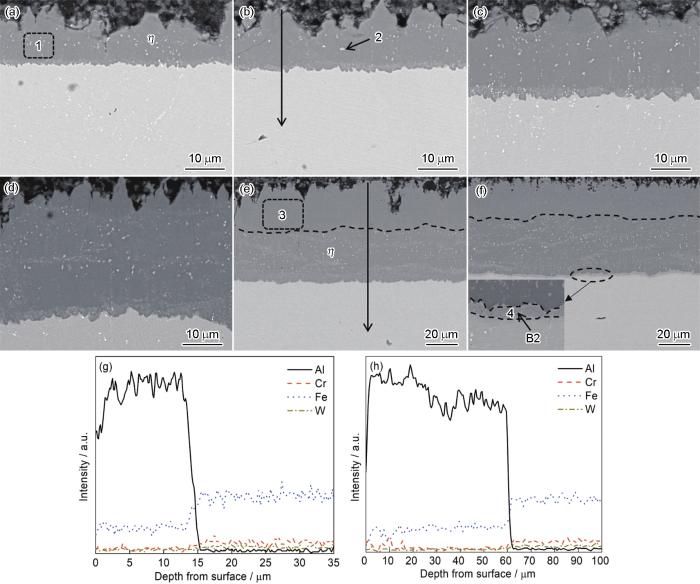
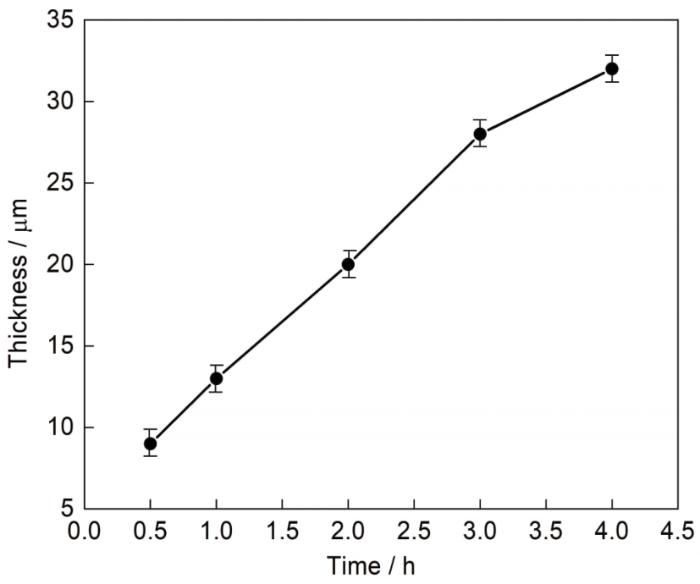
图2为700 ℃下保温不同时间获得的渗铝层截面形貌图及相应线扫描图。从图可知,渗铝层呈锯齿状嵌入基体,可增加渗铝层与基体的结合力。表1为渗铝层中标记区域元素原子含量百分比。在渗铝的前3 h,活性Al原子 ([Al]) 内扩散生成η相。随后渗铝层靠近基体一侧生成不连续的FeAl (B2),但厚度很薄,10 h后渗铝层中可以清晰地观察到连续的B2相层。此时,渗铝层的可观察到由η和θ混合相组成的较厚外层[11]。在η和θ混合相组成的外层中不含有白色析出相,通过对白色析出相EDS检测发现,白色析出相中Cr、W含量较渗层与基体高出许多。外层不含W含量相对较高的白色析出相,这是由于W向外扩散需通过基体中的金属Fe、Cr形成的壁垒,在700 ℃时可以认为金属W基本不向外扩散[12]。据此可以推断外层是由Fe向外扩散形成。[Al]内扩散距离随时间变化动力学曲线如图3所示。由图可知,[Al]内扩散速度生成η相随保温时间的延长逐渐减小。保温4 h与保温10 h的渗铝层形貌和厚度基本相同,因此,在渗铝温度为700 ℃料浆层厚度约80 μm保温4 h即可获得理想厚度的渗层。
图2
图2
T92钢在700 ℃下不同时间渗铝后的截面形貌及相应元素分布
Fig.2
Cross-sectional morphologies of T92 steel after aluminizing at 700 ℃ for 0.5 h (a), 1 h (b), 2 h (c), 3 h (d), 4 h (e) and 10 h (f), and EDS analysis results for 1 h (g) and 4 h (h) aluminized samples
表1 图2中标记区域的元素成分分析结果
Table 1
| Area | Fe | Al | Cr | W |
|---|---|---|---|---|
| 1 | 26.14 | 70.32 | 3.07 | 0.47 |
| 2 | 10.28 | 34.76 | 50.13 | 4.83 |
| 3 | 22.87 | 74.53 | 2.60 | - |
| 4 | 42.47 | 49.59 | 7.10 | 0.84 |
图3
图3
η相层厚度 (x) 随时间变化曲线
Fig.3
Thickness (x) of η phase layer as a function of time
2.2 渗铝动力学分析
样品表面生成渗铝层后,渗铝层增厚需要由Fe向外扩散或[Al]向内扩散实现,因此,渗铝层增厚速度由Fe、[Al]通过渗层的扩散控制,该扩散属于非稳态扩散[13],根据Fick定律有:
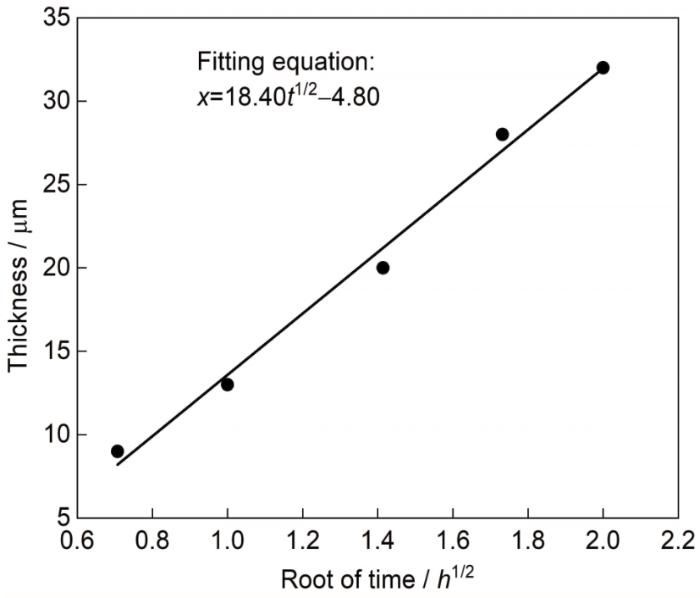
x为[Al]内扩散形成的η相层厚度,t为保温时间,两边取平方根,得到:
渗铝层厚度x正比于t1/2,其中k2=k11/2。
图4
图4
渗铝层中η相层厚度 (x) 随时间的平方根变化曲线
Fig.4
