目前改善热障涂层的抗CMAS腐蚀能力的研究,主要在以下几种方面:在热障涂层表面添加一层惰性保护层[9,10],优化原有涂层的组分[11,12],设计新的热障涂层材料[13],改变热障涂层表面的微观结构[14]等。Zhang等[15]通过在热障涂层表面镀铝,能够封堵热障涂层表面的孔洞与裂纹,限制CMAS的扩散。Kang等[16]利用飞秒激光在热障涂层表面加工出凹型微结构,改变了表面与熔融CMAS的润湿性,降低了其与CMAS的接触面积,从而限制了CMAS在热障涂层表面的沉积。上述方法虽然能够有效地改善了热障涂层的抗CMAS能力,但是受到匹配性差,工艺繁琐,增重明显,成本高昂的限制,仍处于研究阶段。Kramer等[17]报道了Gd2Zr2O7能够与渗入的CMAS迅速反应,并生成高稳定性的Ca2Gd8(SiO4)6O2磷灰石硅酸盐相和Zr(Gd,Ca)O x 萤石相沉淀,形成了致密的封堵层,抑制了CMAS的进一步渗入。Gd2Zr2O7还具有较低的热导率和较好的相稳定性,有望成为代替YSZ的材料之一[18]。但是,Gd2Zr2O7的抗CMAS性能需要进一步提高,以满足服役要求。
本文提出了一种新的提高热障涂层抗CMAS腐蚀的方法。首次将预腐蚀的方法引入到提高热障涂层材料抗CMAS腐蚀性能研究中,通过设计的腐蚀粉末 (CaO-Al2O3-SiO2-Gd2O3-TiO2,ATSCG) 来预腐蚀Gd2Zr2O7,加速了在反应初期磷灰石相为主的高熔点的化合物沉淀,形成致密的封堵层,抑制CMAS的进一步渗入。同时,研究了预腐蚀温度对CMAS渗透深度的影响,并探究其腐蚀机理。
1 实验方法
通过固相反应制备了Gd2Zr2O7粉末。首先,将适量的Gd2O3 (国药,99.9%) 和ZrO2 (国药,99.5%) 加入无水乙醇中,置于球磨机中,以120 r/min转速球磨13 h。将得到的湿粉干燥、研磨后,置于1400 ℃高温炉中烧结6 h。随后将所得粉末再次经过湿法球磨、干燥,然后在约35 GPa的压力 (GK-15粉末压片机) 下冷压并保持1 min,得到直径为7 mm、厚度约2 mm的片状样品。最后放在1400 ℃高温炉中无压烧结6 h,即得到本实验的Gd2Zr2O7块体。
预腐蚀粉末ATSCG为28.5CaO-11.1Al2O3-32.4SiO2-3Gd2O3-11.1TiO2 (摩尔分数,%)。将混合粉末加入过量无水乙醇后,球磨13 h,置于120 ℃的干燥箱内烘干10 h,得到干燥粉末;随后将所得粉末研磨,过200目筛,即得到预腐蚀粉末。CMAS粉末为33CaO-9MgO-13Al2O3-45SiO2 (摩尔分数,%)。将粉末混合物中加入无水乙醇放入球磨机中球磨13 h,然后放在50 ℃的烘箱内干燥12 h。将烘干的粉末放入1300 ℃的高温炉中煅烧8 h后,随后加入无水乙醇进行二次球磨。随后将所得粉末干燥,过200目筛,即得到CMAS粉末。
