pH是影响管线钢腐蚀与SCC的重要环境因素之一。X100管线钢化学成分、微观结构上与低级别管线钢有一定区别,这导致pH对X100管线钢电化学与SCC行为的影响作用发生变化。研究[7,8]表明,pH影响金属表面电化学活性;此外,高pH环境中,管线钢表面会形成一层FeCO3的保护膜,pH可影响表面膜层状态,进而改变其腐蚀行为,探讨pH对X100管线钢电化学行为的影响是认识其腐蚀行为的重要内容。土壤SCC是管线钢的另一安全隐患,可分为两类,近中性pH-SCC和高pH-SCC。其中,高pH-SCC是发现最早、次数较多的埋地管线失效形式,被称为埋地管线的经典SCC类型。文献[9-11]指出,由管线钢表面膜层破损,引起局部腐蚀,造成局部应力集中,在载荷与腐蚀作用下导致最终SCC开裂;高pH-SCC主要受阳极溶解 (AD) 控制,扩展方式主要为沿晶开裂,实验室常用CO32-/HCO3-溶液作为埋地管线高pH-SCC的模拟环境。pH可影响金属表面钝化膜的状态,改变高pH-SCC的萌生过程[12,13];同时,pH的变化将影响AD作用,进而影响SCC裂纹的扩展过程。关于钝化膜的研究主要集中于不锈钢领域,对于管线钢表面保护膜报道并不常见[14-16]。综上分析,探讨pH对X100管线钢高pH-SCC行为的影响对高强管线钢SCC机理的认识与管道安全运行均有积极意义。
本文通过电化学测试方法分析X100管线钢在不同浓度的CO32-/HCO3-溶液中的表面状态;并利用慢应变速率拉伸 (SSRT) 实验研究pH对X100管线钢高pH-SCC行为的影响;探讨pH、保护膜与X100管线钢高pH-SCC的内在关系。
1 实验方法
本文使用实验材料为X100管线钢,化学成分 (质量分数,%) 为:Mn 1.66,Mo 0.37,Ni 0.35,Si 0.29,Cu 0.27,Al 0.091,Nb 0.048,Cr 0.02,Ti 0.011,余量为Fe。将X100管线钢用砂纸逐级打磨至3000#,用粒径为2.5 μm的金刚石抛光膏抛光8 min,在酒精硝酸比例为1∶9的侵蚀液中浸泡20 s,取出洗净后在金相显微镜下观察主要由粒状贝氏体组成。
本文测试溶液为高pH-SCC研究实验溶液—NaHCO3/Na2CO3缓冲液。保持CO32-和HCO3-总量为1.5和0.15 mol/L,调整CO32-与HCO3-之间的比例,如表1所示。所有测试均在常温常压下进行 (25 ℃,0.1 MPa)。
表1 NaHCO3/Na2CO3缓冲液的配比与pH值
Table 1
| Proportion | NaHCO3 | Na2CO3 | pH |
|---|---|---|---|
| 1 mol/L | 0.15 | 0 | 8.55 |
| 2 mol/L | 0.1 | 0.05 | 9.62 |
| 3 mol/L | 0.05 | 0.1 | 10.53 |
| 4 mol/L | 0 | 0.15 | 11.58 |
