本文通过电化学方法和表面分析技术分别测试单一缓蚀剂和复配缓蚀剂的性能,并采用动力学分析了缓蚀机理。使用协同参数S衡量协同效应的强弱。考虑到缓蚀剂分子多通过自组装在金属表面上发生吸附,因此本文采用此方法开展相关研究。
1 实验方法
1.1 实验步骤
以紫铜 (25 mm×25 mm×3 mm) 为实验材料,依次用800#,1000#和2000#金相砂纸打磨,去离子水清洗,乙醇脱脂。室温条件下,在Cu表面分别自组装2-氨基苯并噻唑 (ABT),苯并三氮唑 (BTA),以及ABT与BTA的混合物10 h。分别考察混合缓蚀剂的比例和浓度两个变量对缓蚀性能的影响。ABT和BTA的结构如图1所示。本文中ABT和BTA的混合物用ABT-BTA表示。
图1
1.2 电化学测试
电化学测量采用PGSTAT302N电化学工作站完成。在室温 (25 ℃) 下三电极体系中进行。紫铜样品作为工作电极,有效接触面积为1 cm×1 cm,Pt为对电极,饱和甘汞电极作为参比电极。实验试剂为3.5% (质量分数) NaCl溶液。所有阻抗实验均在频率范围为105~10-2 Hz进行,振幅10 mV。极化曲线测量从低电位扫描至高电位,扫速为0.5 mV/s。
1.3 表面分析
分别采用S-4800型场发射扫描电镜 (SEM),XE-70原子力显微镜 (AFM) 进行表面形貌观察,采用Datephysics OCA 15光学接触角测量仪进行接触角测试,采用LabRAM HR Evolution Raman光谱仪测试样品在铜片表面是否发生吸附。
2 结果与讨论
2.1 动电位极化曲线分析
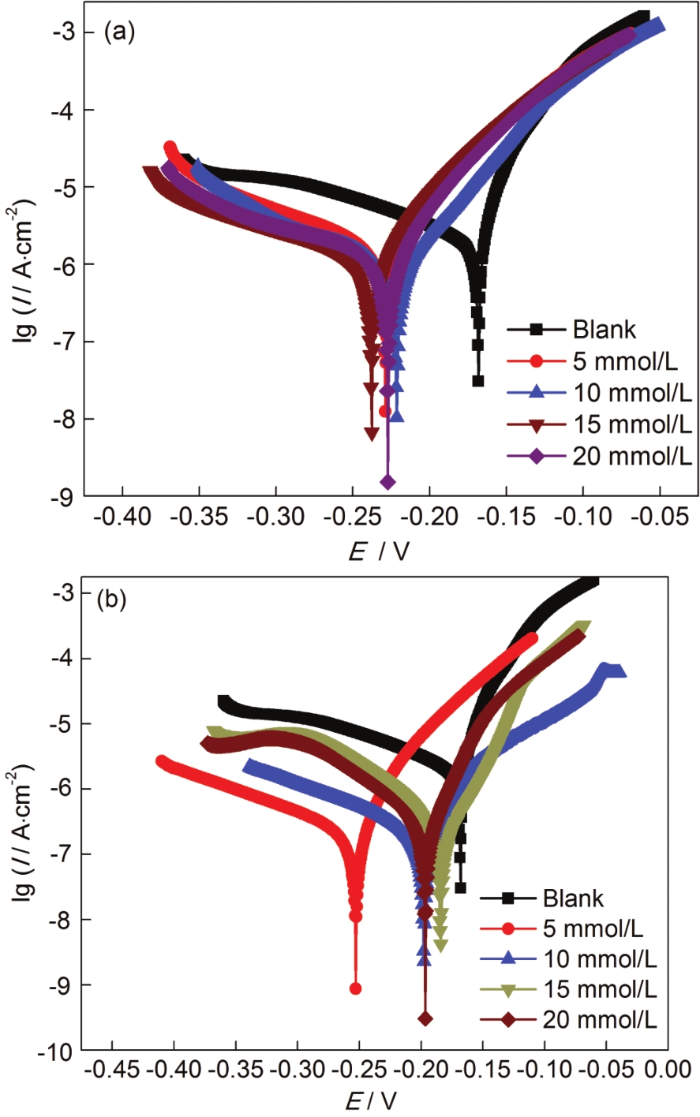
图2
图2
Cu电极分别经不同浓度ABT和BTA组装后在3.5%NaCl溶液中的动电位极化曲线
Fig.2
Potentiodynamic polarization curves of Cu with surface self-assembly of different concentrations of ABT (a) and BTA (b) in 3.5%NaCl solution
表1 Cu电极分别经不同浓度ABT和BTA组装后的极化曲线拟合参数
Table 1
| Inhibitor | Cmmol·L-1 | IcorrμA·cm-2 | βcV·dec-1 | βamV·dec-1 | IE % | θ |
|---|---|---|---|---|---|---|
| Blank | 0 | 5.735 | 0.375 | 0.035 | --- | --- |
| ABT | 5 | 1.730 | 0.140 | 0.051 | 69.80 | 0.698 |
| 10 | 0.958 | 0.162 | 0.040 | 83.28 | 0.832 | |
| 15 | 1.191 | 0.166 | 0.045 | 79.22 | 0.792 | |
| 20 | 1.350 | 0.206 | 0.042 | 76.46 | 0.764 | |
| BTA | 5 | 0.440 | 0.149 | 0.058 | 92.32 | 0.923 |
| 10 | 0.375 | 0.152 | 0.081 | 93.47 | 0.934 | |
