大型原油储罐是石油石化工业的重要装备,然而腐蚀问题一直是威胁储罐安全运行的关键因素。尽管原油含水率一般都小于0.5%,但是原油在存储过程中存在“水沉油浮”现象,最终罐底被沉积水浸没,为储罐底板的腐蚀创造了电解质环境。沉积水中的Cl-浓度高、同时存在微生物、含有少量的溶解O2和H2S/CO2,因此腐蚀性较强,最快甚至不到1 a就导致储罐底板腐蚀失效。研究表明,微生物腐蚀是储罐底板腐蚀的主要原因,通常表现为严重的局部点蚀穿孔,而点蚀坑周围材料的腐蚀往往很轻微[1]。
微生物腐蚀 (MIC) 是指因微生物的生命活动导致材料发生或加速腐蚀的现象[2,3]。金属材料在发生MIC时主要以局部腐蚀,尤其以点蚀为主。目前在油气生产领域中,研究MIC主要以硫酸盐还原菌 (SRB)[4-6]、腐生菌 (TGB)[7,8]和铁细菌 (FB)[9,10]为主,其中尤以SRB为甚。迄今,国内外研究者对于SRB导致金属材料发生腐蚀的机理解释主要有阴极去极化理论[11-13]、阳极加速理论[14,15]和直接电子传递理论[16-18]。阴极去极化理论认为由于微生物活动形成的生物膜使得金属材料表面产生阳极区,当环境中含有SRB时,SRB不仅会降低金属材料表面阴极反应中H结合生成H2的活化能,而且还可以利用金属阴极区产生的氢还原硫酸盐,由此加速阴极反应速度,进而加速金属的腐蚀。阳极加速理论在阴极去极化理论的基础上还考虑了SRB对阳极反应的影响,SRB还原硫酸盐会产生大量的H2S和其他有机酸,这些代谢产物附着在金属表面会与Fe2+相结合,加速Fe2+离开金属基体表面,从而加速金属腐蚀。直接电子传递理论认为在一系列酶促反应作用下,附着在金属表面的SRB可以通过跨膜电子传递直接从金属中获得电子从而加速金属的阳极溶解。
原油储罐受自然环境影响明显,导致储罐内部的温度会存在一定波动,在这种工况条件下微生物的腐蚀变化规律对于评估储罐底板的腐蚀程度具有重要的参考价值。本文采用储罐底部沉积水,测试了不同温度下罐底板钢的微生物腐蚀导致的均匀速率和点蚀速率变化规律,结合沉积水中的微生物种属分析,探讨了温度对微生物腐蚀影响机制。
1 实验方法
实验水样为白沙湾储油库罐底水,水溶液中含 (mg/L):Cl- 42637,HCO3- 2.43,SO42- 55,Na+ 15600,Ca2+ 1021,K+ 326,Mg2+ 730,细菌浓度104~105个/mL。
采用16S rRNA基因测序分析微生物的丰度。高通量测序文库的构建和基于Illumina MiSeq平台的测序由 GENEWIZ 公司完成。使用 Qubit 2.0 Fluorometer检测DNA样品的浓度,使用 MetaVx™文库构建试剂盒构建测序文库。
以30~50 ng DNA为模板,使用PCR引物扩增原核生物16S rDNA上包括V3及V4的2个高度可变区。采用包含"CCTACGGRRBGCASCAGKVRVGAAT”序列的上游引物和包含" GGACTACNVGGGTWTCTAATCC"序列的下游引物扩增V3和V4区。另外,通过PCR向16S rDNA的PCR产物末端加上带有Index的接头,以便进行NGS测序。
使用Agilent 2100生物分析仪检测文库质量,并且通过Qubit2.0 Fluorometer检测文库浓度。DNA文库混合后,按Illumina MiSeq仪器使用说明书进行PE250/300双端测序,由MiSeq自带的MiSeq Control Software (MCS) 读取序列信息。
试样为罐底板用钢Q235A,其化学成分 (质量分数,%) 为:C 0.15,Si 0.25,Mn 0.50,S 0.015,P 0.01,Cr 0.25,Ni 0.15,余量为Fe。将板材加工成100 mm×30 mm×10 mm的试片,并用砂纸将试片逐级打磨至800目,并用酒精和丙酮进行清洗后备用。
实验前在无菌箱内采用紫外线对试样和容器灭菌1 h,然后将试片水平放在底部固定有两根玻璃棒的500 mL广口瓶内,试片不与瓶底部接触。随后注入罐底水,再用橡胶塞密封广口瓶。考虑到实际工况下罐底微生物菌落会形成局部厌氧环境,采用高纯氮气通过橡胶塞上的玻璃导管通入广口瓶内除氧2 h,然后密封玻璃导管。所有缝隙处涂抹704胶密封。操作均在无菌箱内操作。最后将广口瓶放入烘箱内,分别在25、35、45、55、65和85 ℃下腐蚀7 d。
腐蚀结束后,取出试片,利用KYKY-6900扫描电镜 (SEM) 观察腐蚀产物形貌,利用OXFORD-Ultim Max能谱仪 (EDS) 分析元素组成。去除腐蚀产物后,测量腐蚀失重,计算均匀腐蚀速率。通过Olympus OLS5000-SAF激光共聚焦显微镜 (CLSM) 测量试样表面点蚀坑深度,计算均匀腐蚀速率。
2 结果与讨论
2.1 罐底板腐蚀特征
图1
图1
罐底板微生物腐蚀形貌
Fig.1