将制备好的ATCSG粉末与无水乙醇混合,涂覆在Gd2Zr2O7块体表面,涂覆量为20 mg/cm2。在50 ℃干燥箱中干燥3 h后,在不同温度下 (900,1000,1100 ℃) 保温0.5 h,以研究温度对预腐蚀效果的影响。CMAS热腐蚀实验:以同样的方式将CMAS涂覆至Gd2Zr2O7样品表面,涂覆量为20 mg/cm2,然后在1250 ℃下腐蚀3 h。
表面和截面形貌均通过场发射扫描电子显微镜 (SEM,JSM-7001F) 观察,化学成分通过能量色散光谱法 (EDS,Apollo XLT) 确定。通过X射线衍射仪 (XRD,D8 ADVANCE) 来分析CMAS、Gd2Zr2O7以及腐蚀后的样品表面相组成。通过使用差示扫描量热法 (DSC,STA 449F3 Jupiter) 确定CMAS热腐蚀的温度。腐蚀深度计算公式为h=S/L,S为腐蚀反应层面积 (通过Image J软件测量),L为腐蚀反应层的长度。
2 结果与讨论
2.1 Gd2Zr2O7与CMAS的表征
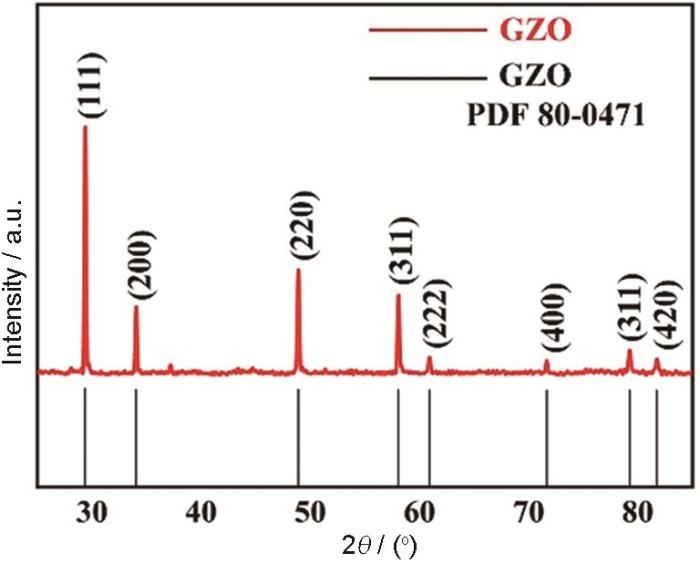
图1为所制备的Gd2Zr2O7陶瓷块体样品的XRD图。表明所制备的Gd2Zr2O7块体样品是具有烧绿石结构的单相。
图1
图2
图2
CMAS粉末的XRD图谱和TG-DSC图谱
Fig.2
XRD pattern (a) and TG-DSC curves (b) of CMAS powders
2.2 腐蚀后的截面形貌
图3
图3
不同温度预腐蚀Gd2Zr2O7在1250 ℃下CMAS腐蚀5 h的截面形貌
Fig.3
Cross sections of Gd2Zr2O7 un-treated (a) and pre-treated at 900 ℃ (b), 1000 ℃ (c) and 1100 ℃ (d) after 3 h CMAS corrosion at 1250 ℃
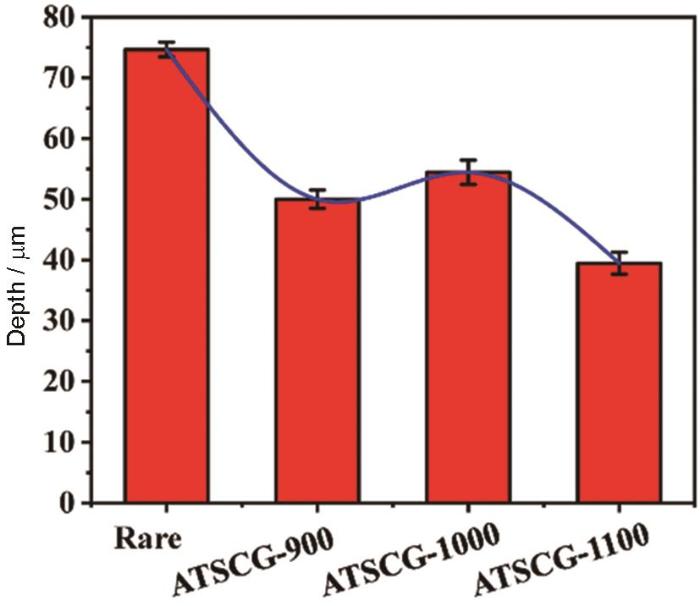
此外,预腐蚀后Gd2Zr2O7的抗CMAS性能可以通过反应层的深度来评价。如图4所示,预腐蚀温度为900、1000和1100 ℃时,反应层深度分别为50.01、54.48和39.46 μm。由于常见的高温合金以及单晶材料其服役极限温度不超过1150 ℃,未对超过1100 ℃之后的预腐蚀展开详细研究,以符合真实工况的需要。
图4
图4
经不同温度预腐蚀的Gd2Zr2O7在1250 ℃下CMAS腐蚀3 h的反应层深度
Fig.4
