碳钢因其性价比高而在工程设备上获得了广泛的应用,但由于海洋大气环境极为复杂,温度、相对湿度、紫外线、臭氧、氯化钠溶胶等环境因素众多,因此一旦处于海洋大气环境中,极易吸附大气中水份,从而在碳钢表面形成一层肉眼看不见的薄液膜,尤其是表面含有氯离子等可溶盐污染物时,更容易吸附水份而在其表面形成不同离子、不同浓度和不同pH的薄液膜,薄液膜层的化学变化以及金属的溶解、表面粗糙度、表面腐蚀产物的聚集、生长和增厚都对使役的材料或构件表面化学环境产生重要影响[1],从而诱发电化学腐蚀过程,进而直接影响其腐蚀行为及其后期使用寿命。
1 实验方法
实验所用材料为1018#碳钢,其化学成份 (质量分数,%) 为:C 0.15,Mn 0.70,P 0.005,S 0.035,余为Fe。将碳钢切割成尺寸为20mm×12 mm×2 mm的片状试样,然后分别用400#、800#、1200#的SiC水磨砂纸逐级打磨,随后依次使用无水乙醇和去离子水清洗干净,干燥后放入真空干燥器中备用。
模拟腐蚀暴露方法参见文献[6]。预先在碳钢样品表面滴加一滴0.6 mol/L的氯化钠溶液 (溶剂采用无水乙醇与去离子水体积比为7∶3的混合物),均匀铺展后,用高纯氮气轻轻吹干,放入相对湿度为85%的大气腐蚀模拟箱中暴露24 h。本实验碳钢样品表面所辐照的紫外线波长分别为365、245和185 nm,光线强度均为0.15 W/m2,表面没有紫外光线辐照的部分样品,用作对比试样。
大气腐蚀模拟箱中暴露的样品取出后,采用带扫描电镜 (SEM) 和能谱仪 (EDS) 双束聚焦离子束系统 (FIB,FEI Helios 650 NanoLab) 在样品表面特征区域进行切割微加工,同时进行形貌观察和元素分析。将双束聚焦离子束系统与微机械手联用,对样品表面特征区域的腐蚀产物进行加工传送出样品腔,进入X射线三维透视成像系统 (XRM,Versa XRM-500) 进行三维立体透视观察。
采用LK3200A电化学工作站进行测试。碳钢样品为工作电极,铂电极为对电极,饱和甘汞电极作为参比电极 (其相对于氢标准电极电位为+0.2415 V)。样品表面积为0.25 cm2,扫描速度为2 mV·s-1,扫描区间为-0.8~0.3 VSCE。电化学实验所用电解液为0.6 mol·L-1 NaCl溶液 (pH=6.8,室温)。
2 结果与讨论
2.1 实验结果
图1
图1
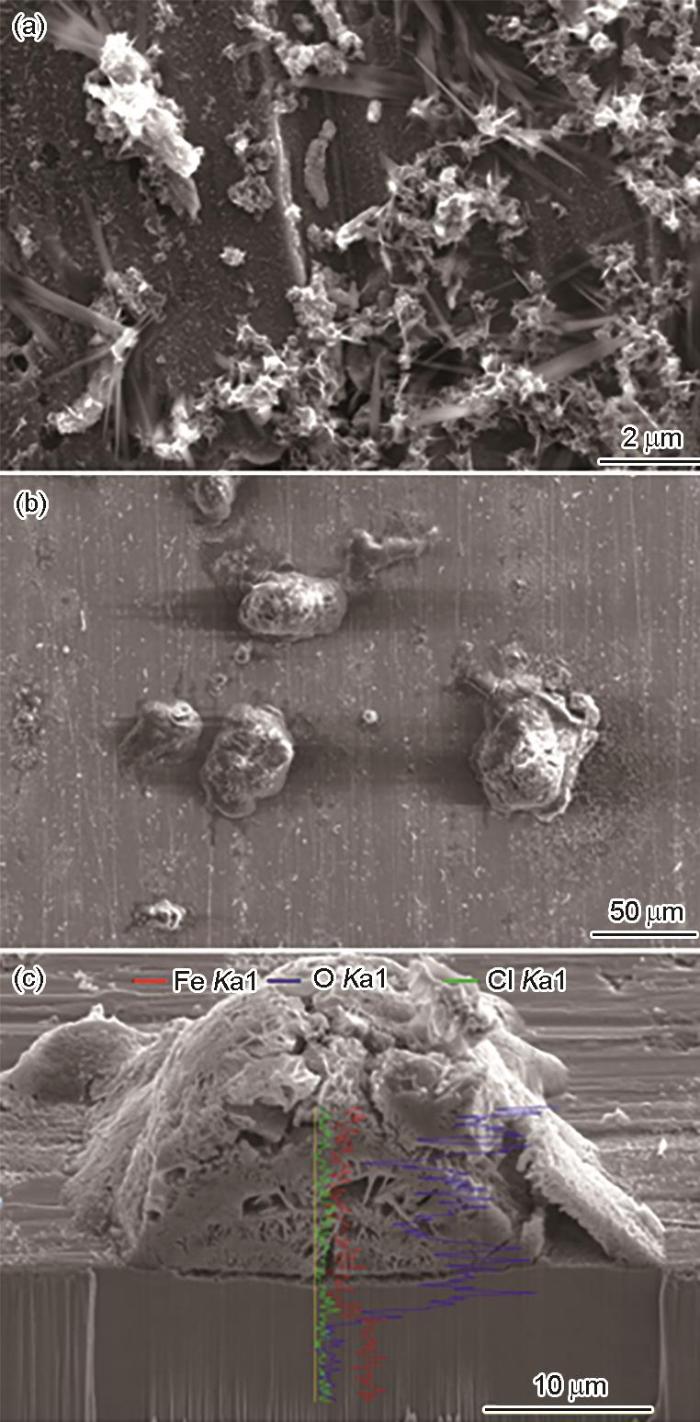
NaCl沉积样品在净化空气中暴露24 h后的表面形貌,腐蚀瘤中间截面三维形貌及元素线扫描曲线
Fig.1