电化学试样用环氧树脂封装于PVC管中,电化学测试的工作面积1 cm2;电化学试样逐级打磨至2000#砂纸后进行测试。电化学测试在Princeton 4000电化学工作站上进行,采用三电极体系,电化学试样为工作电极,饱和甘汞电极 (SCE) 为参比电极,2 cm2铂片为辅助电极。为研究X100管线钢高pH-SCC机理,采用慢速 (0.5 mV/s) 和快速扫描 (50 mV/s) 动电位极化曲线测试 (PDP) 研究SCC电化学机制。EIS扫描频率范围为105~10-2 Hz,振动幅值为10 mV。将X100管线钢试样在不同测试溶液中恒电位极化1 h,恒电位选取0.4 Vvs SCE (根据PDP选取),成膜后进行M-S测试。
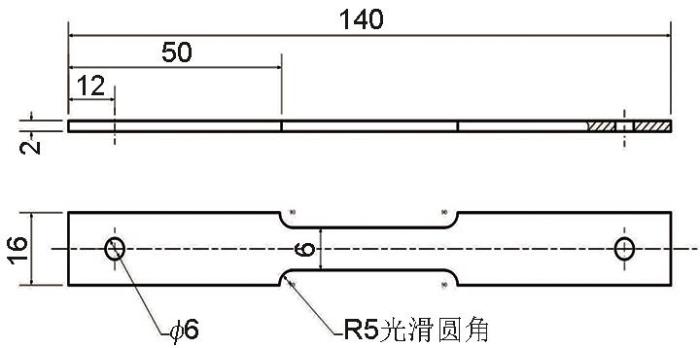
SSRT试样遵循GB/T15970标准制备,由电火花切割成型,经铣床表面处理,尺寸如图1所示。用砂纸打磨至2000#,打磨方向平行与拉伸方向。SSRT样品在400N载荷下浸泡1 d进行预腐蚀,用以减小试验系统误差,后以1×10-6 s-1的速率在WDML-30KN拉伸机上进行SSRT测试,SSRT测试完成,用游标卡尺测量并计算延伸率和断面收缩率,并利用
图1
式中,IΨ 为断面收缩率损失;Iδ 为延伸率损失;Ψs 为溶液中断面收缩率;Ψ0为空气中的断面收缩率;δs 为溶液中断面延伸率;δ0为空气中的断面延伸率。
SSRT实验结束后,在采用FEI Quanta 250扫描电子显微镜 (SEM) 中观察SSRT测试后试样的断口微观形貌。
2 分析与讨论
2.1 X100管线钢在不同pH溶液中电化学行为
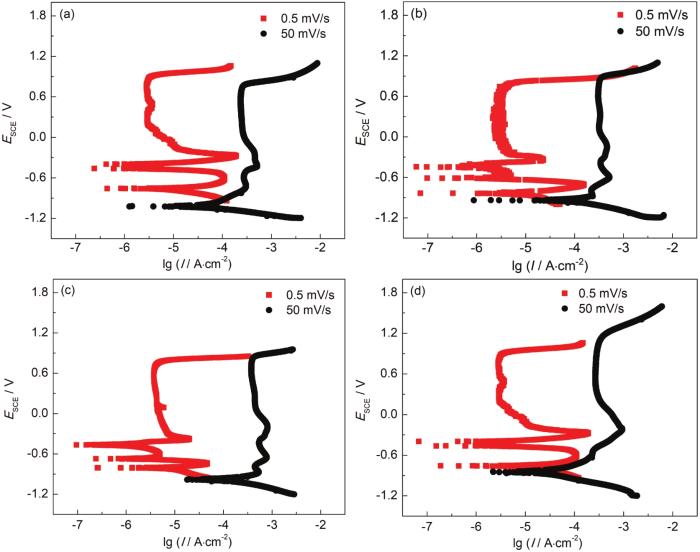
不同pH对X100管线钢高pH-SCC中电化学效应的影响如图2所示。由图可知,在不同pH的CO32-/HCO3-溶液中,X100管线钢的维钝电流密度并无明显差异;破钝电位随pH的提高而升高,点蚀更容易在pH较低的环境中萌生,这将影响到AD机制下SCC的萌生过程。X100管线钢在慢速扫描过程中电位处于-0.6到-0.3 V vs SCE之间时,阳极过程为复杂的活化-钝化状态;此时极化曲线出现多个零电流峰;这一现象并未出现在快速扫描曲线中。根据Liu的理论[17,18],快速扫描极化曲线可反映裂纹尖端的非稳态电化学特征;而慢速扫描极化曲线代表远离裂间位置的稳态电化学行为。X100管线钢在不同pH溶液中的快慢速扫描极化曲线电位,如图3所示,其中pH为8.55时,快慢扫极化曲线的电位差最大,裂尖与远离裂纹区域的微电偶作用最强,从侧面反映出此时的SCC扩展的驱动力最大。
图2
图2
X100管线钢在不同pH的CO32-/HCO3-溶液中的快慢速扫描动电位极化曲线
Fig.2
Fast and slow scanning dynamic potential polarization curves of X100 pipeline steel in CO32-/HCO3- solutions with pH values is 8.55 (a), 9.62 (b), 10.58 (c) and 11.55 (d)