Microbial corrosion images of the bottom plate of a stock tank, showing the local corrosion in the weld area (a) and pitting pit in the middle of the plate (b)
观察罐底板点蚀坑形貌可以发现,点蚀坑都具有口大底小的特征。通常闭塞电池机制可以解释点蚀坑快速发展的原因,即,由于腐蚀产物在点蚀坑的开口处堆积,最终导致点蚀坑内出现自催化酸化和浓差腐蚀电偶,结果点蚀坑内发生快速溶解,通常会形成口小底大的形貌特征,这也是含氧环境下点蚀坑的普遍特征。而罐底板的微生物菌落会团簇状生长,其代谢的酸性产物会导致生物膜下的基底金属快速腐蚀,因此罐底板的口大底小的点蚀形貌与微生物腐蚀的特征相吻合。
2.2 罐底水中微生物分析
图2
图2
罐底水微生物的丰度16S rRNA基因测序分析
Fig.2
16S rRNA gene sequencing analysis of the abundance distributions of phylum (a) and genus (b) of microorganisms in water at the tank bottom
表1 罐底水微生物丰度与特性
Table 1
| Content / % | Nature | ||
|---|---|---|---|
| Thermotogae | Mesotoga | 3.08 | G+Anaerobic or facultative, thermophilic bacteria, metabolize sugar, protein, oxidize sulfate |
| Proteobacteria | Desulfovibrio | 3.12 | G- Anaerobic, non-spore, mesophilic bacteria, oxidized sulfide |
| Desulfobacter | 0.57 | ||
| Bacteroidetes | Macellibacteroides | 6.38 | G+Anaerobic, no spores, metabolize sugar to produce acid, mesophilic bacteria |
| Proteiniphilum | 6.7 | G-Anaerobic, non-spore, metabolizing organic product propionic acid, mesophilic bacteria | |
| Firmicutes | Bacillus | 11.64 | G+Aerobic or anaerobic, with spores, metabolizing sugar to produce acid, mesophilic bacteria |
| Sporotomaculum | 0.9 | G+Anaerobic Spore Metabolism of Benzoate Lipids Methanogenic, Mesophilic Bacteria | |
| Gracilibacter | 0.19 | G-Anaerobic, non-spore, metabolizing organic matter, thermophilic bacteria | |
| Hydrogenoanaerobacterium | 0.6 | G+Anaerobic, hydrogen or methane production, mesophilic bacteria | |
| Ruminococcus | 0.33 | G+Anaerobic, metabolizing cellulose, acid producing, spherical, mesophilic bacteria | |
| Tyzzerella | 4.7 | G+Anaerobic, acid-producing, mesophilic bacteria | |
| Clostridium | 16.18 | G+Anaerobic, metabolize glycoprotein, produce acid or alcohol, thermophilic bacteria | |
| Tepidimicrobium | 0.36 | G+Anaerobic, sporulation, metabolizing organic matter, utilization of sulfide, thermophilic bacteria | |
| Proteiniclasticum | 7.67 | G-Anaerobic, non-spore, metabolizing protein, acid producing, mesophilic bacteria | |