Surface images of the carbon steel deposited with NaCl and exposed in the cleaned air for 24 h (a, b), and 3D cross-sectional image with line scans of O, Cl and Fe elemental distributions along the yellow line passing through one corrosion swell of Fig.1b (c)
在海洋大气腐蚀模拟箱 (RH85%,30 ℃) 中设置波长为185 nm的紫外光灯,控制其到达样品表面的紫外光线强度为0.15 W/m2,将沉积NaCl的碳钢样品放置于腐蚀暴露箱中暴露24 h后,可以观察到样品表面出现明显的腐蚀产物。
图2
图2
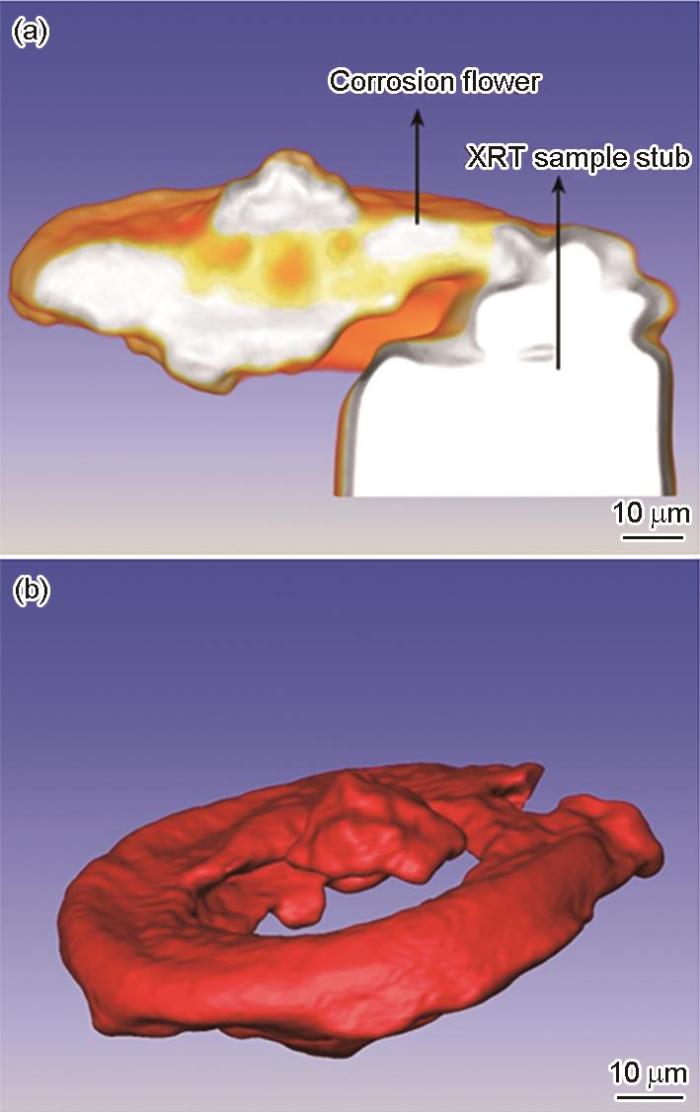
镶在样品台上点蚀花的截面主视图和扣除样品台后点蚀花的侧视图
Fig.2
Front view of the 3D cross-sectional XRT image of the corrosion flower with the XRT sample stub (a), and vertical views of the corrosion flower without the sample stub (b)
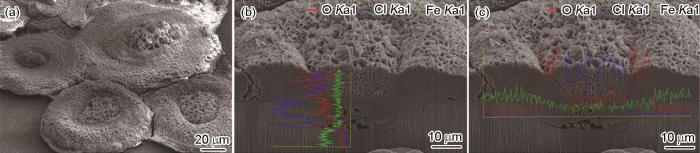
同样将经NaCl沉积的碳钢暴露在净化空气中,同时辐照波长为185 nm、强度为0.15 W/m2的紫外光线,暴露24 h后,在FIB系统里将其中碳钢表面的一个点蚀花纵向切割后,进行元素线扫描,所得结果如图3b和c所示。可以明显看到,点蚀花中间花柱Cl含量很高,而花瓣上Cl含量低,并且花柱从坑底向外,Cl含量逐渐降低,而O含量逐渐增加。相反地,点蚀花中间花柱部分O含量很低,而花瓣上O含量高,几乎不含Cl。蚀孔底部,有很多孔洞产生。点蚀花的花柱十分疏松,上部为片状腐蚀产物。由花瓣形貌可以看出,该腐蚀产物十分疏松。
图3
图3
NaCl沉积碳钢样品在紫外光线辐照 (185 nm) 的净化空气中暴露24 h后的表面形貌,点蚀花纵向截面三维形貌,元素纵向和横向及元素的线扫描曲线
Fig.3
Surface image of carbon steel deposited with NaCl in the exposure chamber with cleaned air with the UVA light (185 nm, 0.15 W/m2) for 24 h (a), 3D cross-sectional image with longitudinal (b) and horizontally (c) line scans of O, Cl and Fe elemental distributions along the respectively yellow lines passing through the corrosion swell
