1 实验方法
采用TRP-450高真空多靶磁控溅射镀膜仪在304不锈钢 (
采用配备能量色散X射线 (EDX) 光谱附件的JSM-7900F型场发射扫描电子显微镜 (SEM) 表征涂层的腐蚀前后的表面形貌。采用D8 Advance X射线衍射仪 (XRD) 分析涂层物相组成,入射X射线采用Cu靶的Kα特征谱线,电压40 kV,电流40 mA,扫描角度范围为10°~90°,步长0.02°,扫描速度为1°/min。使用T Thermo Scientific K-Alpha+进行X射线光电子能谱 (XPS) 测试。
电化学测试在Zahner Zennium电化学工作站上进行,采用三电极体系,工作电极为测试样品,对电极为铂片,参比电极为饱和甘汞电极 (SCE),所有测试均在室温下进行。腐蚀介质为0.01 mol/L H2SO4溶液。测试前,所有样品均在电解质溶液中浸泡至稳定的开路电位。动电位极化电位扫描范围为-250~800 mVSCE,扫描速率为1 mV/s。在阴极 (600 mVSCE)、阳极 (-240 mVSCE) 进行恒电位极化测试。电化学阻抗谱频率测量范围为105~10-2 Hz,振幅为10 mV/s,测量在开路电位下进行。
2 结果与分析
2.1 涂层的微观结构
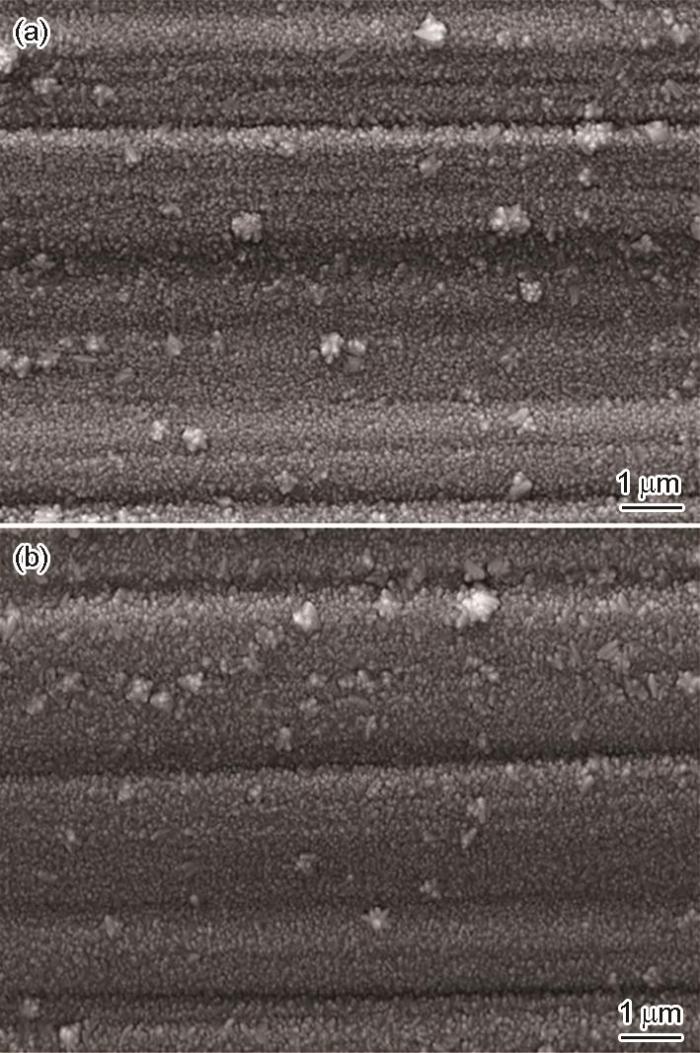
图1
图1
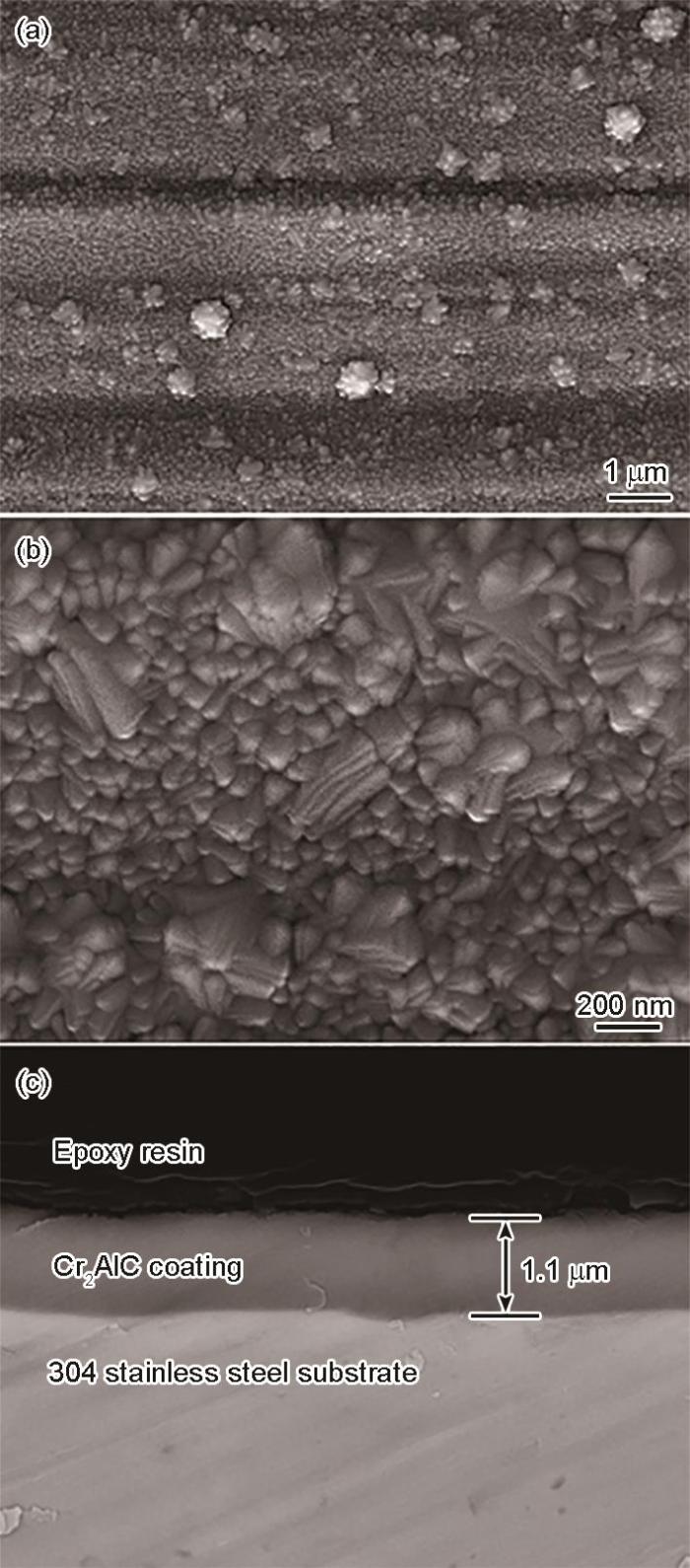
304不锈钢表面Cr2AlC MAX相涂层的表面和截面形貌
Fig.1
Surface (a, b) and cross-sectional (c) morphologies of Cr2AlC coating deposited on 304 stainless steel
2.2 物相分析
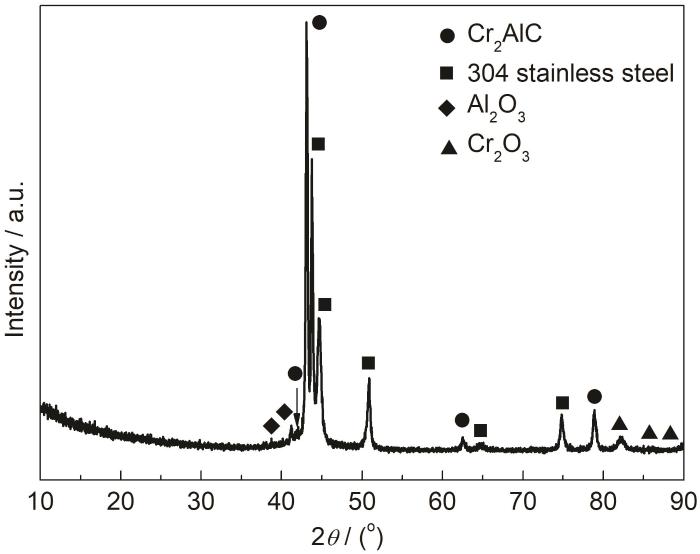
图2为磁控溅射沉积的Cr2AlC涂层的XRD图谱。涂层主要由Cr2AlC MAX相和少量杂质相Cr2O3、Al2O3组成,这是由于溅射过程中Al、Cr原子被真空腔室残留氧气氧化所致。
图2
图2
304不锈钢表面Cr2AlC MAX相涂层的XRD图
Fig.2
