磁场对金属腐蚀影响的研究结论不尽相同。多数研究[5-8]表明,Fe在溶液中受磁场影响时产生磁致过电位,使电极电位正移;也有研究[9]表明铝镁合金在NaCl溶液中受磁场影响时的腐蚀电位负移。研究发现磁场对腐蚀分布具有重要影响,磁场会改变自然腐蚀的阴阳极状态[10],磁场与金属腐蚀面的相对方向[11,12]明显影响腐蚀区域的分布。磁场对金属的腐蚀速率影响也不一致,Ghabashy[13]研究认为,Fe在氯化铁溶液中腐蚀速率受外加磁场的影响,外加磁场较小时腐蚀速率较自然腐蚀速率降低,外加磁场较大时腐蚀速率较自然腐蚀速率增大;张康南等[14]研究表明X80钢在沈阳草甸土中腐蚀速率随着外加磁场强度增强而增大;惠海军等[15]研究表明磁场加速了X60钢在长沙地区土壤中的腐蚀速率;Jackson等[4]研究表明磁场作用加重了试样表面的点蚀和裂纹等缺陷;Espina-Hernández等[16]研究却表明磁场抑制了点蚀的发展;卫晓阳等[17]研究表明磁场可以抑制Cu的微生物腐蚀进程。关于磁场对腐蚀行为产生影响的机理,一般都认为其对腐蚀电极的活化反应过程影响甚微[18,19],主要是对反应物和反应产物的传质具有重要影响[19-21],但Sueptitz等[22]在研究稀硫酸中Fe腐蚀受磁场影响时认为,垂直于电极反应面的磁场可以使Fe2+远离电极、H+靠近电极,从而电极表面溶液pH值减小,造成活化反应电流密度增大。Lorentz力和磁场梯度力是影响传质的主要因素[21,23,24],磁场与带电粒子运动的相对方向决定Lorentz力方向,磁场梯度及粒子磁性决定磁场梯度力的方向,综合影响电极反应速率[25,26]。然而,以上除了Espina-Hernández之外的研究都是将实验试样及电解溶液放置于外加的均匀磁场中,与漏磁内检测的剩余磁场分布明显不同,难以反映客观状况,且未见管道海水腐蚀受磁场影响的研究文献。
本文以开路电位 (OCP)、电位极化曲线、电化学阻抗谱 (EIS) 测量以及腐蚀形貌观察等技术研究磁场对3.5% (质量分数) NaCl溶液中X52管线钢腐蚀行为的影响,并探讨了磁场对腐蚀影响的机理。
1 实验方法
试样材料为X52管线钢,其化学成分 (质量分数,%) 为:C 0.20,Si 0.45,Mn 1.60,P 0.02,S 0.01,Fe余量。试样为
电化学测试采用三电极体系在Gamry Reference 600+电化学工作站上完成。辅助电极为铂电极,饱和甘汞电极 (SCE) 为参比电极。将试样在-1.2 V电位下阴极极化3 min后测试开路电位 (OCP),待电位稳定后,分别开展静、动电位极化曲线和电化学阻抗谱 (EIS) 测试。以腐蚀电位测试静电位极化曲线;以1 mV/s扫描速率测试动电位极化曲线;EIS正弦波激励信号振幅为±10 mV,扫描频率范围为105~10-2 Hz。用软件Origin2017拟合极化曲线动力学参数,软件ZsimpWin3.60拟合电化学阻抗谱曲线。利用光学显微镜观察腐蚀形貌。
线圈中分别施加0、1、2、3 A直流电流以感应出不同的磁场强度。磁场强度由
式中,H为磁场强度 (A/m);N为线圈匝数;I为线圈中电流值 (A);L为磁路长度 (m),本文中为圆环试样周长。
线圈中施加1、2和3 A直流电流产生的计算磁强度分别为0.9、1.9及2.8 kA/m。相关研究[27]表明,在4.5 kA/m的磁场强度下,X52钢磁感应强度不大于1.5 T,则本研究中的试样均处于不同程度的非饱和磁化状态。
2 结果与讨论
2.1 OCP
图1
图1