Thicknesses of the reaction layers formed on Gd2Zr2O7 pre-corroded at different temperatures after CMAS corrosion at 1250 ℃ for 3 h
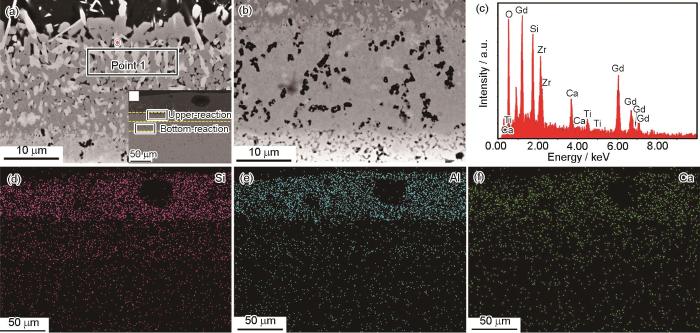
腐蚀反应层呈现上、下两个不同的微观结构特征。一层靠近CMAS,由棒状或针状结构组成的上反应层,另一层为与Gd2Zr2O7接触的下反应层。随着熔体CMAS在高温下不断渗透到预腐蚀后的陶瓷块中,腐蚀产物不断形成并析出。这些腐蚀产物最终会聚集在反应层的上半部分,形成致密层。腐蚀反应层的上部相对致密,而下部相对疏松。通过上层的部分放大图显示,在致密的上反应层中存在许多细长的晶粒 (图5a和b)。根据EDS分析 (图5c),该晶相主要由Ca,Si,Gd和O组成,这表明Ca2Gd8(SiO4)6O2在CMAS侵蚀预腐蚀后的Gd2Zr2O7的致密反应层中存在。图5d~f为CMAS腐蚀1100 ℃/0.5 h预腐蚀Gd2Zr2O7陶瓷块体截面对应的Ca、Al、Si EDS图。Ca、Al和Si主要集中在CMAS的残留层和上反应层中,说明在CMAS腐蚀过程中,CaO、Al2O3和SiO2被有效地阻隔。
图5
图5
上下反应层的 SEM形貌,EDS谱及 Si、Al、Ca的元素分布图
Fig.5
SEM images of the upper (a) and bottom (b) reaction layers, EDS analysis (c) at the point 1 in Fig.5a and EDS mappings of Si (d), Al (e), Ca (f)
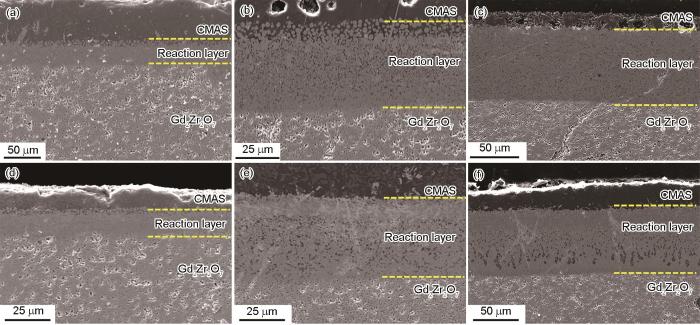
图6为未处理和经1100 ℃/0.5 h预腐蚀的Gd2Zr2O7块体在1250°C下CMAS腐蚀不同时间后的截面形貌。由图看出,在1250 ℃下,CMAS在Gd2Zr2O7内渗透5 min后形成的反应层厚度约为16.85 μm。当CMAS侵蚀达5和10 h后,形成了约48.85和100.35 μm厚度的反应层。因为在本试验中采用了大量腐蚀性物质 (~25 mg/cm2) 来加速热化学相互作用,因此随着时间的推移,涂层的渗透深度增加。而且根据文献[19],CMAS浓度高于4 mg/cm2不适合工作。但是ATSCG预腐蚀后的Gd2Zr2O7在5、300和600 min CMAS腐蚀后,其反应层深度分别为13.38,43.2和70.49 μm,较原始Gd2Zr2O7有明显的降低。因此,采用ATSCG预腐蚀的方法提高了Gd2Zr2O7表面生成磷灰石相层的能力和速率,比原始Gd2Zr2O7具有更小的渗透深度。
图6
图6