XRD pattern of the Cr2AlC coating on 304 stainless steel
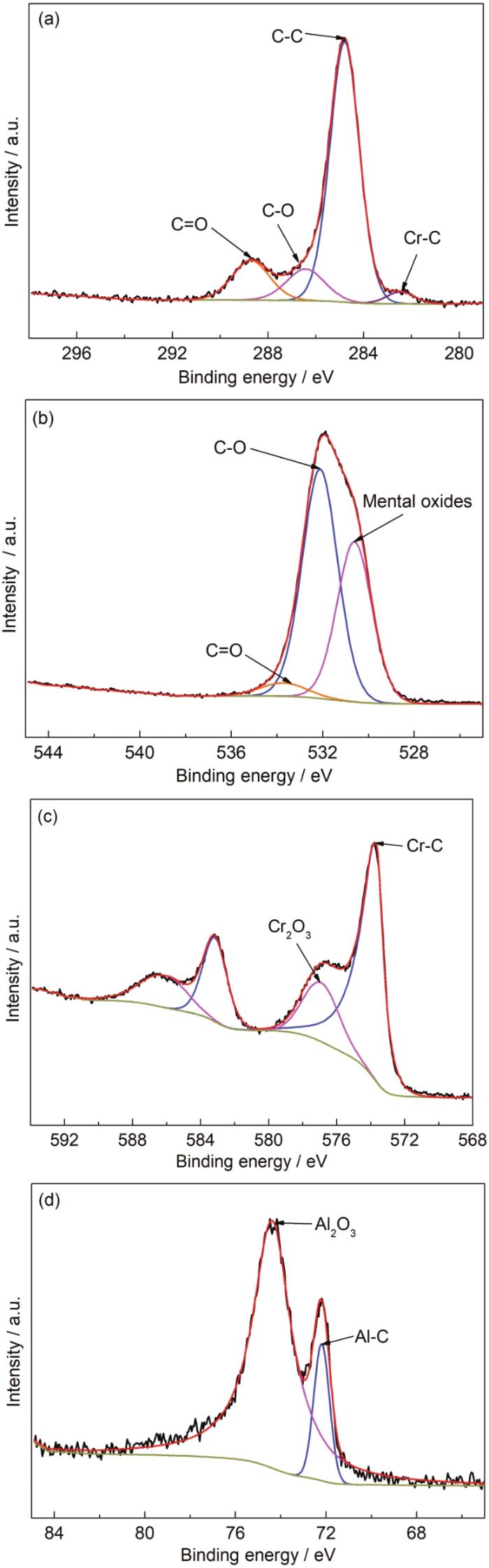
图3为Cr2AlC MAX相涂层C 1s、Cr 2p、Al 2p和O 1s高分辨率XPS能谱。如图3a所示,C 1s结合能为282.46 eV的峰对应于金属碳化物中的Cr-C键,而284.80 eV的峰主要归因于沉积过程中吸附的单质碳[22,23]。图3b中O 1s中结合能为530.62 eV的峰对应金属氧化物[24,25],这在Al 2p轨道光谱 (74.39 eV) 和Cr 2p3/2 (576.93 eV) 轨道光谱中也有体现。在图3d的Al 2p光谱中,在72.19 eV处的结合能不仅小于Al4C3(73.4 eV),甚至小于金属Al (72.9 eV),相对于Al4C3的Al 2p结合能73.4 eV有1.4 eV的负位移,这是由于Cr2AlC相对于碳化铝来说含有更多Al-C键。Cr 2p轨道光谱中结合能为573.67 eV的峰接近但低于Cr7C3中Cr 2p2/3的结合能 (574.2 eV),一般认为是Cr2AlC中形成的Cr-C键[23]。因此,Cr 2p2/3和Al 2p在573.67和72.19 eV下的结合能以及光谱结合能的负位移证实了涂层主要由Cr2AlC MAX相组成[26-28]。
图3
图3
304不锈钢表面Cr2AlC涂层的XPS光谱图
Fig.3
High-resolution XPS spectra of Cr2AlC coating deposited on 304 stainless steel: (a) C 1s, (b) O 1s, (c) Al 2p and (d) Cr 2p
2.3 电化学腐蚀行为
2.3.1 极化曲线
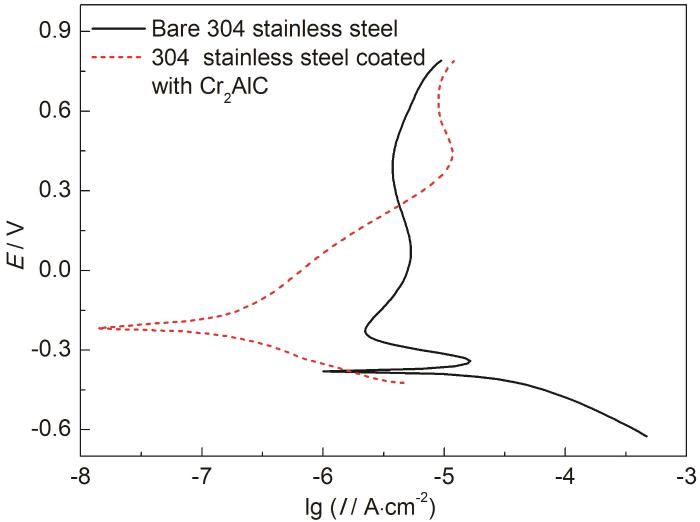
图4为未沉积和沉积Cr2AlC涂层的不锈钢在0.01 mol/L H2SO4中的动电位极化曲线。由图可知,304不锈钢在自腐蚀电位(-378 mVSCE) 下处于活化状态,随着电极电位升高进入钝化区。根据Tafel 公式计算,未沉积和沉积Cr2AlC涂层的不锈钢样品的腐蚀电流密度分别为1.46×10-5和2.43×10-7 A·cm-2。沉积Cr2AlC涂层后,不锈钢样品的腐蚀电流密度降低将近两个数量级,表明Cr2AlC涂层能够显著提高304不锈钢的耐腐蚀性能。