Thickness (x) of η phase layer in the aluminized coating as a function of square root of time
从拟合结果可知,料浆渗铝符合非稳态扩散机制;截距为-4.80,则表明进行活化反应形成[Al]需要一定时间,这段时间与渗剂中生成气相铝化物有关。
如果料浆中Al含量较高,可将Al原子扩散看作是在半无限长物体中的扩散,根据Fick第二定律有:
根据上式可求出Al在基材中的扩散系数。其中,C0为[Al]在距原始界面x处的扩散浓度,D为扩散系数。根据Fick第二定律的Grube方程[14]将
式中,C0为原始界面处[Al]浓度,C为扩散偶中[Al]浓度,C1为渗铝层与基体界面处的[Al]浓度,在原始界面处可认为C0=1[15],C1=0,将C0、C1代入
C在最远处可认为不存在[Al]与C1等同[16],对
可以得到:
通过
2.3 渗铝热力学分析
根据Gibbs函数中的ΔG大小和正负可判断反应发生的方向与倾向,根据Gibbs函数定义:
式中,ΔH为相变热,T为反应温度,ΔS为熵变。
由于实验过程为等压过程,ΔH可根据热容 (Cp,m) 和kirchhoff公式计算:
式中,ΔH为焓变,Qp为反应吸收热,
对
表2是根据热力学数据库制作的渗层中各相热力学数据,其中
| Substance | ||
|---|---|---|
| Fe | 0 | 27.15 |
| Al | 0 | 28.33 |
| B2 | -48483.14 | 50.74 |
| η | -201636.29 | 153.61 |
| θ | -111368.88 | 95.29 |
铁铝化合物可用反应式xFe+yAl→Fe x Al y 表示,反应相变ΔH相等于生成物的焓变 (ΔH生) 减去反应物的焓变 (ΔH反),即:
其中,ΔH相为整个反应过程的焓变、ΔH生为生成物的焓变、ΔH反为反应物的焓变,当298 K<T<933 K时,处于Al的熔点以下,联合式 (
联合式 (
表3 Fe和Al反应摩尔相变焓与温度的关系式
Table 3
| Reaction | T / K | ΔrH相 / (J·mol-1) (933-1298 K) |
|---|---|---|
| Fe+Al→B2 | 298-933 | -48483.14-11.20(T-298)-0.0338(T 2-2982) |
| 933-1298 | -58950.14-11.20(T-298)-0.0338(T 2-2982) | |
| 2Fe+5Al→η | 298-933 | -201636.29-109.17(T-298)-0.1152(T 2-2982) |
| 933-1298 | -253971.29-109.17(T-298)-0.1152(T 2-2982) | |
| Fe+3Al→θ | 298-933 | -111368.88-50.74(T-298)-0.0638(T 2-2982) |
| 933-1298 | -142769.88-50.74(T-298)-0.0638(T 2-2982) |
根据熵变定义,熵变可由
其中,δQp为等压过程中热量随温度的变化值。
当298 K<T<933 K时,对于等压过程有:
将
对
其中,ΔS相为整个反应过程的熵变、ΔS生生成物的熵变、ΔS反反应物的熵变
等温等压下Al从固态转变为液态时ΔG=0,Al从固态转变为液态时熵变值可由下式求算:
当933 K<T<1298 K,联合
表4 Fe和Al反应的摩尔熵变与温度的关系式
Table 4
| Reaction | T / K | ΔrS相 / (J·mol-1) (933-1298K) |
|---|---|---|
| Fe+Al→B2 | 298-933 | -4.74-11.20lnT / 298-0.0338(T-298) |
| 933-1298 | -4.74-11.20lnT / 298-0.0338(T-298)-10467 / T | |
| 2Fe+5Al→η | 298-933 | -42.34-109.17lnT / 298-0.1152(T-298) |
| 933-1298 | -42.34-109.17lnT / 298-0.1152(T-298)-52335 / T | |
| Fe+3Al→θ | 298-933 | -16.85-50.74lnT / 298-0.0638(T-298) |
| 933-1298 | -16.85-50.74lnT / 298-0.0638(T-298)-31401 / Τ |
相变反应Gibbs自由能可由下式表示:
根据式 (
表5 Fe和Al反应的摩尔Gibbs自由能变化与温度的关系式
Table 5
| Reaction | ΔrG相 / (J·mol-1) (298-1298 K) |
|---|---|
| Fe+Al→B2 | -48483.14-21.2724(T-298)+4.74+11.20TlnT / 298 |
| 2Fe+5Al→η | -201636.29-143.4996(T-298)+42.34+109.17TlnT / 298 |
| Fe+3Al→θ | -111368.88-69.7524(T-298)+16.85+50.74TlnT / 298 |
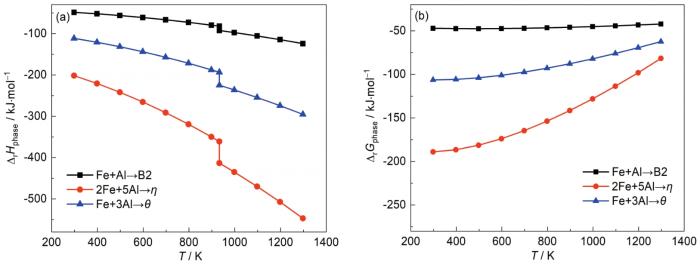
图5是通过式表3和5中的相变表达式及Gibbs自由能变化表达式绘制的铁铝化合物的ΔrH相-T和ΔrG相-T图,B2计算结果差距与文献报道的不大,而η、θ差距较大[23,24],这是由于相关文献中并没有如上文所示对热容积分求算,只通过标准状态下
图5
图5
不同Fe-Al化合反应的ΔrH相-T
Fig.5
ΔrHphase-T (a) and ΔrGphase-T (b) curves for the different reactions of Fe and Al
2.4 渗铝扩散机制分析
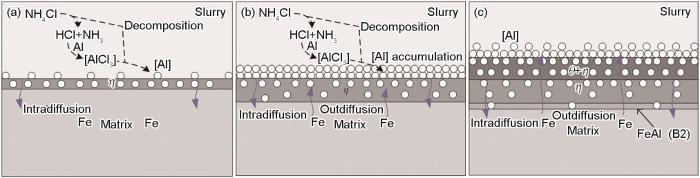
图6
图6
T92钢渗铝层的生长模型
Fig.6
Growth model of aluminizing coating on T92 steel: (a) VD>VP, (b) VD=VP, (c) VD<VP
