高温金属结构材料通常必须满足服役环境对性能的苛刻要求,包括机械强度、韧性和表面化学稳定性等。通过添加一定量的Cr、Al、Si,利用这些元素的选择性氧化可在合金表面形成生长缓慢且连续致密的Cr2O3、Al2O3或SiO2保护性氧化膜,从而显著提高合金的抗氧化性能,延长使役寿命[1]。长期以来,人们致力于氧化机制的研究,以寻求改善合金抗氧化性能的有效途径。
基于上述背景,本文选择Ni-12Cr (质量分数,%) 合金作为研究对象,采用高压扭转变形[17]方法制备了纳米晶 (NC) 结构;采用聚焦离子束显微镜和扫描透射电镜研究了不同氧化时间 (特别是氧化初期) 粗晶 (CG) 和NC Ni-12Cr合金的氧化动力学、氧化膜的微观结构,澄清了晶粒粗化和形成保护性氧化膜之间的竞争关系。这有助于合理设计纳米晶镍基合金以权衡其晶粒尺寸和抗氧化性之间的关系。
1 实验方法
原始Ni-12Cr (名义成分) 合金由高纯Ni (99.999%) 和高纯Cr (99.99%) 熔炼而成。熔炼合金直接浇铸成
利用FEI Verios 460场发射扫描电子显微镜 (SEM) 观察粗晶氧化膜微观结构并测量其厚度,加速电压为15 kV,电流为1.6 nA。SEM样品制备过程为:先在氧化样品表面进行化学镀Ni,镀层厚度为1~2 μm,树脂镶嵌后进行机械研磨和抛光,酒精清洗吹干后利用精密离子抛光仪去除表面应力层,离子束电压为4.5 kV,抛光时间为1 h。采用双束聚焦离子束显微镜 (FIB-SEM) 制备氧化膜截面透射样品,电子束加速电压为18 kV,电流为1.6 nA,离子束加速电压为30 kV,电流为40 pA~9.7 nA。采用FEI Talos F200X扫描透射电镜 (STEM) 表征纳米晶合金氧化前后的微观结构和氧化膜厚度等,利用选区电子衍射 (SAED) 和纳米束衍射 (NBD) 分析氧化膜晶体结构,在STEM模式下利用自带的能谱仪 (EDS) 获得元素的面分布和线分布并分析氧化膜成分组成。STEM的加速电压为200 kV。
2 实验结果
2.1 纳米晶Ni-12Cr合金的微观组织
图1
图1
变形态Ni-12Cr合金微观组织
Fig.1
TEM microstructure (a) and grain size distribution (b) of as-deformed Ni-12Cr alloy
2.2 粗晶和纳米晶Ni-12Cr合金的氧化动力学曲线
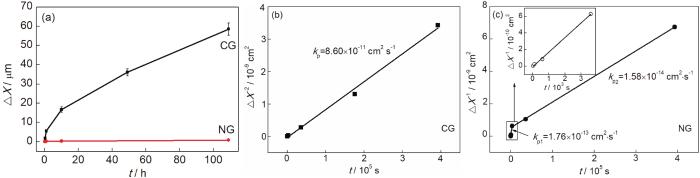
图2为粗晶和纳米晶合金在800 ℃空气中氧化109 h氧化膜厚度时间的动力学曲线。可以看出,纳米晶氧化膜厚度增加的速率明显低于粗晶。根据公式[2]
图2
图2
粗晶和纳米晶结构Ni-12Cr合金在800 ℃空气中氧化109 h的氧化动力学曲线和抛物线速率常数
Fig.2