图4
图4
未沉积和沉积Cr2AlC涂层的304不锈钢样品在0.01 mol/L H2SO4中室温下的动电位极化曲线
Fig.4
Potentiodynamic polarization curves of 304 stainless steel without and with Cr2AlC coating in 0.01 mol/L H2SO4 solution
图5
图5
沉积Cr2AlC涂层的304不锈钢样品在模拟PEMFC阳极和阴极环境中的恒电位极化曲线
Fig.5
Potentiostatic polarization curves of 304 stainless steel with Cr2AlC coating at -240 mVSCE and 600 mVSCE
图6
图6
沉积Cr2AlC涂层的304不锈钢在-240 mVSCE和600 mVSCE恒电位极化后的表面形貌
Fig.6
SEM surface images of Cr2AlC coated 304 stainless steel after potentiostatical polarization at -240 mVSCE (a) and 600 mVSCE (b) in 0.01 mol/L H2SO4 solution at room temperature
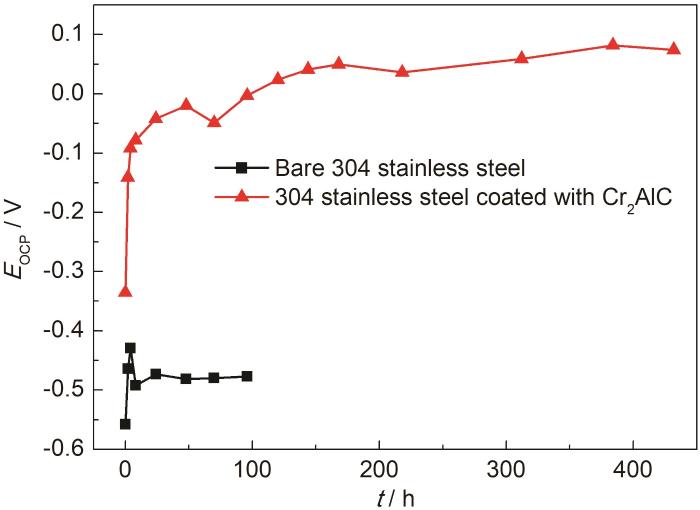
2.3.2 开路电位-时间曲线
图7为未沉积和沉积Cr2AlC涂层的304不锈钢样品的开路电位-时间曲线。在腐蚀过程中,304不锈钢的开路电位由-558 mVSCE增加到-460 mVSCE后趋于稳定,表明不锈钢已达到稳定的腐蚀状态。沉积Cr2AlC涂层的不锈钢样品浸泡初期,腐蚀电位由-335 mVSCE升至82 mVSCE后一直保持稳定,表明在腐蚀过程中涂层保持着良好的防护性能。
图7
图7
未沉积和沉积Cr2AlC涂层的304不锈钢样品在0.01 mol/L H2SO4溶液中的开路电位-时间曲线
Fig.7