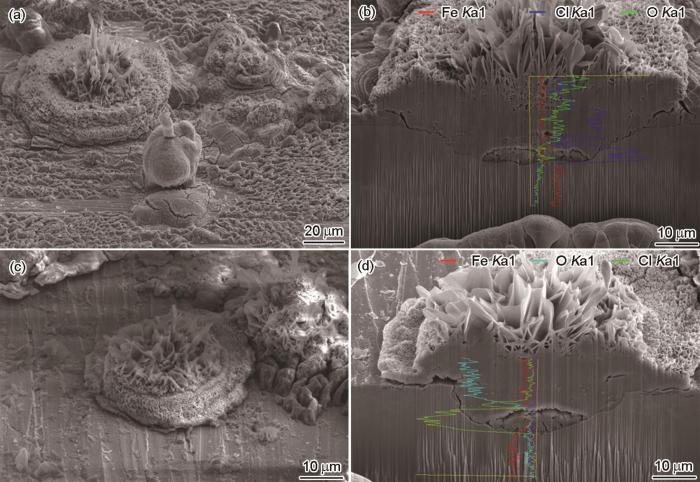
同样将经NaCl沉积的碳钢暴露在净化空气中,同时辐照波长为245 nm、强度为0.15 W/m2的紫外光线,暴露24 h后,将碳钢样品置入FIB系统观察,可以看到样品表面也出现很多花状的点蚀区 (图4a)。将其中碳钢表面的一个点蚀花纵向切割后,进行元素线扫描,所得结果如图4b所示。可以明显看到,在该环境中的点蚀花与在波长为185 nm紫外光线辐照下的点蚀 (图3b和c) 具有类似的特征:点蚀花中间花柱部分Cl含量很高,而花瓣上几乎没有Cl。相反地,点蚀花花瓣上O含量高,而中间花柱部分氧含量很低,并且从坑底向外O含量逐渐增加。点蚀蚀孔底部,有很多的孔洞产生。点蚀花的花柱十分疏松,花柱上部为片状腐蚀产物。花瓣表面的腐蚀产物也是十分疏松的结构。
图4
图4
NaCl沉积碳钢样品在紫外光线辐照 (245和365 nm) 的净化空气中暴露24 h后的表面形貌,点蚀花纵向截面三维形貌及元素的纵向线扫描曲线
Fig.4
Surface images of carbon steel deposited with NaCl in the exposure chamber with cleaned air with 245 nm (a) and 365 nm (c) UVA light (0.15 W/m2) for 24 h respectively and corresponding to the 3D cross-sectional image with longitudinal line scans of O, Cl and Fe elemental distributions along the respectively yellow line passing through the corrosion swell (b, d)
同样将经NaCl沉积的碳钢暴露在相对湿度为85%、温度为30 ℃的净化空气中,同时辐照波长为365 nm、强度为0.15 W/m2的紫外光线,暴露24 h后,将碳钢样品置入FIB系统观察,可以看到样品表面也出现花状的点蚀区 (图4c)。将其中碳钢表面的一个点蚀花纵向切割后,进行元素线扫描,所得结果如图4d所示。将图4d与图3b,3c、4b进行比较,可以明显看到,在该环境中的点蚀花与在波长为185和245 nm紫外光线辐照下的点蚀具有类似的特征:点蚀花中间花柱部分Cl含量很高,而花瓣上几乎没有Cl含量很低。相反地,点蚀花花瓣上O含量高,而中间花柱部分O含量很低,并且从坑底向外O含量是逐渐增加。点蚀蚀孔底部,有很多的孔洞产生。点蚀花的花柱十分疏松,花柱上部为片状腐蚀产物。花瓣表面的腐蚀产物也是十分疏松的结构。