由ΔG计算得到相变反应驱动力B2<θ<η,可将渗铝层的形成分为3个阶段:首先如图6a所示,在渗铝的初始阶段,经气相反应形成的[Al]进入基体与Fe反应在样品表面生成η相,此时,固态扩散速度 (VD)>气相反应速度 (VP),固态扩散速度较快,[Al]不会在原始表面堆积,致使Fe难以向外扩散,无外生长层的形成,渗铝由生成[Al]的气相反应控制;然后如图6b所示,样品表面的[Al]进入基体需要通过第一阶段形成的η相层向内扩散,随着η相增厚,导致能进入基体的[Al]开始逐渐减少,此时,η相层依然在增厚,但由于[Al]需通过渗铝层扩散速度降低,致使[Al]生成速度与扩散速度逐渐相近,直至VD=VP,此阶段反应由生成[Al]的气相反应和固态扩散同时控制;最后如图6c所示,原始界面处的[Al]进入基体的阻力进一步增大,η相层的增厚速度减慢,因为生成[Al]的速度基本不变,所以VD<VP,反应由固态扩散控制,[Al]在表面堆积,此时,Fe依然会以较快的速度向外扩散形成η和θ混合相外层,而[Al]进入基体的扩散阻力进一步增大,到达渗铝层与基体界面处的[Al]进一步减少,形成了Al含量低的B2相内层。
3 结论
(1) 保温时间对渗铝层形貌和厚度有显著影响。渗铝的前3 h,[Al]内扩散形成Fe2Al5 (η) 相;随后[Al]开始在料浆层与渗铝层界面堆积,此时,Fe以较快的速度向外扩散并形成η和θ混合相外层;10 h后,在渗铝层内部可以清晰的观察到B2相层。
(2) 随着保温时间的延长,渗铝层厚度增加,T92钢中700 ℃下[Al]在的扩散系数为1.87
(3) 对Gibbs自由能 (ΔG) 的计算发现在[Al]充足的情况下,渗铝层中形成Fe-Al金属间化合物的反应驱动力按照B2<θ<η依次增加,Gibbs自由能 (ΔG) 的计算结果能很好的解释渗铝层中相分布情况。
参考文献
Research status of main steam pipe candidate material characteristics and service performance for 700 ℃ ultra supercritical unit
[J].
700 ℃超超临界机组主蒸汽管道候选材料特性与服役性能研究现状
[J].
Long exposure steam oxidation testing and mechanical properties of slurry aluminide coatings for steam turbine components
[J].
The formation, microstructure and hot corrosion behaviour of slurry aluminide coating modified by Ni/Ni-Co electrodeposited layer on Ni-base superalloy
[J].
Reactive element modified chemical vapor deposition low activity platinum aluminide coatings
[J].
Dynamic behavior of low temperature aluminizing coating on T91 steel surface
[J].