Open circuit potential-time curves for 304 stainless steel without and with Cr2AlC coating in 0.01 mol/L H2SO4 solution
2.3.3 电化学阻抗谱
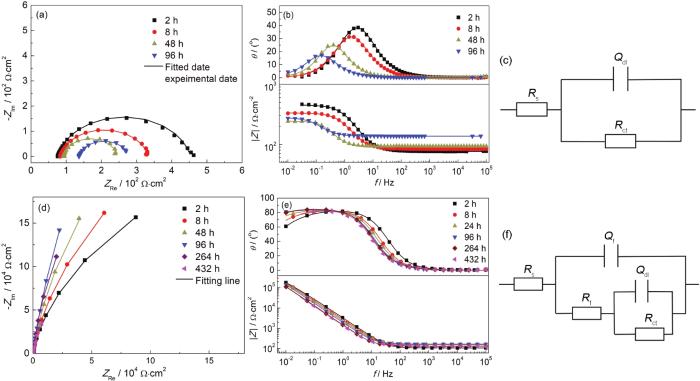
图8a,b为304不锈钢在0.01 mol/L H2SO4溶液中腐蚀的电化学阻抗谱。阻抗谱由单一的容抗弧组成。在腐蚀过程中,容抗弧半径逐渐减小,表明表面形成的腐蚀产物层逐渐溶解。腐蚀96 h后基体表面有肉眼可见的腐蚀坑。图8d,e为沉积Cr2AlC涂层的不锈钢在0.01 mol/L H2SO4溶液中的电化学阻抗谱。由图8d可知,沉积Cr2AlC涂层的不锈钢样品阻抗谱由两个不易区分的容抗弧组成。腐蚀过程中阻抗谱保持着相同的特征。低频段的阻抗模值高于105 Ω·cm2,表现出优异的抗腐蚀性。Bode图的高频区域表示溶液与电极之间的电阻,中频区代表涂层本身的信息,低频区域代表的是基体合金的腐蚀反应[4,29-32]。由图8e的 Bode图可见,在中低频端 (<100 Hz),lg|Z|与lgf几乎为线性关系,且幅角接近80°。这是由于涂层致密,在腐蚀过程中介质未扩散至不锈钢表面,表现近纯容抗特征。
图8
图8
未沉积及沉积Cr2AlC涂层的304不锈钢在0.01 mol/L H2SO4溶液中的电化学阻抗谱及其等效电路
Fig.8
Nyquist (a, d) and Bode (b, e) plots of 304 stainless steel (a, b) Cr2AlC coated 304 stainless steel (c, d) after immersion in 0.01 mol/L H2SO4 solution for different time, equivalent circuits for fitting impedance diagrams of 304 stainless steel without (c) and with (f) Cr2AlC coating in 0.01 mol/L H2SO4 solution (symbol: experimental data; line: fitted data)