Oxidation kinetic curves (a) and parabolic rate constants of CG (b) and NC (c) Ni-12Cr alloy at 800 ℃ in air for 109 h. The inset in (Fig.2c) shows the enlarged view at the initial oxidation stage
2.3 粗晶和纳米晶Ni-12Cr合金的氧化膜截面的微观结构与成分分布
图3
图3
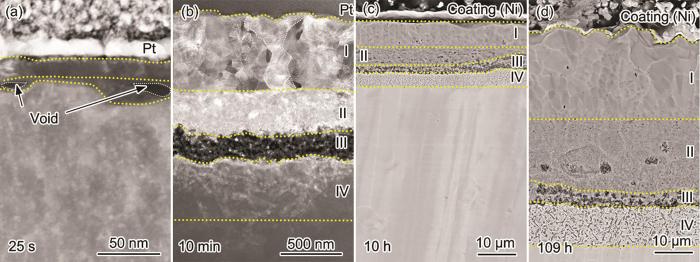
粗晶Ni-12Cr合金在800 ℃下空气中氧化不同时间截面形貌
Fig.3
Cross-sectional morphologies of coarse-grained Ni-12Cr alloy at 800 ℃ in air for 25 s (a), 10 min (b), 10 h (c) and 109 h (d)
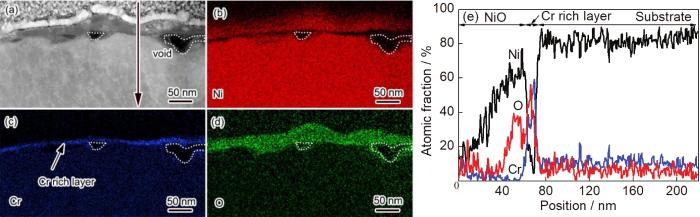
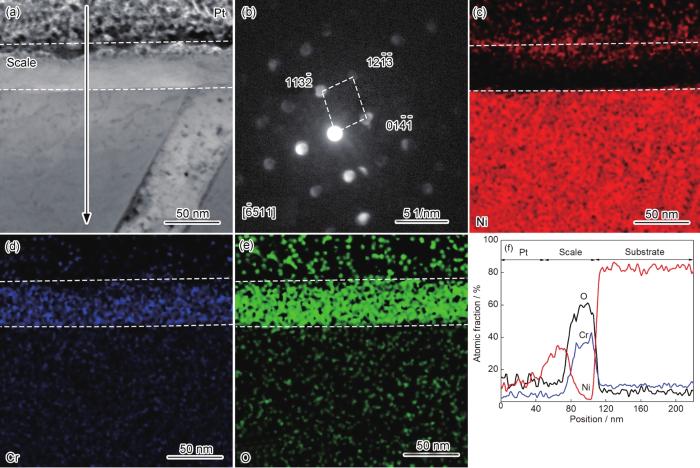
为了进一步研究粗晶氧化膜的微观结构,分别对氧化25 s和10 min时氧化膜的截面组织进行了元素分布和选区电子衍射分析。图4a~d为粗晶氧化25 s时截面的TEM形貌和EDS分析结果。从图中可以看出,氧化25 s时粗晶外层为富Ni和O的NiO,平均厚度约为21 nm;内层为富Cr层,平均厚度约为10 nm。图5为粗晶氧化10 min时截面的TEM形貌、选区电子/纳米束衍射和EDS分析结果。从图中可以看出,第I层富Ni和O (图5b、d和i),纳米束衍射分析该层结构为NiO (图5e);第II层富Ni、Cr和O (图5b、c、d和i),选取电子衍射分析显示该区为NiO和NiCr2O4尖晶石的混合结构 (图5f);第III层富Cr和O (图5c,d和i),纳米束衍射分析显示该层主要为Cr2O3 (图5g);第IV层内第二相Cr和O含量较高 (图5c和d),纳米束衍射分析显示该相为Cr2O3 (图5h)。随着反应的进行,基体Ni除了向外扩散与O发生反应形成NiO之外,少量Ni和向内扩散的O与第III层的Cr2O3在II、III层界面发生反应形成NiCr2O4[19],使第II层界面不断向合金侧推移。文献中通常将第II层和第III层视为一层,即NiO+Cr2O3+NiCr2O4氧化层[19]。Ni向外扩散的同时,空位向氧化膜和基体界面扩散和团聚,导致界面处形成空洞[20] (图4a)。对比粗晶氧化25 s和10 min时的氧化膜结构可以发现,氧化25 s时在氧化膜/基体界面形成了富Cr层,但该富Cr层并未有效抑制内氧化,导致氧化膜最终发展为多层 (NiO/NiO+NiCr2O4/多孔Cr2O3/内氧化) 结构,说明该条件下形成的富Cr层不能有效抑制Ni向外扩散和O相内扩散。