T91钢表面低温渗层的动力学行为
[J].研究了T91钢在渗铝温度区间650~750℃,活化剂添加量2%,渗铝时间2~8 h时的渗铝层形成与生长过程。结果表明:渗铝温度为650℃和700℃时,渗层为单一的FeAl相;当渗铝温度为750℃,渗层为FeAl与少量的FeAl<sub>2</sub>相;渗层在渗铝温度高于700℃时,渗铝层内部出现空洞与裂纹等缺陷,裂纹的产生可能与渗层Al浓度有关。保温时间只对渗铝层厚度产生影响,不会改变渗铝层成分;渗铝层厚度会随着渗剂中的Al含量不同而发生变化。在渗铝温度650℃,保温8 h,活化剂含量2%时,得到厚度约为11.5 μm、致密的FeAl渗层。在650~750℃温度区间渗铝温度(T)、渗铝时间(t)、渗剂Al含量(W)与渗层厚度(h)的关系可由方程式h=77.44W<sup>1/2</sup>t<sup>1/2</sup>exp(-3178.92/T)来描述。
Comparison between field and laboratory steam oxidation testing on aluminide coatings on P92
[J].
The influence of slurry aluminising on SCC of 304 stainless steel
[J].
料浆渗铝对304不锈钢应力腐蚀开裂的影响
[J].
Study on slurry aluminizing process with refractory clay protective case
[J].
用耐火粘土做保护层的料浆渗铝工艺研究
[J].
Preparation of FeAl penetration layer on G115 and T92 steel surface and its oxidation resistance to high temperature steam
[J].
G115和T92钢表面FeAl渗层制备及其抗高温水蒸汽氧化性能
[J].
Overview of steam oxidation behaviour of Al protective oxide precursor coatings on P92
[J].
The Al-rich part of the Fe-Al phase diagram
[J].
Diffusion of tungsten in α-iron
[J].
Experimental study on aluminizing of carbon steel surface by pack cementation
[J].
碳钢表面粉末包埋法渗铝的实验研究
[J].
Fe and Al atoms diffusion in intermetallic formation
[J].
铁铝原子在金属间化合物形成中的扩散
[J].
Preparation method and diffusion mechanism of Fe-Al coating on Q235 Low Carbon steel by Pack aluminizing
[J].The Fe-Al coating, with compactness, stiffness, and continuity, could be prepared on Q235 low carbon steel by pack aluminizing. The phase structure, morphology, composition, and hardness of the prepared coating were characterized by XRD, SEM, EDS, and micro-hardness tester respectively. Results indicate that the Fe-Al coating is composed of Fe2Al5 and FeAl3 phases, whilst, the coating fabricated at 750℃ is particularly rich in Fe2Al5 phase. With the rising temperature, the thickness of Fe-Al coating increases, whereas the micro-hardness decreases. As a result of aluminizing for different time, the formed coatings are composed of the two phases Fe2Al5 and FeAl3 as well. However, with the increasing aluminizing time, the content of FeAl3 phase decreases, while the micro-hardness of the coating decreases slightly. Finally, a diffusion mechanism related with the formation of Fe-Al coating is proposed based on the comprehensive analysis on the thermodynamics and kinetics of pack aluminizing process.
包埋渗铝法制备Fe-Al渗层及其扩散机制
[J].包埋渗铝法可在钢基体表面制备出一层致密、坚固、连续的Fe-Al渗层,以改善基体性能。本文在不同温度和不同时间下对Q235低碳钢进行包埋渗铝,形成Fe-Al渗层,采用X射线衍射、扫描电镜及能谱分析等方法研究了渗铝层的物相结构、表面及截面形貌和成分,采用显微硬度仪测量了截面硬度。结果表明,不同渗铝温度下获得的渗铝层,主要含有Fe<sub>2</sub>Al<sub>5</sub>和FeAl<sub>3</sub>两相,且750℃得到的渗层存在较多Fe<sub>2</sub>Al<sub>5</sub>相;随着渗铝温度升高,Fe-Al渗层厚度增加,Al原子扩散系数增大,但显微硬度降低;不同渗铝时间下制备的渗铝层,物相仍以Fe<sub>2</sub>Al<sub>5</sub>和FeAl<sub>3</sub>为主,但随着渗铝时间延长,FeAl<sub>3</sub>含量减少,且Al原子扩散系数变大,渗层显微硬度略有降低。在进一步分析Fe-Al渗层形成的热力学与动力学基础上,总结了渗铝层形成的扩散机制。
Preparation of Fe-Al intermetallic compound layer and its corrosion resistance to seawater
[D].