采用图8c,f所示的等效电路分别对未沉积和沉积Cr2AlC涂层的304不锈钢阻抗谱进行拟合。其中,Rs为溶液电阻,Rct和Rf分别为电荷转移电阻和涂层电阻。Cf和Cdl分别为涂层电容和双层电容。考虑到弥散效应,采用恒相位角原件CPE (Q) 代替纯电容C,其表达式为 (1):
表1 304不锈钢在0.01 mol/L H2SO4溶液中的电化学阻抗谱拟合结果
Table 1
| Time / h | Rs / Ω·cm2 | CPE1 | Rct / Ω·cm2 | |
|---|---|---|---|---|
| Ydl / S-α Ω-1 cm-2 | n | |||
| 2 | 39.62 | 1.053×10-3 | 0.877 | 195.7 |
| 8 | 42.19 | 1.869×10-3 | 0.893 | 126.9 |
| 48 | 47.45 | 8.892×10-3 | 0.963 | 76.33 |
| 96 | 69.76 | 2.118×10-2 | 0.911 | 71.48 |
表2 沉积Cr2AlC涂层的304不锈钢在0.01 mol/L H2SO4溶液中的电化学阻抗谱拟合结果
Table 2
| Time / h | Rs / Ω·cm2 | CPE1 | Rct / Ω·cm2 | CPE2 | Rf / Ω·cm2 | ||
|---|---|---|---|---|---|---|---|
| Ydl / S-α Ω-1 cm-2 | ndl | Yf / S-α Ω-1 cm-2 | nf | ||||
| 2 | 106.0 | 1.604×10-5 | 0.676 | 4.228×105 | 5.565×105 | 0.939 | 1.900×105 |
| 8 | 152.8 | 1.116×10-5 | 0.673 | 6.316×105 | 6.675×105 | 0.942 | 2.799×105 |
| 48 | 141.1 | 3.687×10-5 | 0.872 | 1.841×106 | 5.141×105 | 0.985 | 59.27 |
| 96 | 165.0 | 3.299×10-5 | 0.880 | 2.600×106 | 5.984×105 | 0.975 | 100.50 |