图4
图4
粗晶在800 ℃空气中氧化25 s截面TEM形貌和EDS分析结果
Fig.4
Cross-sectional STEM morphology (a) of coarse-grained Ni-12Cr alloy at 800 ℃ in air for 25 s, EDS mappings of Ni (b), Cr (c) and O (d) and elemental line scannings (e)
图5
图5
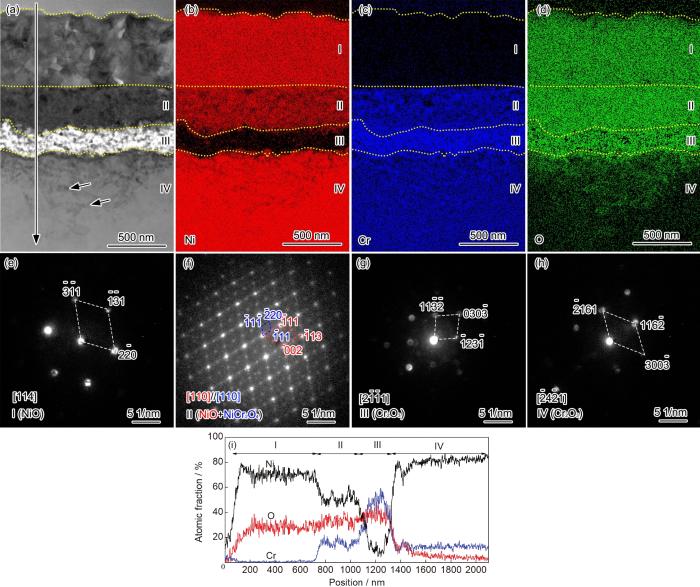
粗晶样品在800 ℃空气中氧化10 min后的截面TEM形貌、选区电子/纳米束衍射和EDS分析结果
Fig.5
Cross-sectional STEM morphology (a) of coarse-grained Ni-12Cr alloy at 800 ℃ in air for 10 min, EDS mappings of Ni (b), Cr (c) and O (d), NBD of layer I (e), SAED of layer II (f), NBDs of layer III (g) and layer IV (h), and EDS line scannings (i)
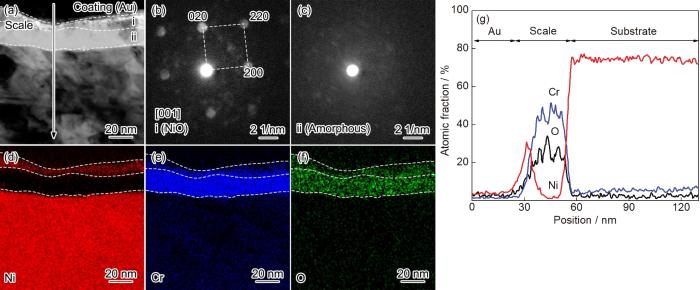
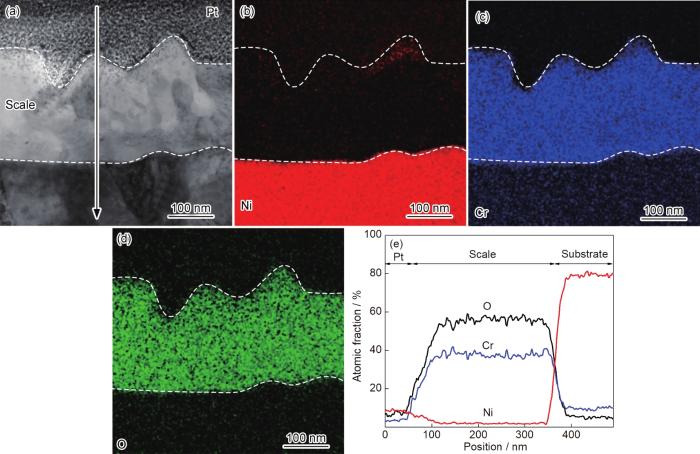
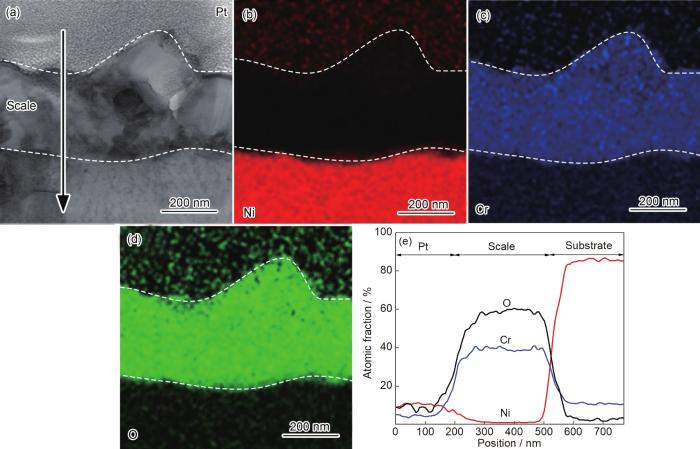
图6为纳米晶Ni-12Cr合金在800 ℃空气中氧化25 s后氧化膜截面的TEM形貌、纳米束衍射和EDS分析结果。可以看出,纳米晶表面形成厚度约为30 nm的氧化膜 (图6a)。Ni、Cr和O的面扫描 (图6d~f) 和线扫描 (图6g) 结果显示氧化膜分为两层:外层富Ni,厚度约为10 nm;内层富Cr,不含Ni,厚度约为20 nm。纳米束衍射分析显示外层为NiO (图6b),内层为非晶 (图6c)。对比粗晶和纳米晶氧化25 s后的组织可以看出,纳米晶氧化膜的厚度和分层结构与粗晶相似,不同的是纳米晶NiO层较薄,富Cr层较厚。图7为纳米晶在800 ℃下空气中氧化2 min后氧化膜截面的TEM形貌、纳米束衍射和EDS分析结果。可以看出,氧化膜的厚度增加至60 nm (图7a)。纳米束衍射分析显示富Cr层由非晶转变为Cr2O3晶态结构 (图7b)。图8为纳米晶在800 ℃下空气中氧化1 h后氧化膜截面的TEM形貌和EDS分析结果。此时,氧化膜的厚度增加至252 nm (图8a),氧化膜内平均晶粒尺寸约为82 nm。纳米晶在800 ℃下空气中氧化10 h后,氧化膜的厚度进一步增加至320 nm (图9a),而氧化膜内平均晶粒尺寸 (约为77 nm) 与氧化1 h时的样品相近,说明氧化1 h时,纳米晶的氧化膜进入稳态增厚阶段。值得说明的是,纳米晶氧化2 min、1 h和10 h后的样品在FIB制备过程中受离子束轰击的影响,外层NiO的结构受到破坏,形貌显示不清晰,因此统计氧化膜厚度时未统计NiO的厚度。相应地,图2中动力学数据中均未包含NiO的厚度。