| 15 | 0.237 | 0.076 | 0.025 | 95.87 | 0.958 | |
| 20 | 0.336 | 0.081 | 0.028 | 94.15 | 0.941 |
θ为表面覆盖度,由以下方程计算得出:
其中,Icorr(blank) 和Icorr(inh) 分别表示无缓蚀剂和添加缓蚀剂时测量的动电位极化曲线上的腐蚀电流密度。
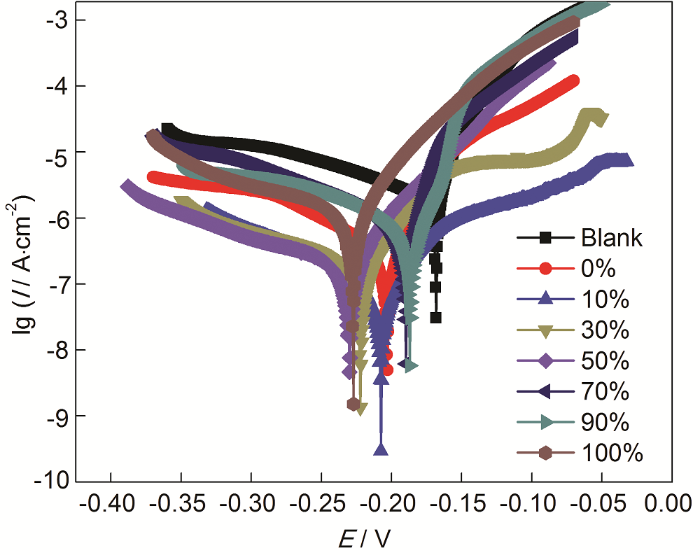
图3
图3
在ABT和BTA总浓度为20 mmol/L而ABT浓度不同时,Cu电极在3.5%NaCl溶液中的动电位极化曲线
Fig.3
Potentiodynamic polarization curves of Cu with surface self-assembly of ABT and BTA (their total concentration is 20 mmol/L, but the concentration of ABT varies) in 3.5%NaCl solution
表2 对图3中极化曲线拟合所得电化学参数
Table 2
| Concentration of ABT% | IcorrμA·cm-2 | βcV·dec-1 | βamV·dec-1 | IE % | θ |
|---|---|---|---|---|---|
| Blank | 5.735 | 0.375 | 0.035 | --- | --- |
| 0 | 0.336 | 0.081 | 0.028 | 94.15 | 0.941 |
| 10 | 0.254 | 0.129 | 0.160 | 95.58 | 0.955 |
| 30 | 0.218 | 0.179 | 0.041 | 96.20 | 0.962 |
| 50 | 0.210 | 0.204 | 0.035 | 96.34 | 0.963 |
| 70 | 2.336 | 0.127 | 0.066 | 59.20 | 0.592 |
| 90 | 2.882 | 0.242 | 0.070 | 49.75 | 0.497 |
| 100 | 1.350 | 0.206 | 0.042 | 76.47 | 0.764 |
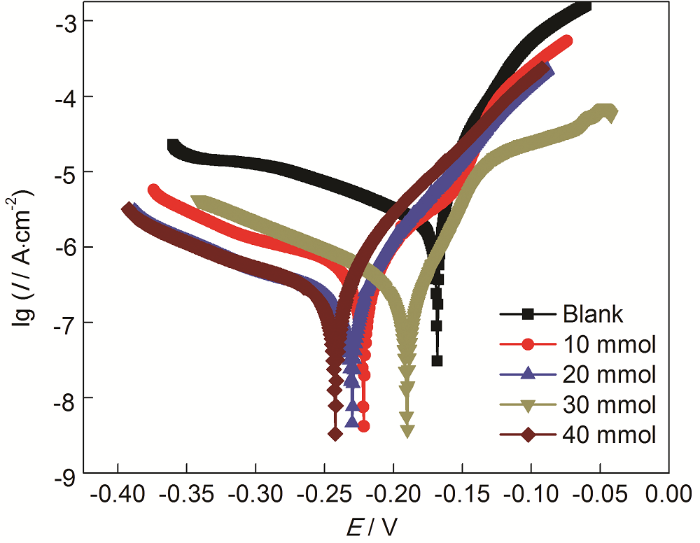
图4
图4
在ABT浓度为50%时而ABT-BTA总浓度不同的自组装条件下,Cu电极在3.5%NaCl溶液中的动电位极化曲线
Fig.4
Potentiodynamic polarization curves of Cu with surface self-assembly of ABT and BTA (the concentration of ABT is 50%, but the total concentration of ABT and BTA varies) in 3.5% NaCl solution
表3 对图4中极化曲线拟合所得电化学参数
Table 3
| Cmmol·L-1 | IcorrμA·cm-2 | βcV·dec-1 | βamV·dec-1 | IE % | θ |