| 264 | 130.8 | 4.116×10-5 | 0.936 | 1.122×106 | 8.114×105 | 0.960 | 85.28 |
| 432 | 131.4 | 7.643×10-5 | 0.942 | 2.877×105 | 5.363×105 | 0.947 | 41.01 |
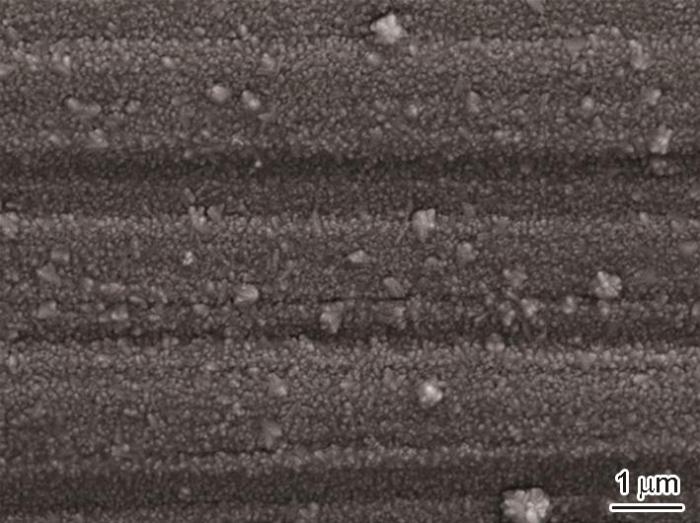
图9
图9
沉积Cr2AlC涂层的不锈钢在0.01 mol/L H2SO4溶液中腐蚀432 h后的表面形貌
Fig.9
Surface morphology of Cr2AlC coated 304 stainless steel after immersion for 432 h in 0.01 mol/L H2SO4 solution
3 结论
(1) 利用直流磁控溅射技术在基体温度480 ℃的304不锈钢表面成功制备了Cr2AlC MAX相涂层。涂层表面均匀致密,主要由Cr2AlC MAX相和少量金属氧化物组成。
(2) 在0.01 mol/L 的H2SO4中,沉积Cr2AlC涂层的304不锈钢腐蚀电流密度为1.46×10-5 A·cm-2,比304不锈钢下降了两个数量级。在600和-240 mVSCE的阴阳极恒电位极化后的腐蚀电流密度分别为2.3×10-7和2.44×10-8 A·cm-2。与304不锈钢相比,沉积Cr2AlC涂层的304不锈钢Rct值提高了4个数量级以上,表明Cr2AlC涂层显著提高了304不锈钢双极板的耐蚀性。
参考文献
Carbon-based coatings for metallic bipolar plates used in proton exchange membrane fuel cells
[J].
Research progress on corrosion behavior and surface protection of stainless steel bipolar plate of proton exchange membrane fuel cell
[J].
质子交换膜燃料电池不锈钢双极板的腐蚀行为及其表面防护的研究进展
[J].