图6
图6
纳米晶在800 ℃空气中氧化25 s氧化膜截面形貌、纳米束衍射和元素分布图
Fig.6
Cross-sectional STEM morphology (a) of nanocrystalline Ni-12Cr alloy at 800 ℃ in air for 25 s, NBDs of layer i (b) and layger ii (c), EDS elemental mappings (d~f) and line scannings (g)
图7
图7
纳米晶在800 ℃空气中氧化2 min氧化膜截面形貌、纳米束衍射和元素分布图
Fig.7
Cross-sectional STEM morphology (a) of nanocrystalline Ni-12Cr alloy at 800 ℃ in air for 2 min, NBD (b), EDS mappings (c-e) and EDS line scannings (g)
图8
图8
纳米晶在800 ℃空气中氧化1 h氧化膜截面形貌和元素分布图
Fig.8
Cross-sectional STEM morphology (a) of nanocrystalline Ni-12Cr alloy at 800 ℃ in air for 1 h, EDS mappings (b-d) and EDS line scannings (e)
图9
图9
纳米晶在800 ℃空气中氧化10 h氧化膜截面形貌和元素分布图
Fig.9
Cross-sectional STEM morphology (a) of nanocrystalline Ni-12Cr alloy at 800 ℃ in air for 10 h, EDS mappings (b-d) and EDS line scannings (e)
3 分析讨论
3.1 纳米晶Ni-12Cr合金形成Cr2O3的氧化机制
氧化25 s时粗晶氧化膜的结构与纳米晶氧化膜的结构相近,均为外层NiO、内层富Cr。但继续氧化,粗晶氧化膜演化为NiO/NiO+NiCr2O4/多孔Cr2O3/内氧化的多层结构,而纳米晶内层富Cr层转变为Cr2O3,说明纳米晶在NiO氧化物/基体界面发生选择性氧化,而粗晶由于Cr含量不足导致内氧化。根据Wagner理论,Ni-Cr合金在800 °C由内氧化向外氧化转变所需的临界Cr含量为[21]:
式中,g*为发生由内氧化向外氧化转变的临界Cr2O3体积分数,通常为0.3[22];
式中,
从
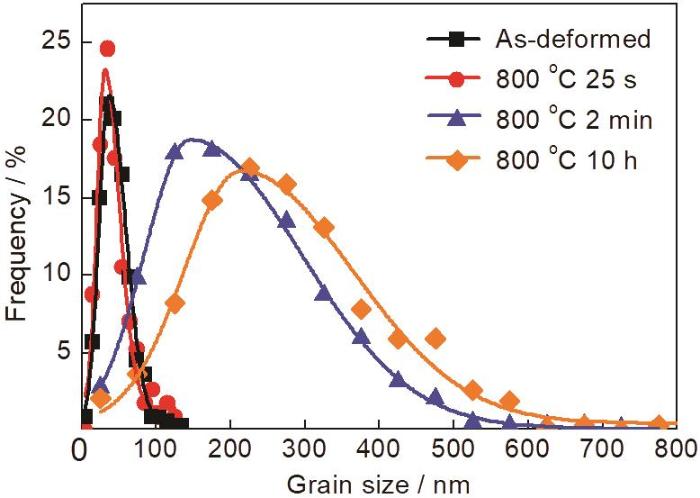
图10
图10
纳米晶Ni-12Cr合金在800 °C下保温不同时间后的晶粒尺寸分布
Fig.10
Grain size distributions of as-deformed and annealed nanocrystalline Ni-12Cr alloy after annealing at 800 ℃ in air for 25 s, 2 min and 10 h
3.2 纳米晶Ni-12Cr合金的抛物线速率常数
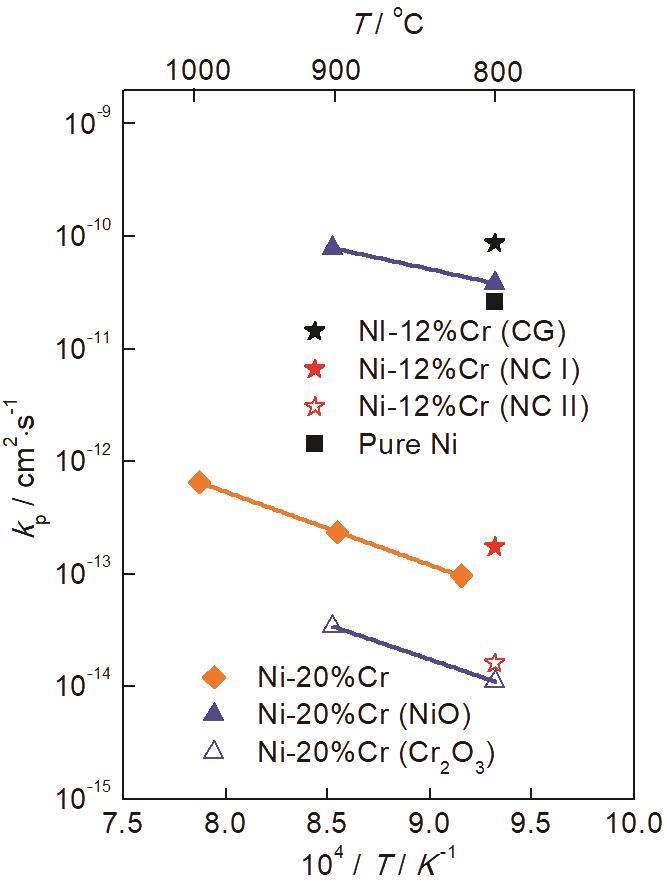
图11
纳米晶Ni-12Cr合金的抛物线速率常数分为两个阶段:kp1低于纯Ni[31],但稍高于粗晶Ni-20Cr合金在临近温度下的抛物线速率常数[32];kp2与Ni-20Cr合金内层Cr2O3[19]的抛物线速率常数相当。说明氧化时间>1 h时,纳米晶Ni-12Cr合金氧化膜生长由阳离子在氧化膜内的传输控制。kp1较高的氧化行为在粗晶Ni-15Cr[34]、纳米晶Ni-20Cr[10]、铸造镍基高温合金K447[35]、Ni-10Cr-5Al[36]、Fe-19Cr-5.5Al[37]、β-NiAl[38]等多种合金中均有发现。一种观点认为[35]氧化初期表面反应为氧化反应的控制步骤,即氧在金属表面被吸附和离子化,之后与合金元素反应形成氧化物晶核,晶核长大并在二维扩展形成连续氧化膜,这一过程结束之后即进入稳态氧化阶段。然而高温氧化反应的形核和二维晶核长大往往发生在几分钟之内[37]。本文中纳米晶Ni-12Cr合金氧化25 s即可在金属表面形成一层连续的氧化膜,而抛物线速率常数转折点发生在氧化1 h左右,文献[10,35-38]中则是在氧化开始数小时之后才出现转折点,与上述机制矛盾。另一种观点认为在Al2O3形成体系中,抛物线速率常数转折点的出现与亚稳态Al2O3向稳态α-Al2O3转变有关[38]。然而,Cr2O3只存在一种晶型,并不存在同素异构转变。
基于Wagner理论推导的抛物线速率常数表达式为[39]:
式中,
4 结论
(1) 800 ℃下恒温氧化25 s时,粗晶和纳米晶Ni-12Cr合金表面均形成表层为NiO和内层富Cr的两层氧化膜结构。继续氧化,粗晶氧化膜演化为NiO/NiO+NiCr2O4/多孔Cr2O3/内氧化层的多层结构,而纳米晶发生Cr的选择性氧化形成Cr2O3,Cr2O3以非晶晶化的方式形核长大。
(2) 纳米晶保护性氧化膜的形成早于纳米晶晶粒粗化的发生,根据有效扩散系数修正的Wagner理论,Ni-12Cr合金发生由内氧化向外氧化转变的临界晶粒尺寸约为94 nm。