未处理和经1100 ℃/0.5 h预腐蚀的Gd2Zr2O7块体在1250 ℃下CMAS腐蚀不同时间后的截面形貌
Fig.6
Cross sections of Gd2Zr2O7 untreated (a-c) and pre-corroded (d-f) under the condition of 1100 ℃/0.5 h after CMAS corrosion at 1250 ℃ for 5 min (a, d), 5 h (b, e) and 10 h (c, f)
2.3 腐蚀机理
由于熔融的CMAS具有复杂的化学成分,因此很难用化学方程式或简单的机制来准确描述反应层是如何形成的。然而,根据其他研究人员的工作[20],ATSCG和Gd2Zr2O7都溶解在熔融的CMAS玻璃中。由于ATSCG和Gd2Zr2O7高稀土元素含量 (>36%),溶解的Gd2Zr2O7晶粒重新沉淀为t-ZrO2晶粒,使得熔融的CMAS成分发生显着改变。在熔融CMAS中掺入大量Gd会显着影响其结晶行为。此外,据报道Ti的加入也可以加速CMAS的结晶[21]。这导致熔融的CMAS迅速结晶化,形成以磷灰石相为主的结晶化合物,从而形成稳定致密的反应层,抑制CMAS的进一步渗透。在ATSCG中1100 ℃/0.5 h预腐蚀,有利于CMAS腐蚀初期在Gd2Zr2O7表面快速形成由高熔点Ca2Gd8(SiO4)6O2 (>2000 ℃) 组成的致密反应层,堵塞CMAS扩散通道[22]。此外,由于在磷灰石相形成过程中Ca:Si的增加,使得熔体粘度降低,避免了CMAS与Gd2Zr2O7的连续接触,阻碍了CMAS的后续反应,从而抑制了CMAS的进一步渗透。
3 结论
Gd2Zr2O7材料作为最有前景取代8YSZ,成为下一代热障涂层的材料,其抗CMAS性能有待进一步提高,以满足更复杂的服役要求。本文首次将预腐蚀工艺引入到Gd2Zr2O7抗CMAS性能的研究中。通过将预腐蚀粉末ATSCG涂覆至Gd2Zr2O7块体表面,研究了预腐蚀温度对Gd2Zr2O7抗CMAS腐蚀性能的影响。结果表明:
(1) 经1100 ℃/0.5 h预腐蚀后的Gd2Zr2O7在1250 ℃下CMAS腐蚀3 h的渗透深度为39.46 μm,腐蚀10 h后为70.49 μm。
(2) 预腐蚀后,CMAS在反应初期更容易形成稳定的高熔点Ca2Gd8(SiO4)6O2相,从而抑制CMAS熔体的持续渗透。
(3) 靠近CMAS的致密反应层能有效阻碍CMAS的持续渗透。
参考文献
Corrosion behavior and failure prediction of YSZ coatings under CMAS attack
[J].
Thermal barrier coatings-a state of the art review
[J].