不同的磁场强度对X52管线钢腐蚀电位影响
Fig.1
Effect of magnetic field on the corrosion potential of X52 pipeline steel: (a) corrosion potential of samples under different magnetic field strengths, (b) the change of sample corrosion potential with different magnetic field strength
2.2 电位极化
图2分别为不同磁场强度下NaCl溶液中X52管线钢恒电位及动电位极化曲线。恒电位极化电位设为自腐蚀电位,磁场强度为0.9 kA/m时,腐蚀电流为阴极电流并达到稳定,但数值很小;磁场强度为1.9和2.8 kA/m时,腐蚀电流变成阳极电流并持续增大。动电位极化曲线显示,有无磁场情况都为活性溶解;有磁场时,随着磁场强度增大,腐蚀电流密度从7.3 μA/cm2增长到12.7、22.8及37.1 μA/cm2,即磁场强度越大腐蚀速率越大。
图2
图2
不同磁场强度下的恒电位及动电位极化曲线
Fig.2
Potentiostatic (a) and potentiodynamic (b) polarization curves with magnetic fields
2.3 EIS
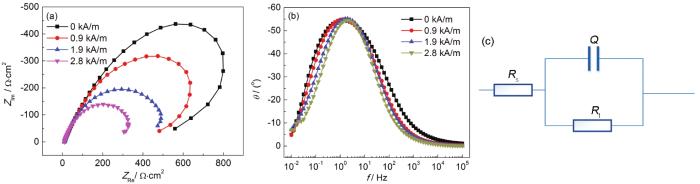
图3a和b为不同磁场强度下3.5%NaCl溶液中X52管线钢EIS曲线。由Nyquist图可见,不同磁场强度下电化学阻抗谱都为单一容抗弧,说明反应过程由电子转移过程控制。由容抗弧半径大小可见,磁场强度越大,电化学反应阻抗越小,耐腐蚀性越弱。结合Bode图,以图3c所示等效电路对EIS数据进行拟合,等效电路中,Rs为溶液电阻,Q为双电层电容常相位角元件,Rt为电荷转移电阻。拟合结果见表1,电荷转移电阻随着磁场强度增大而减小,2.8 kA/m磁场强度下的电荷转移电阻 (369 Ω·cm2) 不到无磁场时的 (1182 Ω·cm2) 三分之一,即耐蚀性随着磁场强度增大而大幅减弱;弥散指数ndl随着磁场强度增大而增大,说明磁场强度越大腐蚀电流密度分布越均匀,应该是由于磁场改变了试样表面阴阳极的分布状态[10]。
图3
图3
不同磁场强度下的EIS Nyquist和Bode图及等效电路
Fig.3
Nyquist (a) and Bode (b) plots of EIS with magnetic fields and equivalent circuit of EIS (c)
表1 不同磁场强度下NaCl溶液中试样EIS等效电路拟合结果
Table 1
| H / kA/m | Rs / Ω·cm2 | Ydl / S·sn·cm-2 | ndl | Rt / Ω·cm2 |
|---|---|---|---|---|
| 0 | 9.4 | 1.55×10-3 | 0.6930 | 1182 |
| 0.9 | 11.0 | 1.74×10-3 | 0.7316 | 827 |
| 1.9 | 9.1 | 2.11×10-3 | 0.7507 | 562 |
| 2.8 | 9.5 | 2.19×10-3 | 0.7793 | 369 |
2.4 腐蚀形貌
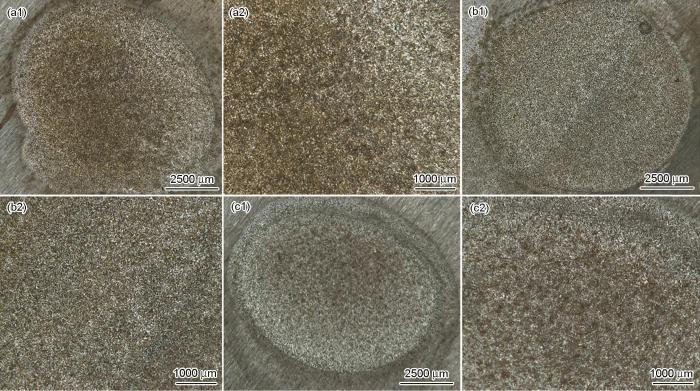