图3
图3
X100管线钢快慢速扫描极化曲线的自腐蚀电位
Fig.3
Corrosion potentials obtained by fitting fast and slow scanning polarization curves of X100 pipeline steel
图4
图4
X100管线钢在不同pH的CO32-/HCO3-溶液中的电化学测试曲线
Fig.4
Nyquist (a), Bode (b) and phase angle (c) plots, equivalent circuit diagram and fitting Rct values (d) of X100 pipeline steel in CO32-/HCO3- solutions with different pH values
2.2 pH对X100管线钢高pH-SCC的影响
式中,E为施加电位;ɛr为膜层介电常数 (15.6);ɛ0为真空介电常数 (8.85×10-14 Fcm-1);κ为Boltzmann常数 (1.38×10-23 J/K) T为温度;e为电子电荷 (1.60218×10-19 C);φfb为Flat-band电位 (CSC2=0时的电位)。
图5
图5
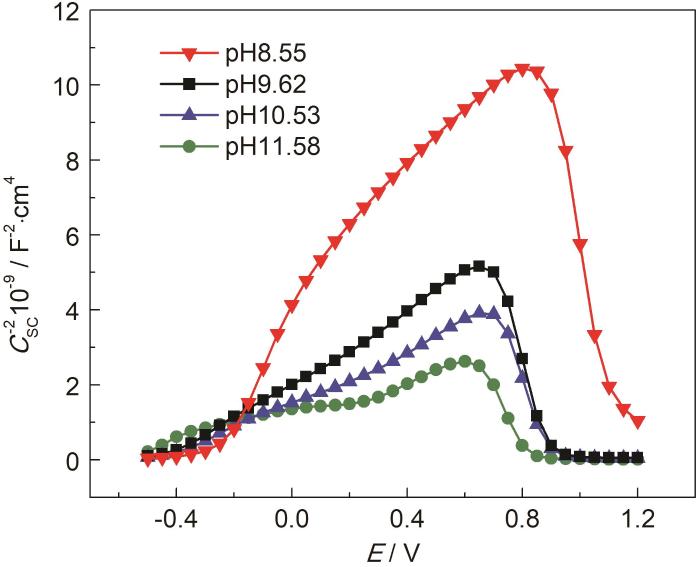
X100管线钢在不同pH的溶液中的Mott-Schottky曲线
Fig.5
Mott-Schottky curves of X100 pipeline steel in CO32-/HCO3- solutions with different pH values
图6
图6
pH对X100管线钢在CO32-/HCO3-溶液中表面膜层密度和厚度的影响
Fig.6
Effects of pH value on density and thickness of surface corrosion layer formed on X100 pipeline steel in CO32-/HCO3- solutions
由图6可知,随着pH的提高,ND 降低,W有一定程度的增加。这表明,pH影响了表面保护膜的致密性和厚度,改变了表面膜层的保护性;当溶液pH较高时,该层保护膜有较少的缺陷,说明此时有较好的致密性,膜层较厚,离子的交互过程受到阻碍,对基体材料的保护性更强。
2.3 X100管线钢在CO32-/HCO3-溶液中的电化学机理
FeCO3、Fe(OH)2继续氧化Fe2O3和Fe3O4,反应如下[17],
根据极化曲线与文献分析可知,阴极为氧去极化反应:
研究表明,在HCO3-/CO32-溶液中,管线钢表面形成FeCO3膜层,对电化学反应有一定的阻碍作用,但该层膜具有不稳定性;根据M-S的分析,pH值影响保护膜的致密性与厚度。HCO3-/CO32-的相对含量决定了溶液的pH值,当溶液中只存在HCO3-时,pH值为8.55,此时FeCO3的形成主要依靠HCO3-反应形成CO32-,因缺少CO32-,阻碍了FeCO3生成,最终导致膜层较薄,缺陷较多;随着CO32-相对含量提高,促进了FeCO3的形成,较多的FeCO3形成厚且致密的膜层结构,这很好的解释了电化学测试结果。