|---|---|---|---|---|---|
| Blank | 5.735 | 0.375 | 0.035 | --- | --- |
| 10 | 0.559 | 0.195 | 0.073 | 90.26 | 0.902 |
| 20 | 0.210 | 0.204 | 0.035 | 96.34 | 0.963 |
| 30 | 0.252 | 0.209 | 0.043 | 95.60 | 0.956 |
| 40 | 0.271 | 0.134 | 0.025 | 95.28 | 0.952 |
Cu在3.5%NaCl溶液中存在如下电化学反应:
阳极反应:
阴极反应:
2.2 电化学阻抗分析
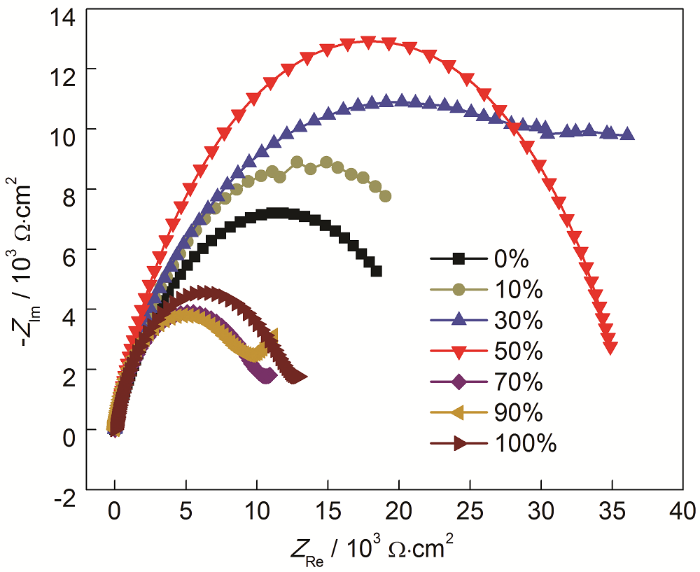
图5
图5
经总浓度为20 mmol/L而ABT浓度不同条件下自组装后,Cu电极在3.5%NaCl溶液中的Nyquist图
Fig.5
Nyquist plots of Cu with surface self-assembly of ABT and BTA (the total concentration of ABT and BTA is 20 mmol/L, but the concentration of ABT varies) in 3.5%NaCl solution
表4 对图5中阻抗谱拟合所得等效电路各元件参数
Table 4
| α (ABT) % | RsΩ·cm2 | RctkΩ·cm2 | QdlμF·cm-2 | N | WμΩ·S-1/2 |
|---|---|---|---|---|---|
| 0 | 13.27 | 23.19 | 8.12 | 0.707 | --- |
| 10 | 10.76 | 27.35 | 6.18 | 0.810 | --- |
| 30 | 12.46 | 28.70 | 6.03 | 0.705 | --- |
| 50 | 13.82 | 36.04 | 3.44 | 0.792 | --- |
| 70 | 14.72 | 9.99 | 20.35 | 0.826 | 712.8 |
| 90 | 14.03 | 8.81 | 23.45 | 0.868 | 330.3 |
| 100 | 14.64 | 12.20 | 18.95 | 0.843 | --- |
图6
图6
经ABT浓度为50%而ABT-BTA总浓度不同条件下自组装后,Cu电极在3.5%NaCl溶液中的Nyquist图
Fig.6
Nyquist plots of Cu with surface self-assembly of ABT and BTA (the concentration of ABT is 50%, but the total concentration of ABT and BTA varies) in 3.5%NaCl solution
表5 对图6中阻抗谱拟合所得等效电路各元件参数
Table 5
| C / mmol·L-1 | Rs / Ω·cm2 | Rct / kΩ·cm2 | Qdl / μF·cm-2 | N2 |
|---|---|---|---|---|
| 10 | 12.03 | 15.39 | 9.22 | 0.773 |
