近几十年来,导电聚合物在防腐涂料中的应用越来越受到关注,多种导电聚合物已被用于提高有机涂层的防腐性能[1],例如聚苯胺 (PANI)[2,3]、聚吡咯 (PPY)[4]、聚噻吩[5]等。聚苯胺除了成本低廉、结构多样、环境稳定性良好等优点外[6],其导电性和独特的氧化还原可逆性促使金属表面钝化是提高涂层耐蚀能力的直接原因[7]。Wessling[8]在Fe表面涂覆PANI,促进了铁表面钝化膜的生成。Liu等[9]将添加不同掺杂聚苯胺 (PANI-SSA、PANI-TSA、PANI-H3PO4) 的3种有机涂层分别刷涂在AZ91D镁合金上服役120 d,涂层防护效果均有变化,并观察到了厚度为1.5 μm的连续致密氧化膜层。刘素云[10]通过研究认为,将磺基水杨酸掺杂聚苯胺 (PANI-SSA) 掺入有机涂层后,在3.5% (质量分数) NaCl溶液中浸泡150 d,可诱导5083铝合金生成约300 nm厚的致密保护膜。然而,引入PANI同时带来的问题是有机涂层致密性的降低:由于PANI分子链骨架刚性强,因此存在与环氧树脂相容性差、二者结合界面不紧密的问题。虽然有研究表明在PANI链上接枝烷基或者烷氧基可实现与环氧树脂更好的复合[11-13],但该接枝点所占N原子会导致PANI导电性的下降,进而降低其促进钝化的能力。
综上所述,本实验提出采用乳液聚合手段一步实现超疏水空心球状聚苯胺胶囊的合成方法,其中磺基水杨酸 (SSA) 作为掺杂酸,十二烷基苯磺酸钠 (SDBS) 作为表面活性剂,过硫酸铵 (APS) 用作氧化剂,乙基苯作为油相溶剂,并探究其在不同表面活性剂浓度下粉末的形貌结构、疏水性。讨论了该聚苯胺粉末对涂层致密性及耐蚀性的影响。
1 实验方法
实验所用铝合金为5083 H112 (明泰铝业),其主要化学成分为 (质量分数,%):Mn 0.5,Mg 4.0,Cr 0.05,Si 0.40,Fe 0.40,Cu 0.10,Al余量。
首先称取1 mmol磺基水杨酸掺杂酸溶于20 mL去离子水中,再分别加入质量125、175、225和275 mg的表面活性剂十二烷基苯磺酸钠形成水相,由5 mmol苯胺溶于2.5 mL乙基苯组成油相部分,随后将两相混合,通过超声波细胞破碎仪进行乳化4 min,并加入一定浓度的聚乙烯醇水溶液作为乳液稳定剂增加体系粘度,减缓油相液滴凝聚速度。最后加入过硫酸铵 (n(ANI)∶n(APS)=1∶1) 氧化剂反应24 h,将反应产物用去离子水、无水乙醇离心数次,真空冷冻干燥后得到墨绿色聚苯胺粉末。将四种粉末分别命名为PANI-125、PANI-175、PANI-225、PANI-275。
取5 g环氧树脂加入烧杯中,加入2 g二甲苯、正丁醇 (7∶3) 混合有机溶剂,搅拌5 min至完全溶解,随后加入540 mg待测粉末,搅拌分散20 min,超声5 min。再将混合均匀的溶液移入5 mL透明样品瓶中,静置观察,拍照记录。
将环氧树脂、有机溶剂 (二甲苯∶正丁醇=7∶3) 按照质量比1∶0.4充分混合后,分别加入质量分数为6%的PANI-125粉末和PANI-275粉末以及不添加任何填料混合搅拌30 min,再将环氧树脂与聚酰胺固化剂按照质量比为1∶0.8混合搅拌20 min,超声处理10 min,制得3种复合涂料。将3种涂料刷涂至5083铝合金试样表面,常温固化12 h、40 ℃下固化3 h,60 ℃下固化24 h,最后在室温下 (25 ℃,RH30%) 固化7 d,以挥发残留溶剂。固化后干态涂层厚度的平均值为 (120±10) µm。