图4为不同磁场强度下X52管线钢在3.5%NaCl溶液中浸泡10 d的腐蚀形貌。自然腐蚀情况下,部分表面明显比其余部分腐蚀严重;0.9 kA/m磁场强度下的腐蚀相对更严重,但腐蚀程度更均匀;1.9 kA/m磁场强度下的腐蚀明显更严重,表面分布许多较深的小腐蚀坑。
图4
图4
不同磁场强度下X52管线钢在3.5%NaCl溶液中浸泡10 d的腐蚀形貌
Fig.4
Optical photographs of X52 steel in 3.5%NaCl solution for 10 d under 0 kA/m (a1, a2), 0.9 kA/m (b1, b2) and 1.9 kA/m (c1, c2) magnetic fields
2.5 磁场作用机理
图5
图5
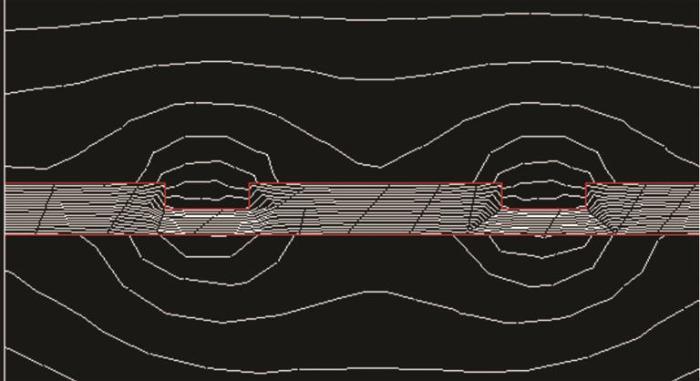
试样表面磁感应强度分布示意图
Fig.5
Schematic diagram of the magnetic flux intensity distribution on the surface of the sample
式中,J为带电离子的能量密度,B为磁感应强度。
本研究中,溶液中的离子主要有Na+、Cl-以及钢腐蚀产生的Fe2+,其中Fe2+为顺磁性离子,且在电极表面不断产生并向溶液中扩散。由图5可见,Fe2+扩散时会切割磁力线从而产生平行于电极表面的FL,加速贴近电极表面的Fe2+扩散。逆磁性离子Na+和Cl-不参与电化学反应,基本不受FL的影响。
在非均匀磁场中,存在使顺磁性离子向高磁感应强度区移动、逆磁性离子向低磁感应强度区移动的Kelvins力FB:
式中,
离子磁性、浓度、磁感应强度和梯度值决定FB大小,磁感应强度分布及离子磁性决定离子的移动方向。电极表面及几何不连续处边缘的磁感应强度更大 (如图5所示),Fe2+倾向于电极表面及几何不连续处边缘集聚,Na+和Cl-倾向于远离这些位置。
另一个重要的磁场力是顺磁性梯度力,表达式见
本研究中的阳极、阴极反应主要为:
Lorentz力加速Fe2+扩散的同时,其引起的微观溶液运动会减小双电层厚度[32],减小了电化学反应速阻抗 (图3);但Kelvins力对Fe2+的集聚作用会抑制腐蚀的发生。Kelvins力使逆磁性离子Cl-在电极表面的浓度减小,一定程度上提高了O的溶解度[33],从而促进阴极反应的进行 (图2b)。可见,磁场对电化学反应过程的影响由多种因素综合决定,取决于电极表面的磁场强度、梯度以及电解质中离子磁性、浓度等。实验显示本研究中磁场促进了腐蚀发展,说明Lorentz力加速Fe2+扩散、减小双电层厚度及Kelvins力增加氧含量等腐蚀促进作用超过了Kelvins力使Fe2+在电极表面聚集的腐蚀抑制作用。
3 结论
(1) 在3.5%NaCl溶液中,磁场使X52管线钢腐蚀电位负移、腐蚀电流密度增大、反应电荷转移阻抗减小,并一定程度上改变腐蚀形貌。
(2) 在3.5%NaCl溶液中,X52管线钢管体磁场强度越大,对电化学腐蚀行为的影响越大。
(3) 磁场对电化学反应过程的影响由电极表面磁感应强度、磁感应梯度以及电解质中离子磁性、浓度等多因素综合决定,Lorentz力加速Fe2+扩散、减小双电层厚度及Kelvins力增加氧含量等腐蚀促进作用大于Kelvins力使Fe2+在电极表面聚集的腐蚀抑制作用,从而总体上促进了电化学腐蚀。
参考文献
Construction progress and existing problems of intelligent pipeline network
[J].