Corrosion resistance of air plasma sprayed thermal barrier coating SrZrO3 on superalloy In718 against CaO-MgO-Al2O3-SiO2 (CMAS)
[J]. J.
等离子喷涂SrZrO3热障涂层的CaO-MgO-Al2O3-SiO2 (CMAS) 腐蚀行为
[J].
Failure mechanism, improvment method and future development direction of thermal barrier coatings
[J].
热障涂层失效机理、改进方法及未来发展方向
[J].
Calcia-magnesia-alumino-silicate (CMAS)-induced degradation and failure of air plasma sprayed yttria-stabilized zirconia thermal barrier coatings
[J].
Composite ceramics thermal barrier coatings of yttria stabilized zirconia for aero-engines
[J].
Step-by-step investigation of degradation mechanisms induced by CMAS attack on YSZ materials for TBC applications
[J].
CMAS corrosion of YSZ thermal barrier coatings obtained by different thermal spray processes
[J].
CMAS (CaO-MgO-Al2O3-SiO2) resistance of Y2O3-stabilized ZrO2 thermal barrier coatings with Pt layers
[J].
CMAS behavior of yttrium aluminum garnet (YAG) and yttria-stabilized zirconia (YSZ) thermal barrier coatings
[J].
Investigation of calcium-magnesium-alumino-silicate (CMAS) resistance and hot corrosion behavior of YSZ and La2Zr2O7/YSZ thermal barrier coatings (TBCs) produced with CGDS method
[J].
CMAS-resistant plasma sprayed thermal barrier coatings based on Y2O3-stabilized ZrO2 with Al3+ and Ti4+ solute additions
[J].
Hot corrosion behavior of Gd2(Zr1- x Ce x )2O7 thermal barrier coating ceramics exposed to artificial particulates of CMAS
[J]. J.
Gd2(Zr1- x Ce x )2O7热障涂层陶瓷层材料的CMAS热腐蚀行为研究
[J].
Volcanic ash infiltration resistance of new-generation thermal barrier coatings at 1150 ℃
[J].
Adsorbability and spreadability of calcium-magnesium-alumino-silicate (CMAS) on Al-modified 7YSZ thermal barrier coating
[J].
High temperature wettability between CMAS and YSZ coating with tailored surface microstructures
[J].
Infiltration-inhibiting reaction of gadolinium zirconate thermal barrier coatings with CMAS melts
[J].
Preparation of nanostructured Gd2Zr2O7-LaPO4 thermal barrier coatings and their calcium-magnesium-alumina-silicate (CMAS) resistance
[J].
Jet engine coatings for resisting volcanic ash damage
[J].
Degradation of La2Zr2O7 and other novel EB-PVD thermal barrier coatings by CMAS (CaO-MgO-Al2O3-SiO2) and volcanic ash deposits
[J].
Titanium substitution in Gd2Zr2O7 for thermal barrier coating applications
[J].The study underlines the impact of Ti(4)(+)substitution in Gd2Zr2O7 for applications in thermal barrier coatings (TBC). Depending on the Ti4+ content, two different crystal structures of Gd2Zr2O7 namely pyrochlore and fluorite were determined. Ti4+ substitutions in the increasing order induced a gradual contraction of Gd2Zr2O7 unit cell; however, with the accomplishment of concentration dependent crystal structures of either single phase pyrochlore or mixtures of pyrochlore and fluorite. Absorption measurements enunciated the enhanced infra-red reflectance behaviour of Gd2Zr2O7 due to Ti4+ substitutions. A gradual increment in the concentration of Ti4+ substitutions in Gd2Zr2O7 envisaged a simultaneous porous to dense morphological features, which reflected in the resultant mechanical data. Hot corrosion studies ensure the critical role of Ti4+ to retain the crystal structure of Gd2Zr2O7.
Gadolinium oxide solubility in molten silicate: dissolution mechanism and stability of Ca2Gd8(SiO4)6O2 and Ca3Gd2(Si3O9)2 silicate phases
[J].