Fe-Al金属间化合物渗层制备及耐海水腐蚀性能研究
[D].
Steam oxidation of aluminide-coated and uncoated TP347HFG stainless steel under atmospheric and ultra-supercritical steam conditions at 700 ℃
[J].The efficiency of ultra-supercritical (USC) steam power plants is limited by the materials properties, in particular, the steam oxidation resistance of the currently used steels at temperatures higher than 600 °C. Under these conditions, steam oxidation results in the development of thick oxide scales which spall and can accumulate in tube bends leading to blockage, overheating and premature creep rupture, as well as erosion of downstream components such as steam valves and turbine blades. Most published work related to oxidation testing is carried out at atmospheric pressure, with significantly less testing of austenitic steels in supercritical steam, and rarely including protective coatings. Indeed, the effect of high-pressure steam in the oxidation process is not quite understood at present. This paper covers a comparison of the behaviour of TP347HFG stainless steel at 700 °C under atmospheric pressure and 25 MPa, with and without slurry-applied diffusion aluminide coatings. The results show a very protective behaviour of the aluminide coatings, which develop a very thin Al-rich protective oxide, and no significant difference between the two environments. In contrast, the uncoated steel exhibited a different behaviour. Indeed, under atmospheric pressure after 3000 h, very thin scales, rich in Cr and not surpassing 5 to 10 µm in thickness, covered the samples along with some much thicker Fe-rich oxide nodules (up to 150 µm). However, under 25 MPa, a thick multilayer scale with a non-homogeneous thickness oscillating between 10 to 120 µm was present. A microstructural investigation was undertaken on the oxidised uncoated and coated substrates. The results suggest that pressure increases the oxidation rate of the chromia former steels but that the oxidation mechanism remains the same. A mechanism is proposed, including early detachment of the outer growing scales under supercritical pressure.
Heat capacity of η-AlFe (Fe2Al5)
[J].
Heat capacity of Fe-Al intermetallics: B2-FeAl, FeAl2, Fe2Al5 and Fe4Al13
[J].
Study on low temperature aluminizing process of 9Cr-3W-3Co steel and its resistance to steam oxidation
[D].
9Cr-3W-3Co钢低温渗铝工艺及其抗水蒸汽氧化性能研究
[D].
Microstructure analysis of interfacial layer with tungsten inert gas welding-brazing joint of aluminum alloy/stainless steel
[J].
铝合金/不锈钢钨极氩弧熔-钎焊接头界面层的微观结构分析
[J].运用OM, SEM和EDS分析了铝合金/不锈钢TIG熔-钎焊接头界面层的结构特征, 并通过微压痕和SEM原位拉伸实验测试了其力学性能. 研究结果表明: 界面处形成了厚度不均一的锯齿状金属间化合物层, 厚度为4-9 μm, 满足界面层的要求(≤10 μm); 界面反应层包括两类化合物层, 即焊缝一侧的τ<sub>5</sub>层和钢基体一侧θ+η+τ<sub>5</sub>层, 在界面处首先形成τ<sub>5</sub>相, 抑制了粗大枝晶状θ+η二元相的生长. 微压痕测试得出: τ<sub>5</sub>层平均硬度值为HV1025, θ+η+τ<sub>5</sub>层硬度值为HV835. τ<sub>5</sub>层压痕处产生裂纹, 表明τ<sub>5</sub>相是一种硬脆相. SEM原位拉伸实验 显示, 界面层起裂于θ+η相, 在外力作用下沿θ+η+τ<sub>5</sub>层迅速开裂, 界面层抗拉强度达到120 MPa.
Experimental and theoretical research on the corrosion resistance of ferrous alloys in aluminum melts
[J].