2.2 讨论
图5
图5
碳钢在0.5 mm薄液膜 (0.6 mol·L-1 NaCl)、波长为365 nm及强度为0.3 mW/cm2的紫外光线辐照条件下的极化曲线
Fig.5
Potentiodynamic scan of steel in 0.6 M NaCl thin electrolyte layer (UV light: 365 nm, 0.3 mW/cm2)
为考察紫外光线对碳钢腐蚀动力学的影响特征,将碳钢置入浓度为0.6 mol·L-1薄液膜 (液膜厚度为0.5 mm) 条件下的模拟海洋大气环境中,并对其碳钢表面进行紫外光线 (波长为365 nm、强度为0.3 mW/cm2) 辐照,测试其动电位极化曲线,结果如图5所示。利用CorrView2软件对图5的极化曲线进行拟合,拟合所得结果如表1所示。根据图5和表1,可知相对于没有紫外光线辐照的条件,碳钢在紫外光线辐照下,腐蚀电位升高,而腐蚀电流相对下降,说明紫外光线对碳钢的腐蚀产生钝化作用,这也说明,一旦碳钢暴露在紫外光线辐照的海洋大气环境中时,碳钢表面会产生一层氧化膜,因此其Rp阻值变大,与表1所示结果吻合。
表1 采用CorrView2软件对图5拟合所得电化学参数
Table 1
| Illumination condition | Icorr / A·cm-2 | Ecorr vs SCE / V | Rp / Ω·cm2 |
|---|---|---|---|
| No UV light | 2.9719×10-5 | -0.53034 | 877.78 |
| With UV light | 1.6304×10-5 | -0.46378 | 1600 |
在点蚀发展过程中,竖直方向具有最短的扩散路径,因此Cl-沿着点蚀花的花柱向下扩散,而Cl-从坑底向外扩散,在点蚀洞口形成Fe2O3[6]。蚀孔底部的高浓度Cl-成为点蚀诱发的前沿,并使点蚀具有自催化效应,而不断发展。
3 结论
(1) 碳钢在模拟海洋大气环境中发生明显点蚀。紫外光线使碳钢表面钝化,形成一层氧化膜,碳钢表面的Cl-穿透氧化膜,诱发其点蚀行为。
(2) 碳钢点蚀具有花样的疏松核壳结构,点蚀花花柱由氯化物组成,花瓣由Fe的氧化物组成。
(3) 蚀孔底部具有高浓度Cl-、极低的氧含量,Cl-浓度从坑底向外逐渐降低,而氧浓度却相反,从坑底向外逐渐增加。
(4) 聚集在蚀孔底部的高浓度Cl-,产生自催化效应,使点蚀继续发展。
参考文献
Influence of environmental factors on corrosion of ship structures in marine atmosphere
[J].