利用INSPECT F50场发射扫描电子显微镜 (SEM) 观察聚苯胺产物形貌。利用FT-330四探针电阻率测试仪进行粉末电导率测试。使用Thermo Scientific Nicolet iS20型傅里叶变换红外光谱 (FTIR) 对粉末进行测量,测试光谱区域波数为400-4000 cm-1。利用XPS Peak对得到的XPS数据进行分峰拟合,其中L-G固定为20%。利用光学接触角测量仪测量室温下去离子水 (4 μL) 在粉末表面接触角。利用PARSTAT 4000A电化学工作站对涂层体系进行电化学阻抗测量,测试频率范围为105~10-2 Hz,正弦扰动信号50 mV。采用三电极体系,工作电极为涂层/金属电极,工作面积10.2 cm2,参比电极为饱和甘汞电极 (SCE),对电极为钛电极。采集后的阻抗数据利用ZSimpWin软件进行拟合。
2 结果与讨论
2.1 粉末微观形貌分析
图1
图1
4种样品SEM图像
Fig.1
SEM images of four types of samples: (a1, a2) PANI-125, (b1, b2) PANI-175, (c1, c2) PANI-225, (d1, d2) PANI-275
当SDBS添加量为125和175 mg时产物尺寸集中分布在500 nm左右,当添加量达到225和275 mg时,小尺寸空心球 (200±100 nm) 的占比逐渐增加,出现这种现象应该是由于单位油相液滴表面活性剂含量增加,界面张力下降,具有更高的乳液稳定性,减少小液滴凝聚成大液滴比例。并且可以发现随着添加量的增大,空心球表面呈现更多的绒毛状突起,形成更加粗糙的球壳表面。
2.2 粉末结构及成分分析
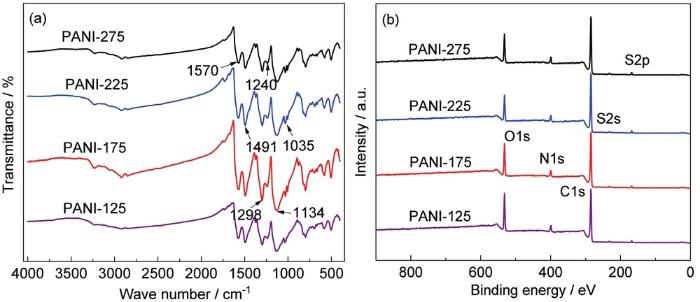
图2
图2
4种粉末样品的红外光谱及XPS能谱
Fig.2
FT-IR spectra (a) and XPS spectra (b) of four types of samples
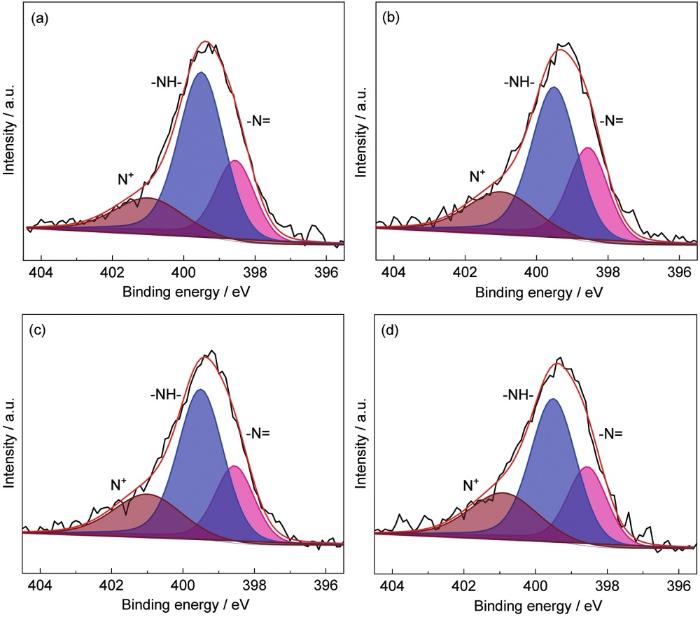
图3
图3
N 1s的高分辨能谱
Fig.3
High-resolution spectra of N 1s: (a) PANI-125, (b) PANI-175, (c) PANI-225, (d) PANI-275
表1 产物中各种状态N的比例
Table 1
| Sample | -N= | -N- | N+ |
|---|---|---|---|
| PANI-125 | 25.0% | 58.2% | 16.8% |
| PANI-175 | 29.8% | 50.5% | 19.7% |
| PANI-225 | 24.2% | 54.2% | 21.6% |
| PANI-275 | 24.7% | 53.0% | 22.3% |
2.3 粉末疏水性能分析
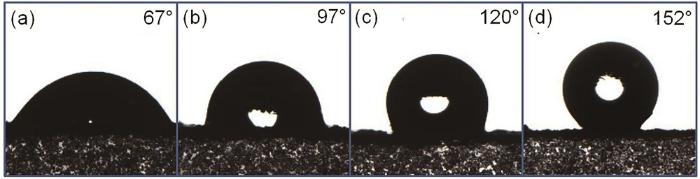
图4所示为4种粉末在常温条件下的接触角测量结果,可以清楚的看到,当SDBS的添加量为125 mg时接触角为67°,表现为亲水性,当随着SDBS的添加量增加到175、225和275 mg时,接触角分别达到了97°、120°的疏水,以及152°的超疏水。出现此现象有以下两方面原因:首先,苯胺在酸性条件下聚合过程中,质子掺杂在醌亚胺结构上的N原子上,为维持电中性,掺杂酸及表面活性剂阴离子也将掺入聚苯胺分子链,其中SDBS的磺酸基亲水基团与聚苯胺分子链结合,而尾部疏水长碳链朝向分子链外侧,当朝向聚苯胺分子链外侧疏水基团达到一定程度后,材料具备超疏水性能。另一方面,由于空心球壳外表面的“绒毛”增多,提升了表面粗糙度,根据Wenzel理论,粗糙的微纳米结构更利于疏水性的提升。
图4
图4
N 1s的高分辨能谱
Fig.4
High-resolution spectra of N 1s: (a) PANI-125, (b) PANI-175, (c) PANI-225, (d) PANI-275