| Terrisporobacter | 25.07 | G+Anaerobic, spore-forming, spherical, metabolizing sugar, acid-producing, mesophilic bacteria | |
| Sedimentibacter | 7.1 | G+Anaerobic, spore production, amino acid metabolism, acid production, mesophilic bacteria |
细菌种群中属于Bacteroidetes和Firmicutes的细菌种类和含量最多,均是异养微生物,代谢产物产生酸,通常会将这类菌属归类为腐生菌 (TGB)。其中除了Bacillus (芽孢杆菌属) 是好氧细菌外,其余均为厌氧菌。另外,腐生菌中的Clostridium (梭菌属) 属于嗜热菌[21],含量达到16.18%,在罐底水中也是优势菌群。
微生物种群特性表明罐底水中不仅含有的丰富的SRB、TGB,而且在室温和高温下均有适合生长的微生物菌种。另外,罐底水中的菌群绝大多数是厌氧菌,说明罐底沉积物中的微生物是在厌氧环境下生存,而且SRB和TGB形成了共生体系。通常,SRB可以利用TGB代谢形成的有机酸作为电子供体进行生理代谢[22],因此,这种共生模式有助于微生物菌群增殖,最终对金属材料造成更严重的腐蚀。
2.3 温度对罐底水微生物腐蚀影响
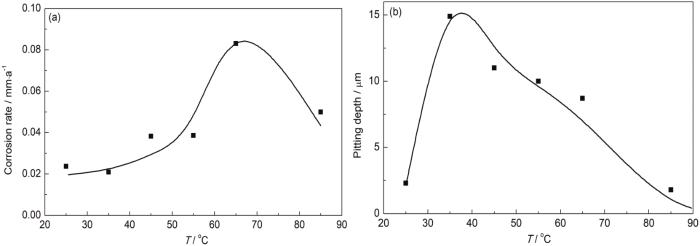
不同温度的沉积水浸泡后,罐底板的均匀腐蚀速率和最大点蚀坑深度变化规律如图3所示。在25~85 ℃温度范围内,均匀腐蚀速率呈现先升后降的变化规律,其中在65 ℃达到最大值,温度超过85 ℃以后均匀腐蚀速率迅速降低,但均匀腐蚀速率整体并不高,属于轻微腐蚀范围 (图3a)。与均匀腐蚀速率类似,局部腐蚀速率也呈现先增大后减小的变化规律,峰值出现在30~40 ℃之间,点蚀坑深度接近15 μm,点蚀速率达到0.8 mm/a;在50~70 ℃之间也有10 μm左右,点蚀速率仍然超过0.5 mm/a,同样当温度超过85 ℃以后,点蚀速率也迅速降低。整体来说,从图3b可以看出30~70 ℃是点蚀快速发生的温度区间。
图3
图3
温度对罐底板微生物腐蚀影响规律
Fig.3
Influences of temperature on the uniform corrosion rate (a) and maximum pitting depth (b) of the microbiological corrosion of the bottom plate of a stock tank
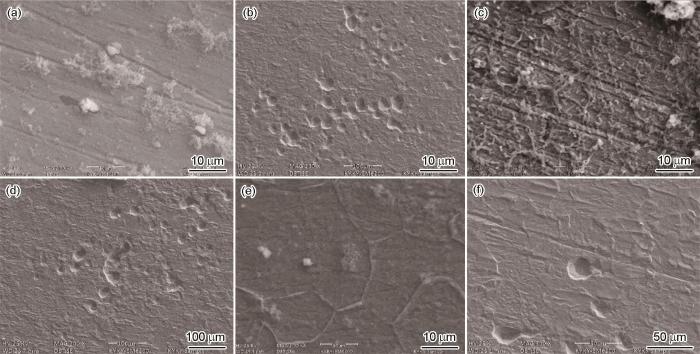
图4
图4
罐底板微生物腐蚀后的表面形貌
Fig.4
Surface morphologies (a, c, e) and pitting morphologies (b, d, f) of the bottom plate of a stock tank after microbial corrosion at 25 ℃ (a, b), 35 ℃ (c, d) and 65 ℃ (e, f)
图5
图5
35 ℃下腐蚀以后点蚀坑内部形貌
Fig.5
Internal view of a pitting pit formed on the bottom plate of a stock tank after corrosion at 35 ℃
图6
图6
不同温度腐蚀后最大点蚀坑轮廓形貌
Fig.6
Contour morphologies of the largest pitting pits formed after corrosion at 25 ℃ (a), 35 ℃ (b) and 65 ℃ (c)
点蚀通常由直径非常微小的蚀坑发展起来,然后才逐渐扩大,一般尺寸小于1 μm的点蚀坑看成是亚稳态蚀坑。从图6点蚀坑的发展过程来看,反映出初期时的点蚀坑直径可能就比较大,这应该与微生物菌落团簇生长有关,先是微生物形成一定尺度的菌落,然后是菌落下逐步发生局部腐蚀,因此点蚀的萌生主要还是由于团簇状的菌落导致的。
温度直接影响硫酸盐还原菌的代谢活性和生长速度。根据SRB适宜生长温度的不同,可将其分为嗜温型,嗜热型及嗜冷型SRB。嗜冷型SRB的适宜生长温度范围为10~18 ℃[23,24]。嗜中温型SRB的最适宜生长温度为37±1 ℃[25],高于45℃停止生长。嗜热SRB的生长温度范围在50~70 ℃,最佳生长温度为65 ℃,在80 ℃或更高温度下仍可以生存。附着在钢铁表面的SRB可以耐受更高温度[26-28]。Liu等[29]研究从渤海油田分离得到的嗜热SRB菌株在不同温度下对碳钢腐蚀的影响,可见60 ℃碳钢的腐蚀速率是37 ℃时的2.2倍,表明嗜热硫酸盐还原菌的代谢活性对适宜生长温度的依赖性。温度太低太高均会抑制SRB的生长代谢活动。