2.4 X100管线钢在不同pH溶液中慢应变速率拉伸测试
图7
图7
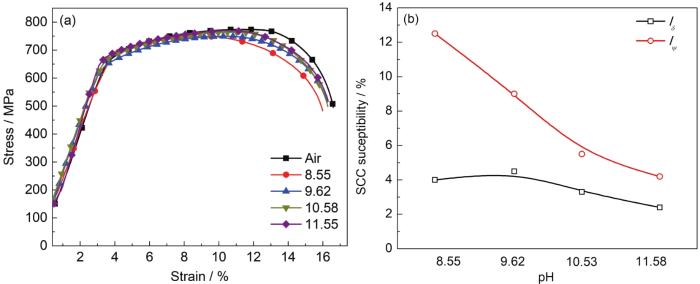
X100管线钢在不同pH的CO32-/HCO3-溶液中的应力-应变曲线及应力腐蚀敏感性
Fig.7
Stress-strain curves (a) and stress corrosion cracking susceptibilities (b) of X100 pipeline steel in CO32-/HCO3- solutions with different pH values
图8为SSRT测试后断口微观形貌图。由图可知,在CO32-/HCO3-溶液测试后,X100管线钢的断口以韧窝为主,为典型的韧性断裂。这表明,pH的变化并未改变X100管线钢的断裂类型。结合电化学分析,pH为8.55时,表面膜层薄且缺陷多,在应力作用下,更容易受到破坏,又无足够的CO32-修复表面膜层,这将导致膜破损位置离子交换速率较快,形成局部微电偶,引起点蚀,点蚀是SCC裂纹萌生的关键位置[21];同时载荷亦会使X100管线钢滑移系启动,导致台阶形成,引起钝化膜破损,夹杂物等也破坏了钝化膜的连续性,膜破损区域裸露的金属和具有连续钝化膜的金属构成腐蚀微电偶,导致局部腐蚀的发生,引起该区域的应力集中,造成高pH-SCC的发生。而当pH较高时,溶液中有充足的CO32-,FeCO3膜具有再形成能力;其次,由于溶液中并不存在膜破坏性离子,这为表面再钝化提供了可能,应力引起的膜损伤将被修复,膜层对传质过程阻碍的区别不能够充分体现,导致pH超过9.62时,SCC敏感性相差不大。
图8
图8
X100管线钢慢应变速率拉伸测试后的断口形貌
Fig.8
Fracture morphologies of X100 pipeline steel after slow strain rate test in CO32-/HCO3- solutions with pH values of 8.55 (a), 9.62 (b), 10.58 (c) and 11.55 (d)
3 结论
(1) pH改变了X100管线钢在CO32-/HCO3-溶液中电化学行为。随着pH值的升高,X100管线钢的破钝电位提高,但维钝电流变化较小,而表面保护膜致密性和厚度提高。
(2) 随pH值的提高,X100管线钢的高pH-SCC敏感性有一定程度的下降,但SCC断裂类型并未发生改变,保持韧性断裂。
参考文献
Research progress and prospect of stress corrosion cracking of pipeline steel in soil environments
[J].
管线钢土壤应力腐蚀开裂研究进展及展望
[J].