(3) 纳米晶Ni-12Cr合金的抛物线速率常数比粗晶低约3~4个数量级。纳米晶的氧化动力学曲线分为两个阶段,均符合抛物线规律。两阶段的抛物线速率常数分别为:氧化1 h内,kp1为1.76×10-13 cm2·s-1;氧化1~109 h,kp2为1.58×10-14 cm2·s-1。
参考文献
Theoretical analysis of the diffusion processes determining the oxidation rate of alloys
[J].
The effect of nanocrystallization on the selective oxidation and adhesion of Al2O3 scales
[J].
On the role of dislocations in bulk diffusion
[J].
The oxidation resistance of fine-grained sputter-deposited 304 stainless steel
[J].
Differences in oxides on large-and small-grained 304 stainless steel
[J].
Oxidation behavior of a fine-grained rapidly solidified 18-8 stainless steel
[J].
Oxidation resistance of sputtered Ni3(AlCr) nanocrystalline coating
[J].
Oxidation behaviour of sputter-deposited Ni-Cr-Al micro-crystalline coatings
[J].
Effect of grain-size reduction on oxidation behavior of Fe-Cr and Ni-Cr alloys
[J].
晶粒细化对Fe-Cr、Ni-Cr合金氧化行为的影响
[J].
Nanocrystalline coatings on superalloys against high temperature oxidation: A review
[J].
Investigation of the growth and stability of (100)[001] NiO films grown by thermal oxidation of textured (100)[001] Ni tapes for coated conductor applications during oxygen exposure from 700 to 1400 ℃
[J].
Nanoscale assembly of high-temperature oxidation-resistant nanocomposites
[J].