Insight into atmospheric corrosion evolution of mild steel in a simulated coastal atmosphere
[J].The corrosion behavior of mild steel in a simulated coastal atmosphere environment has been investigated by the indoor accelerated wet/dry cyclic corrosion acceleration test (CCT), scanning electron microscopy (SEM), Raman spectroscopy and electrochemical measurements. During the CCT test of 60 cycles, the evolution of logarithmic (corrosion rate) vs. logarithmic (CCT cycles) presents a turning point at the 5th cycle, presenting a tendency to increase first and then decrease to gradually stabilize as the CCT cycle prolonged. Before the 5th cycle, γ-FeOOH and β-FeOOH and Fe3O4 were detected, respectively. And then, α-FeOOH as a new chemical composition was detected in the subsequent corrosion cycles. It is found that, after long term corrosion, the rust separated into a relatively dense inner layer rich with α-FeOOH and a loose outer layer rich with γ-FeOOH, both of which have poor electrical conductivity. The rapid increase of corrosion rate in the early stage since reducible corrosion products are involved in the reduction process of the cathode which promotes the dissolution of the anodic metal substrate. Afterward, as the rust layer thickens, the resistance of the rust increases, and the aggressive ions diffusion is blocked, gradually suppressing the electrochemical corrosion process. At last, when the composition and distribution of the rust layer remain stable, the corrosion presents a fluctuating speed around a certain value during the cracking and self-repairing process of the rust layer.
Corrosion evolution characteristics of Q235B steel in O3/SO2 composite atmosphere
[J].
O3/SO2复合大气环境中Q235B钢的腐蚀演化特性
[J].
Atmospheric pitting corrosion of 304L stainless steel: the role of highly concentrated chloride solutions
[J].
Effect of relative humidity on corrosion of steel under sea salt aerosol proxies: I. NaCl
[J].
Insight into atmospheric pitting corrosion of carbon steel via a dual-beam FIB/SEM system associated with high-resolution TEM
[J].Carbon steel experiences serious pitting corrosion in the atmospheres containing chloride ions. The present research is focused on revealing a three-dimensional chemical and microstructural insight into pitting corrosion with high spatial resolution nano-tomography by addressing 1018 carbon steel after exposed in the marine atmosphere. A well-defined core-shell structure for pitting corrosion products was revealed by using a combination of dual-beam focused ion beam system with scanning electron microscope (FIB/SEM) and high-resolution transmission electron microscope (HRTEM). The core is made of ferric chloride and the shell is constituted with iron oxides. Pitting corrosion is a self-catalytic process and the corrosion products grow to be porous flower-type structures. The porosity in the core provides a diffusion path for the ions. Inclusions promote pitting corrosion growth.
Study on corrosion models of structural steel exposed in urban industrial atmospheric and laboratory simulated environments based on the 3D profile
[J].
Improving atmospheric corrosion prediction through key environmental factor identification by random forest-based model
[J].
Atmospheric corrosion of copper and silver
[J].
The distribution of NaCl on Fe during atmospheric corrosion
[J].
Corrosion mechanisms for iron and low alloy steels exposed to the atmosphere
[J].