2.4 粉末与环氧树脂相容性分析
图5为对不同粉末样品的沉降实验结果,为模拟真实涂层中的相容性情况,采用环氧树脂、二甲苯、正丁醇溶液体系。沉降实验结果能够说明填料在溶液中的分散稳定性,一般情况下,分散性越好,填料的沉降越少[25]。5组样品分别为磺基水杨酸掺杂聚苯胺 (PANI-SSA)、PANI-125、PANI-175、PANI-225、PANI-275。实验结果可见PANI-SSA样品在4和8 d时出现明显分层,其相容性最差。而本研究制备的四组样品均表现出较好的稳定性,未出现明显的分层,但PANI-125相较于其余三组自制粉末在沉降8 d后,其颜色更趋向于棕色,相容性稍差。综合以上结果,出现此现象的原因为本实验所采用的制备方法使得长链烷基掺杂于聚苯胺分子中,提升了聚苯胺在环氧树脂中相容性[11-13]。
图5
图5
不同样品在树脂溶液中沉降不同时间的照片
Fig.5
Visual illustrations of different samples dispersed in epoxy resin at
2.5 涂层的耐腐性能分析
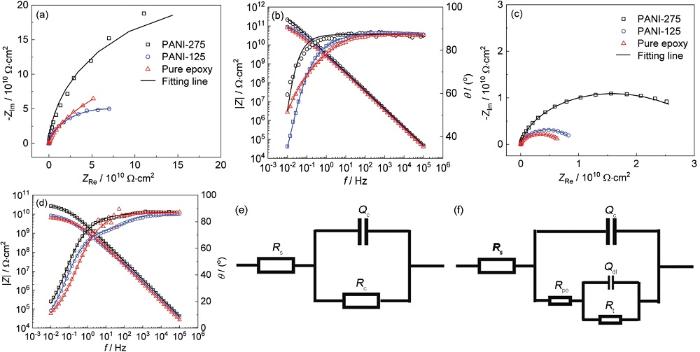
图6
图6
不同浸泡时间下的电化学阻抗谱及拟合电路
Fig.6
Nyquist (a, c) and Bode (b, d) plot at 12 h (a, b) and 14 d (c, d) and the corresponding preliminary stage (e) and mid stage (f) immersion time
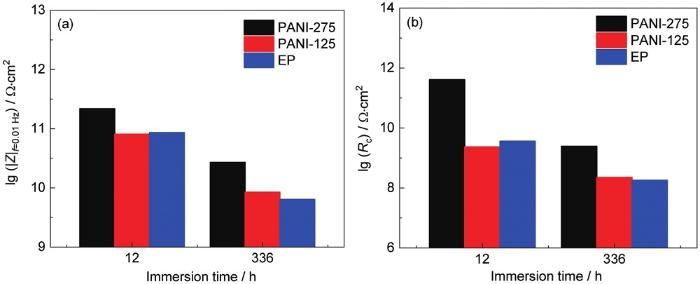
掺有PANI-275的涂层在12 h使用图6e浸泡初期阶段电路图拟合可获得较好结果。这是由于PANI-275粉末的超疏水性,使得腐蚀介质在涂层中的扩散阻力增大,且长链烷基更多的掺杂提升了填料在环氧树脂中的相容性,使得涂层内部缺陷减少,腐蚀介质渗透至涂层/金属界面时间延长。而PANI-125及EP涂层在使用浸泡初期电路拟合时,结果误差较大,因而采用图6f中期阶段电路拟合。低频区阻抗模值 (|Z|0.01 Hz) 通常用来评判涂层整体防护性能,低频区阻抗模值越大,涂层综合耐蚀性能越好。图7分别为3种涂层在不同浸泡时间下的低频阻抗模值及拟合后的涂层电阻结果。数据表明无论在浸泡初期还是浸泡中期,PANI-275涂层均表现出最佳防护效果,具有着最高的低频阻抗模值为2.69×1010,且拟合后的涂层电阻超过其余两组涂层一个数量级以上。
图7
图7
低频阻抗及涂层电阻拟合结果
Fig.7
Fitting results of low-frequency impedance and coating resistance: (a) low-frequency, (b) coating resistance
3 结论
(1) 为改善聚苯胺在环氧树脂中相容性差、腐蚀介质在有机涂层中扩散速度快的问题。采用乳液聚合法成功一步合成具有超疏水性能的聚苯胺空心胶囊。
(2) 通过控制表面活性剂的添加量,可实现对PANI胶囊的疏水性能、掺杂度、微观形貌及尺寸分布的调控。
(3) 由于超疏水粉末的掺入增加了腐蚀介质在涂层中的扩散阻力。且表面活性剂具有长链烷烃尾基,在掺入聚苯胺分子链时能改善聚苯胺在环氧树脂中的相容性,减少涂层内部缺陷,提升涂层致密性。使得在5083铝合金表面刷涂含有PANI-275的有机涂层无论在浸泡初期还是浸泡14 d后均有着最佳的防护效果,低频阻抗模值达2.69×1010。
参考文献
Application potential of conducting polymers
[J].