智慧管网建设进展及存在问题
[J].
Ultrasonic echo detection technology for coating defects on submarine pipeline
[J].
海底管道外涂层缺陷超声回波检测技术
[J].
Magnetic flux leakage internal detection technology of the long distance oil pipeline
[J].
长输油气管道漏磁内检测技术
[J].
The influence of magnetization on corrosion in pipeline steels
[A].
The effect of strong magnetic field on corrosion behavior of iron
[J]. J.
磁场对铁腐蚀过程中阴极析氢和阳极溶解的影响
[J].
Effect of magnetic field and Cl- on anodic polarization behavior of iron in neutral 0.5 mol/L Na2SO4 solution
[J]. J.
磁场和Cl-对铁在中性Na2SO4溶液中阳极极化行为的影响
[J].
Magnetic field induced variation of open circuit state of iron in chloride solutions
[J].
磁场作用下铁在盐酸和氯化钠溶液中自腐蚀状态的变化
[J].
Effect of magnetic field on anodic dissolution of iron in sodium perchlorate solution at different potentials
[J].
磁场对铁在不同电位高氯酸钠溶液中阳极溶解的影响
[J].
Effects of magnetic fieldon electrochemical behavior of AZ31B in NaCl solution
[J].
磁场对AZ31B在NaCl溶液中电化学行为的影响
[J].
Effect of magnetic field on corrosion
[J].
磁场对腐蚀的影响
[J].
Anomalous surface morphology of iron generated after anodic dissolution under magnetic fields
[J].
Effects of an applied magnetic field on the dissolution and passivation of iron in sulphuric acid
[J].
Effect of magnetic field on the rate of steel corrosion in aqueous solutions
[J].
Effect of magnetic field on corrosion of X80 pipeline steel in meadow soil at Shenyang area
[J]. J.
磁场对X80管线钢在沈阳草甸土中腐蚀行为的影响
[J].
Effect of magnetic field on corrosion of X60 pipeline steel in soil of Changsha area
[J].
磁场对X60钢材料在长沙地区土壤中腐蚀行为的影响
[J].
Pitting corrosion in low carbon steel influenced by remanent magnetization
[J].
Influence of magnetic field on corrosion of pure Cu in artificial seawater with multispecies aerobic bacteria
[J]. J.
磁场对纯Cu微生物腐蚀行为的影响
[J].
Effect of high gradient magnetic fields on the anodic behaviour and localized corrosion of iron in sulphuric acid solutions
[J].
Effects of a magnetic field on the anodic dissolution, passivation and transpassivation behaviour of iron in weakly alkaline solutions with or without halides
[J].
Magnetic field effects on mass transport
[J].
Influence of magnetic forces on electrochemical mass transport
[J].
Magnetic field effects on the active dissolution of iron
[J].
Magnetic field effect on the anodic behaviour of a ferromagnetic electrode in acidic solutions
[J].
Effects of the Lorentz force and the gradient magnetic force on the anodic dissolution of nickel in HNO3+NaCl solution
[J].
Effects of magnetic field Directedorthogonally to surfaces on electrochemical processes
[J].
Effects of the magnetic field on the corrosion dissolution of the 304 SS│FeCl3 system
[J].
Magnetic and microstructural investigations of pipeline steels
[J].
Is there a magnetic field effect on electrochemical kinetics?
[J].
Magnetic field-controlled microfluidic transport
[J].
Magnetic fields in electrochemistry: the Lorentz force. A mini-review
[J].
Magnetic fields in electrochemistry: the Kelvin force. A mini-review
[J].
In situ observation of Cu2+ concentration profile during Cu dissolution in magnetic field
[J].