罐底板的微生物主要是嗜温和嗜热菌,因此,从无论是均匀腐蚀速率还是点蚀速率在25 ℃时都不高,说明25 ℃以下罐底水中的微生物不活跃。均匀腐蚀速率在65 ℃达到峰值说明微生物在这一温度也有较高的代谢活性,微生物代谢出来的酸性产物在高温下与金属基体会有较快的反应速率,当温度升高到85 ℃以后,微生物活性大大降低,此时虽然温度有利于腐蚀速率加快,但由于代谢产物较少,因此均匀腐蚀速率也降低。
由于点蚀的发展与菌落的相关性更大,因此,温度升高到65 ℃时,主要是嗜温菌生长,但其数量要小于嗜温菌,因此在试样表面的菌落不够发达,这从试样表面的扫描电镜也能看出来 (图4e),因此点蚀坑深度和密度都降低。当超过85 ℃以后,绝大部分的微生物的活动受到抑制,因此点蚀也迅速减小。
3 结论
(1) 大型原油储罐的罐底水中微生物种群含有嗜温和嗜热的SRB和TGB,它们在室温到60~70 ℃范围内均能生长。
(2) 罐底板钢的微生物腐蚀的均匀腐蚀速率在65 ℃时达到峰值,但均匀腐蚀总体属于轻微腐蚀。而点蚀速率在35 ℃时达到峰值,为0.8 mm/a,属于严重腐蚀;65 ℃时也有较高的点蚀速率,温度超过65 ℃以后点蚀速率迅速降低。30~70 ℃是点蚀快速发生的温度区间,温度对罐底板钢微生物腐蚀的影响与微生物种类耐温性有关。
(3) 罐底板微生物腐蚀形成的点蚀坑与菌落的团簇生长有关,微生物先是在金属表面形成团簇状的菌落,然后代谢过程中使得菌落下的金属发生快速点蚀。
参考文献
Corrosion mechanism and protective measures of inner wall baseplate of large crude oil storage tank
[J].
大型原油储罐内壁底板腐蚀机理及防护措施
[J].
Microbiologically influenced corrosion (MIC)
[A].
Microbiologically influenced corrosion of pipeline steels
[J].
管线钢的微生物腐蚀
[J].
Case analysis of microbial corrosion in product oil pipeline
[J].
成品油输送管道微生物腐蚀案例分析
[J].
Study on the microbiological corrosion behavior of X65 pipeline steel by SRB and IB
[J].
SRB和IB对X65管线钢的微生物腐蚀行为研究
[J].
Research progress on sulfate-reducing bacteria induced corrosion of steels
[J].
硫酸盐还原菌对钢材腐蚀行为的研究进展
[J].
Experimental study on microbiologically influenced corrosion of natural gas gathering pipelines
[J].
天然气集输管道的微生物腐蚀试验研究
[J].
Corrosion failure analysis on an underground pipeline for a gas gathering station
[J].
某集气站埋地管道腐蚀失效原因分析
[J].
Corrosion behavior of L245 pipeline steel in shale gas fracturing produced water containing iron Bacteria
[J].
铁细菌对L245钢腐蚀行为的影响研究
[J].
Discussion on the causes of gas field corrosion and anti-corrosion measures
[J].
关于气田腐蚀原因及防腐措施探究
[J].
A synergistic D-tyrosine and tetrakis hydroxy‐methyl phosphonium sulfate biocide combination for the mitigation of an SRB biofilm
[J].
Corroding iron as a hydrogen source for sulphate reduction in growing cultures of sulphate-reducing bacteria
[J].
Sulphate respiration from hydrogen in Desulfovibrio bacteria: a structural biology overview
[J].
Influence of thermal aging on sulfate-reducing bacteria (SRB)-influenced corrosion behaviour of 2205 duplex stainless steel
[J].