Recent research progress on nanocrystalline materials
[J].
纳米晶体材料的研究现状
[J].本文综述国内外在纳米晶体材料研究领域的最新研究进展,包括纳水晶体材料的制备、微观结构特证、热稳定性、结构性能关系及纳米品体材料的应用
Stabilizing nanostructures in metals using grain and twin boundary architectures
[J].
Suppressing atomic diffusion with the Schwarz crystal structure in supersaturated Al-Mg alloys
[J].High atomic diffusivity in metals enables substantial tuneability of their structure and properties by tailoring the diffusional processes, but this causes their customized properties to be unstable at elevated temperatures. Eliminating diffusive interfaces by fabricating single crystals or heavily alloying helps to address this issue but does not inhibit atomic diffusion at high homologous temperatures. We discovered that the Schwarz crystal structure was effective at suppressing atomic diffusion in a supersaturated aluminum-magnesium alloy with extremely fine grains. By forming these stable structures, diffusion-controlled intermetallic precipitation from the nanosized grains and their coarsening were inhibited up to the equilibrium melting temperature, around which the apparent across-boundary diffusivity was reduced by about seven orders of magnitude. Developing advanced engineering alloys using the Schwarz crystal structure may lead to useful properties for high-temperature applications.Copyright © 2021, American Association for the Advancement of Science.
The effect of coating grain size on the selective oxidation behaviour of Ni-Cr-Al alloy
[J].
High temperature oxidation behavior of a novel Ni-Cr binary alloy coating prepared by cathode plasma electrolytic deposition
[J].
Oxidation mechanism of Ni-20Cr foils and its relation to the oxide-scale microstructure
[J].
In situ observations of early stage oxidation of Ni-Cr and Ni-Cr-Mo alloys
[J].
Types of reactions in the oxidation of alloys
[J].
The transition from internal to external oxidation and the formation of interruption bands in silver-indium alloys
[J].
Temperature effect on oxidation behavior of Ni-Cr alloys in CO2 gas atmosphere
[J].
A review of the role of short-circuit diffusion in the oxidation of nickel, chromium, and nickel-chromium alloys
[J].
A novel method to promote selective oxidation of Ni-Cr alloys: Surface spreading α-Al2O3 nanoparticles
[J].
A new theoretical approach to the thermodynamic calculation of high-temperature oxidation of Ni-Cr alloys
[J].
Diffusion and surface alloying of gradient nanostructured metals
[J].
High temperature corrosion of nanocrystalline metallic materials
[J].
纳米晶金属材料的高温腐蚀行为
[J].一些金属基结构材料, 不需要增加Cr和Al含量而只需“纳米晶化”, 就能够在高温环境下形成保护性Cr<sub>2</sub>O<sub>3</sub>或Al<sub>2</sub>O<sub>3</sub>氧化膜. 纳米晶化是施加高Cr高Al涂层之外提高金属材料抗高温腐蚀性能的另一途径. 近20年来, 纳米晶金属材料的高温腐蚀行为已广泛报道. 本文简要评述了纳米晶金属材料的高温腐蚀特性、纳米晶化提高金属抗氧化性能的根本原因以及亟待澄清的问题.
Grain boundary diffusion mechanisms in metals
[J].
The effect of silicon and manganese on the oxidation mechanism of Ni-20 Cr
[J].
The effects of yttrium ion implantation on the oxidation of nickel-chromium alloys. II. Oxidation of yttrium implanted Ni-20Cr
[J].
Transport properties of Al or Cr-doped nickel oxide relevant to the thermal oxidation of dilute Ni-Al and Ni-Cr alloys
[J].
Mechanism of oxidation of dilute nickel-chromium alloys
[J].
Isothermal oxidation behavior of a cast Ni-base superalloy K447
[J].
铸造镍基高温合金K447的高温氧化行为
[J].
Effect of surface roughness on oxidation behavior of Ni-Cr-Al alloy at high temperatures
[J].
不同表面粗糙度镍铬铝合金的高温氧化行为
[J].
Growth rates of alumina scales on Fe-Cr-Al alloys
[J].
Effect of the θ-α-Al2O3 transformation on the oxidation behavior of β-NiAl+Zr
[J].