Corrosion and electrical properties of carbon/ceramic multilayer coated on stainless steel bipolar plates
[J].
Effects of Mo content on the interfacial contact resistance and corrosion properties of CrN coatings on SS316L as bipolar plates in simulated PEMFCs environment
[J].
Surface modification of TA1 bipolar plate for proton exchange membrane fuel cell
[J].
质子交换膜燃料电池中TA1双极板的表面改性研究
[J].
Comparison of corrosion behavior between coarse grained and nanocrystalline Ni-Fe alloys in chloride solutions and proton exchange membrane fuel cell environment by EIS, XPS and Raman spectra techniques
[J].
Conducting polymer-coated stainless steel bipolar plates for proton exchange membrane fuel cells (PEMFC)
[J].
Study of electrodeposited polypyrrole coatings for the corrosion protection of stainless steel bipolar plates for the PEM fuel cell
[J].
Superior conducting polypyrrole anti-corrosion coating containing functionalized carbon powders for 304 stainless steel bipolar plates in proton exchange membrane fuel cells
[J].
Effect of conducting composite polypyrrole/polyaniline coatings on the corrosion resistance of type 304 stainless steel for bipolar plates of proton-exchange membrane fuel cells
[J].
A study of the corrosion behaviour and protective quality of sputtered chromium nitride coatings
[J].
An investigation into TiN-coated 316L stainless steel as a bipolar plate material for PEM fuel cells
[J].
Site-selective, low-loading, Au nanoparticle-polyaniline hybrid coatings with enhanced corrosion resistance and conductivity for fuel cells
[J].
Silver implanted 316L stainless steel as bipolar plates in polymer electrolyte membrane fuel cells
[J].
Metallic bipolar plates for PEM fuel cells
[J].
New materials for polymer electrolyte membrane fuel cell current collectors
[J].
Towards large area deposition of Cr2AlC on steel
[J].
Synthesis and characterization of conductive ceramic MAX-phase coatings for metal bipolar plates in simulated PEMFC environments
[J].
Characterization of Ti3SiC2-coating on stainless steel bipolar plates in simulated proton exchange membrane fuel cell environments
[J].
Variation of microstructure and composition of the Cr2AlC coating prepared by sputtering at 370 and 500 ℃
[J].
Crystallization kinetics of amorphous Cr2AlC thin films
[J].
Non-isothermal oxidation kinetics of nano-laminated Cr2AlC MAX phase
[J].A highly pure Cr2AlC MAX phase is prepared through the sintering method by varying the aluminum content. The non-isothermal oxidation kinetics of the Cr2AlC phase is examined through a TGA/DTA technique, at variable heating rates (10, 20, 30, 40 degrees C/min). The TGA/DTA results show that the oxidation of the Cr2AlC phase occurred in two stages. The multi-stage kinetic analysis is performed to establish the nature of the oxidation process. The activation energy is calculated by following the Kissinger-Akahira-Sunose (KAS) and the Friedman (FR) iso-conversional kinetic methods. The reaction mechanism involved during the oxidation process is proposed through the integral master-plots method. (C) 2018 Elsevier B.V.
Nano-structured CrN/CN x multilayer films deposited by magnetron sputtering
[J].
Plasma spray of Ti2AlC MAX phase powders: effects of process parameters on coatings' properties
[J].
Structural characterization of TiC x films prepared by plasma based ion implantation
[J].
Charge distribution in the single crystalline Ti2AlN thin films grown on MgO (111) substrates
[J].
Temperature-dependent microstructural evolution of Ti2AlN thin films deposited by reactive magnetron sputtering
[J].
Use of tetraneopentylchromium as a precursor for the organometallic chemical vapor deposition of chromium carbide: a Reinvestigation
[J].
Corrosion resistant and conductive TiN/TiAlN multilayer coating on 316L SS: a promising metallic bipolar plate for proton exchange membrane fuel cell
[J].
Synthesis and properties of novel N/Ta-co-doped TiO2 coating on titanium in simulated PEMFC environment
[J].
Effect of Al content on the corrosion resistance and conductivity of metal nitride coating in the cathode environment of PEMFCs
[J].
Corrosion and interfacial contact resistance behavior of electrochemically nitrided 316L SS bipolar plates for proton exchange membrane fuel cells
[J].