The corrosion of mild steel by biogenic sulfide films exposed to air
[J].
Corrosion of carbon steel influenced by anaerobic biofilm in natural seawater
[J].
Extracellular electron transfer is a bottleneck in the microbiologically influenced corrosion of C1018 carbon steel by the biofilm of sulfate-reducing bacterium Desulfovibrio vulgaris
[J].
Characterizing the effect of carbon steel exposure in sulfide containing solutions to microbially induced corrosion
[J].
Obligate sugar oxidation in Mesotoga spp., phylum Thermotogae, in the presence of either elemental sulfur or hydrogenotrophic sulfate‐reducers as electron acceptor
[J].Mesotoga prima strain PhosAc3 is a mesophilic representative of the phylum Thermotogae comprising only fermentative bacteria so far. We show that while unable to ferment glucose, this bacterium is able to couple its oxidation to reduction of elemental sulfur. We demonstrate furthermore that M. prima strain PhosAc3 as well as M. prima strain MesG1 and Mesotoga infera are able to grow in syntrophic association with sulfate-reducing bacteria (SRB) acting as hydrogen scavengers through interspecies hydrogen transfer. Hydrogen production was higher in M. prima strain PhosAc3 cells co-cultured with SRB than in cells cultured alone in the presence of elemental sulfur. We propose that the efficient sugar-oxidizing metabolism by M. prima strain PhosAc3 in syntrophic association with a hydrogenotrophic sulfate-reducing bacterium can be extrapolated to all members of the Mesotoga genus. Genome comparison of Thermotogae members suggests that the metabolic difference between Mesotoga and Thermotoga species (sugar oxidation versus fermentation) is mainly due to the absence of the bifurcating [FeFe]-hydrogenase in the former. Such an obligate oxidative process for using sugars, unusual within prokaryotes, is the first reported within the Thermotogae. It is hypothesized to be of primary ecological importance for growth of Mesotoga spp. in the environments that they inhabit.© 2017 Society for Applied Microbiology and John Wiley & Sons Ltd.
Cellular systems
[A].
The ecology and biotechnology of sulphate-reducing bacteria
[J].
Adaptation of psychrophilic and psychrotrophic sulfate-reducing bacteria to permanently cold marine environments
[J].
Community size and metabolic rates of psychrophilic sulfate-reducing bacteria in arctic marine sediments
[J].
Research on screening and physiological characteristics of sulfate reducing bacteria
[J].
硫酸盐还原菌的筛选及生理特性研究
[J].
Characterization of a new thermophilic sulfate-reducing bacterium
[J].A thermophilic sulfate-reducing vibrio isolated from thermal vent water in Yellowstone Lake, Wyoming, USA is described. The gram-negative, curved rod-shaped cells averaged 0.3 micrometer wide and 1.5 micrometers long. They were motile by means of a single polar flagellum. Growth was observed between 40 degrees and 70 degrees C with optimal growth at 65 degrees C. Cultures remained viable for one year at 27 degrees C although spore-formation was not observed. Sulfate, thiosulfate and sulfite were used as electron acceptors. Sulfur, fumarate and nitrate were not reduced. In the presence of sulfate, growth was observed only with lactate, pyruvate, hydrogen plus acetate, or formate plus acetate. Pyruvate was the only compound observed to support fermentative growth. Pyruvate and lactate were oxidized to acetate. Desulfofuscidin and c-type cytochromes were present. The G + C content was 29.5 mol%. The divergence in the 16 S ribosomal RNA sequences between the new isolate and Thermodesulfobacterium commune suggests that these two thermophilic sulfate-reducing bacteria represent different genera. These two bacteria depict a lineage that branches deeply within the Bacteria domain and which is clearly distinct from previously defined phylogenetic lines of sulfate-reducing bacteria. Strain YP87 is described as the type strain of the new genus and species Thermodesulfovibrio yellowstonii.
The growth and the sulfur metabolisms of thermophilic sulfate-reducing bacteria at different temperatures
[J].
嗜热硫酸盐还原菌在不同温度下的生长及其硫代谢
[J].
Growth characteristics of thermophile sulfate-reducing bacteria and its effect on carbon steel
[J].
嗜热硫酸盐还原菌生长特征及其对碳钢腐蚀的影响
[J].
Growth characteristics of thermophile sulfate-reducing bacteria and its effect on carbon steel
[J].
A review of microbiologically influenced corrosion of metals
[J].
金属的微生物腐蚀
[J].
A new mechanistic model for mic based on a biocatalytic cathodic sulfate reduction theory
[A].