| 20 | 13.82 | 36.04 | 3.44 | 0.792 |
| 30 | 13.73 | 24.79 | 4.86 | 0.772 |
| 40 | 13.87 | 19.27 | 5.91 | 0.766 |
图7为不同阻抗谱的等效电路图。其中,Rs为溶液电阻;Qdl为双层电容;Rct表示电荷转移电阻;W为Warburg阻抗。
图7
图7
表面自组装不同缓蚀剂的Cu电极在3.5%NaCl溶液中的等效电路图
Fig.7
Equivalent circuits of Cu with surface self-assembly of different inhibitors in 3.5%NaCl solution: (a) the concentrations of ABT is 30%, 70%, 90%; (b) the concentrations of ABT is 0%, 10%, 50%, 100%
2.3 动力学分析
Temkin's模型
Freundich's 模型
Frumkin's模型
Langmuir's模型
图8
图8
不同浓度的ABT和BTA在Cu表面的Langmuir吸附等温曲线
Fig.8
Langmuir adsorption plots of (a) ABT and (b) BTA on copper surface
式中,R是理想气体常数 (8.314 J·mol-1·K-1),T是热力学温度,17.13是1 L溶液中乙醇的物质的量。通常认为,△G0ad在约-20 kJ/mol时,缓蚀剂分子通过静电作用吸附在金属表面,属于物理吸附;△G0ad在约-40 kJ/mol时,缓蚀剂分子通过孤对电子和金属空轨道结合形成配位化合物的方式吸附于金属表面,属于化学吸附。计算得到ABT的△G0ad值为-24.248 kJ/mol,BTA的△G0ad值为-33.941 kJ/mol。-40 kJ/mol<-33.941 kJ/mol<-24.248 kJ/mol<-20 kJ/mol,说明两种缓蚀剂同时存在物理吸附和化学吸附。
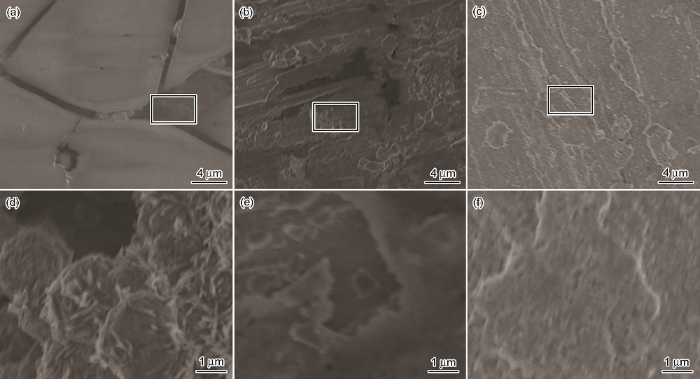
2.4 SEM观察结果
图9为分别经ABT,BTA和ABT-BTA组装的铜片在NaCl溶液中腐蚀后的SEM像。可知,ABT单独存在时,铜片表面存在腐蚀产物裂痕,并伴有腐蚀坑;BTA单独存在时,由于铜片在预处理过程中被金相砂纸磨损,表面有划痕,存在轻微的腐蚀坑;ABT-BTA共同存在时,在铜片表面形成了致密且有序的保护膜,无明显腐蚀现象,说明两者之间存在协同作用,具有良好的缓蚀性能。
图9
图9
铜片分别经ABT,BTA和ABT-BTA组装后在3.5%NaCl溶液中腐蚀后的SEM像
Fig.9
Surface micrographs of Cu samples with surface self-assemblies of ABT (a), BTA (b) and ABT-BTA (c) after corrosion in 3.5%NaCl solution and the magnified images of square areas in Fig.9a (d), Fig.9b (e) and Fig.9c (f)