Corrosion protection of steel by polyaniline blended coating
[J].
Protection of epoxy coatings containing polyaniline modified ultra-short glass fibers
[J].
Electrochemical preparation and characterization of polypyrrole/stainless steel electrodes decorated with gold nanoparticles
[J].
Steel protection of two composite coatings: polythiophene with ash or MCM-41 particles containing iron (III) nitrate as inhibitor in chloride media
[J].
Growth behavior of initial product layer formed on Mg alloy surface induced by polyaniline
[J].
Growth kinetics of oxide films at the polyaniline/mild steel interface
[J].
Passivation of metals by coating with polyaniline: corrosion potential shift and morphological changes
[J].
Studies of different acid doped polyaniline incorporated into epoxy organic coatings on the Mg alloy
[J].
Preparation of multifunctional anti-corrosion polyaniline composite coating on Al alloy under marine environment
[D].
海洋环境用Al合金表面多功能耐蚀聚苯胺复合涂层的研制
[D].
Electrically conductive composites based on epoxy resin with polyaniline-DBSA fillers
[J].
Effects of N-alkylation on anticorrosion performance of doped polyaniline/epoxy coating
[J].N-alkylation of sulfosalicylic acid-doped polyaniline (PANI-SSA) was used to promote the anticorrosion performance of PANI-SSA/epoxy coating for 5083 Al alloy. PANI-SSA was modified with C5H11Br and C12H25Br in polar solvents IPA (isopropanol) and DMF (dimethylformamide), and then characterized by FTIR, XPS and sedimentation experiments. Results showed that alkanes were successfully linked onto the PANI-SSA chains. The compatibility between N-alkylated PANI-SSA and epoxy/xylene solution was improved. SEM results proved a better dispersion performance of N-alkylated PANI-SSA in epoxy coatings, with less holes and aggregations. Corrosion protection of the epoxy coatings incorporating PANI-SSA and N-alkylated PANI-SSA on 5083 Al alloy was studied by EIS and adhesion measurements in 3.5% NaCl solution. It turned out that the epoxy coating including C12H25Br-modified PANI-SSA in DMF has yielded the highest values of impedance modulus and best protective properties.
Structures and properties of the soluble polyanilines, N-alkylated emeraldine bases
[J].
Superhydrophobic nanocontainers for passive and active corrosion protection
[J].
A novel high anti-corrosion performance polymer based composite coating with new functional fillers
[J].
Anticorrosive behavior of epoxy coating modified with hydrophobic Nano-silica on phosphatized carbon steel
[J].
Different morphologies of polyaniline nanostructures synthesized by interfacial polymerization
[J].
Acid and base dual-controlled cargo molecule release from polyaniline gated-hollow mesoporous silica nanoparticles
[J].
Conducting-polymer nanotubes for controlled drug release
[J].
Inhibitor-loaded conducting polymer capsules for active corrosion protection of coating defects
[J].
Redox responsive release of hydrophobic self-healing agents from polyaniline capsules
[J]
Sub-micron calcium carbonate as a template for the preparation of dendrite-like PANI/CNT nanocomposites and its corrosion protection properties
[J].
Highly hydrophobic polyaniline nanoparticles for anti-corrosion epoxy coatings
[J].
A newly designed graphite-polyaniline composite current collector to enhance the performance of flow electrode capacitive deionization
[J].
Reinforcing the corrosion protection property of epoxy coating by using graphene oxide-poly (urea-formaldehyde) composites
[J].
Study and evaluation on organic coatings by electrochemical impedance spectroscopy
[J].
电化学阻抗谱方法研究评价有机涂层
[J].
Preparation and anticorrosion performance of M-phenylenediamine-graphene oxide/organic coating
[J].
间苯二胺-氧化石墨烯/有机涂层的制备及防腐性能研究
[J].