Effect of applied potential on stress corrosion behavior of X80 pipeline steel and its weld joint in a simulated liquor of soil at Lunnan area of Xinjiang
[J].
外加电位对X80管线钢在轮南土壤模拟溶液中应力腐蚀行为的影响
[J].
Effect of alternating current on corrosion behavior of X100 pipeline steel in a simulated solution for soil medium at Korla district
[J].
交流电流对X100管线钢在库尔勒土壤模拟液中腐蚀行为的影响
[J].
Stress corrosion cracking behavior and mechanism of Fe-Mn-Al-C-Ni high specific strength steel in the marine atmospheric environment
[J].
Characteristics of hydrogen embrittlement in high-pH stress corrosion cracking of X100 pipeline steel in carbonate/ bicarbonate solution
[J].
Effect of cathodic potential on stress corrosion cracking behavior of different heat-affected zone microstructures of E690 steel in artificial seawater
[J].
Electrochemical behavior of passive films formed on X80 pipeline steel in various concentrated NaHCO3 solutions
[J].
X80管线钢钝化膜在各种高浓度NaHCO3溶液中的电化学行为
[J].
Stress corrosion cracking of X80 pipeline steel exposed to high pH solutions with different concentrations of bicarbonate
[J].
Corrosion behavior and surface analysis of 690 MPa-grade offshore steels in chloride media
[J].
The effect of simulated concrete pore solution composition and chlorides on the electronic properties of passive films on carbon steel rebar
[J].
The influence of ppb levels of chloride impurities on the strain-induced corrosion cracking and corrosion fatigue crack growth behavior of low-alloy steels under simulated boiling water reactor conditions
[J].
A study of X100 pipeline steel passivation in mildly alkaline bicarbonate solutions using electrochemical impedance spectroscopy under potentiodynamic conditions and Mott-Schottky
[J].
Passive film growth on carbon steel and its nanoscale features at various passivating potentials
[J].
Effect of pH on corrosion behavior of 14Cr12Ni3WMoV stainless steel in chlorine-containing solutions
[J].
pH对14Cr12Ni3WMoV不锈钢在含氯溶液中腐蚀行为的影响
[J].
A new study for healing pitting defects of 316L stainless steel based on microarc technology
[J].
The study of microbiologically influenced corrosion of 2205 duplex stainless steel based on high-resolution characterization
[J].
Mechanistic aspect of non-steady electrochemical characteristic during stress corrosion cracking of an X70 pipeline steel in simulated underground water
[J].
Effect of cathodic polarisation on stress corrosion cracking behaviour of a Ni (Fe, Al)-maraging steel in artificial seawater
[J].
Fundamentals of hydrogen evolution reaction and its implications on near-neutral pH stress corrosion cracking of pipelines
[J].
Electrochemical polarization behavior of X70 steel in thin carbonate/bicarbonate solution layers trapped under a disbonded coating and its implication on pipeline SCC
[J].
Fundamental investigation of stress corrosion cracking of E690 steel in simulated marine thin electrolyte layer
[J].The mechanism of stress corrosion cracking (SCC) of E690 high-strength steel in a marine thin electrolyte layer (TEL) was investigated by performing in-situ mechanical-electrochemical tests, slow strain rate tensile (SSRT) tests, and characterization of corrosion morphology. It was concluded that E690 steel was highly sensitive to SCC, which was jointly determined by local anodic dissolution (AD) and hydrogen embrittlement (HE) both caused by dissolved O-2. In addition to these functions, hydrogen oxidation catalyzed by ferric ion was found. There was a critical oxygen concentration, approximately 21% by volume, between these two different roles. Below this value, the increase in the oxygen concentration promoted the synergistic effect of AD and HE, resulting in the increase in SCC susceptibility. However, above this value, worse general corrosion offset crack initiation as well as the oxidation of hydrogen catalyzed by ferric ions reduced the SCC susceptibility.