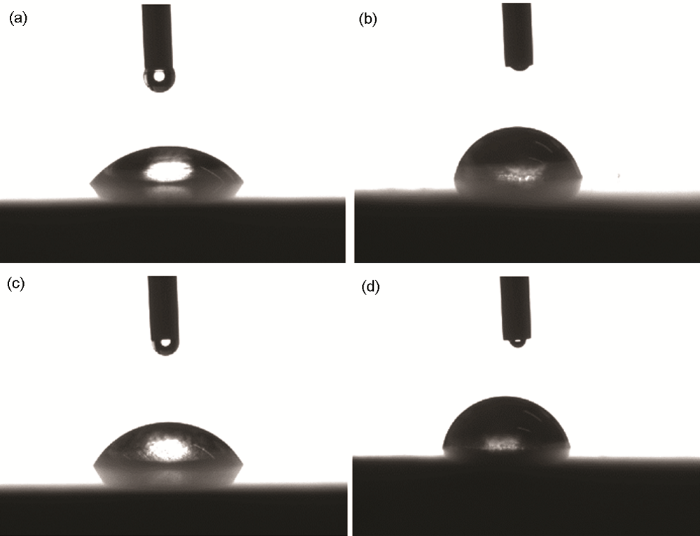
图10
图10
裸铜,分别经ABT,BTA和ABT-BTA 组装后于Cu表面测得的接触角
Fig.10
Optical contact angle values of Cu samples without (a) and with surface self-assemblies of ABT (b), BTA (c) and ABT-BTA (d)
2.5 OCA测量
使用光学接触角测量仪测定不同缓蚀剂在Cu表面的接触角。可知,裸铜的接触角为52.51º,ABT处理后为78.68º,BTA处理后为61.07º,ABT-BTA处理后为79.48º。可以看出,经ABT-BTA组装的Cu表面的接触角最大,表明两种缓蚀剂具有良好的协同作用,此结果与SEM观察结果一致。
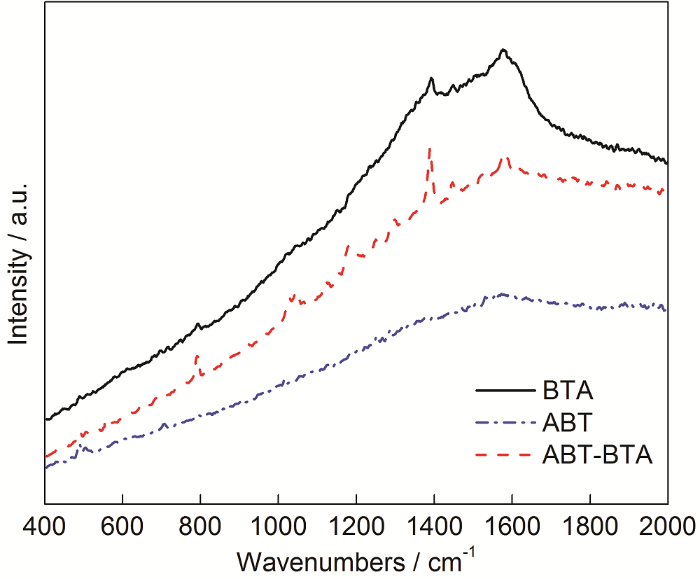
2.6 Raman光谱分析
图11分别为铜片经BTA,ABT-BTA和ABT组装后测得的Raman光谱图。在经BTA组装的Cu表面,790 cm-1处为苯环呼吸的谱峰,BTA的抑制机制是三唑环上氮原子上的孤电子对与Cu原子通过配位作用发生吸附,形成铜络合物[22],即Cu+BTA
图11
图11
铜片表面分别经BTA,ABT和ABT-BTA组装后的Raman光谱
Fig.11
Raman spectra of Cu samples with surface self-assemblies of ABT, BTA and ABT-BTA
2.7 AFM分析
图12为ABT,ABT-BTA和BTA组装铜表面的AFM像。可知,ABT在铜片表面分布不均匀,发生了团聚现象,粗糙度Ra为63.4 nm;BTA在铜片表面可以观察到凸起很均匀地吸附到Cu表面,Ra为92 nm;经ABT-BTA混合吸附的Cu表面,整体比较平整,Ra为63 nm,说明ABT与BTA发生了良好的协同作用,彼此间相互促进空间占位。此结果与光学接触角测量结果相一致。
图12
图12
ABT,ABT-BTA和BTA组装铜表面的AFM像
Fig.12
AFM images of Cu samples with surface self-assemblies of ABT (a), BTA (b) and ABT-BTA (c)
2.8 缓蚀剂的协同效应
复配缓蚀剂的缓蚀性能与缓蚀剂类型、复配比例等多种因素有关,通常用协同参数S来说明协同程度的大小。对于复配使用的缓蚀剂ABT (A) 与BTA (B),协同参数S的计算公式如下[29]:
式中,ηA和ηB分别代表ABT与BTA单独存在时的缓蚀率;ηAB代表ABT与BTA混合时的缓蚀率。若S>1,表示复配体系具有明显的协同效应。若S≤1,表示复配体系协同作用不显著或存在拮抗效应;S值越大,复配缓蚀剂的协同作用程度越强。可知,S50%=25.32>S30%=24.61>S10%=20.51>1>S70%=0.745>S90%=0.283,表明ABT浓度为50%时,协同作用较强;ABT浓度分别为70%和90%时,协同作用不显著,甚至出现了拮抗作用。
3 结论
ABT和BTA复配具有良好的协同作用,ABT浓度为50%,总浓度为20 mmol/L时,对Cu在3.5%NaCl溶液中的缓蚀率达到了96.34%,缓蚀性能较好。两种缓蚀剂同时存在物理吸附与化学吸附。复配缓蚀剂成膜比较完整,具有良好的协同效应。
参考文献
The roles of mercapto, benzene, and methyl groups in the corrosion inhibition of imidazoles on copper: II. Inhibitor-copper bonding
[J].
Water soluble corrosion inhibitors for copper in 3.5 wt% sodium chloride solution
[J].
Corrosion inhibition of copper, mild steel and galvanically coupled copper-mild steel in artificial sea water in presence of 1H-benzotriazole, sodium molybdate and sodium phosphate
[J].
2-amino-5-(4-pyridinyl)-1, 3, 4-thiadiazole monolayers on copper surface: observation of the relationship between its corrosion inhibition and adsorption structure
[J].
Corrosion inhibition and olecular structure of thiadiazole derivatives in sulfur-ethanol system
[J].
噻二唑衍生物分子结构与其缓蚀性能的关系
[J].-1硫溶液中对金属银的缓蚀性能。实验结果表明:缓蚀剂成功地吸附到了金属表面,金属腐蚀受到明显的抑制,且4种缓蚀剂的缓蚀效率的大小顺序是:MATD>PATD>ATD>DPTD。位于噻二唑环2,5位置上非极性和极性基团结构的变化,极性基团均对缓蚀剂的缓蚀性能有较大影响。因极性基团更容易吸附到金属表面,所以当噻二唑环上存在极性基团时,其抗腐蚀性能明显增强;当环上存在非极性基团时,与芳基相比,非极性基团为烷基时,其缓蚀性能更好,原因可能是由于芳基的体积较大,在吸附过程中受到的阻力较大。通过动力学分析可知:4种缓蚀剂在金属表面的吸附遵循Langmuir吸附等温方程,吸附类型属于化学吸附为主的混合吸附。通过分子动力学模拟,进一步研究了4种缓蚀剂的抗腐蚀机理,结果表明缓蚀剂与金属界面发生吸附时,4种缓蚀剂的噻二唑环和环上亲水支链中的极性基团优先吸附到金属银表面,理论计算和实验结果一致。]]>
Synthesis of 2, 5-diaryl-1, 3, 4-thiadiazole corrosion inhibitors and their performance
[J].
2, 5-二芳基-1, 3, 4-噻二唑衍生物的合成及缓蚀性能
[J].
Inhibition behavior of self-assembled films of Schiff bases for copper
[J].
自组装席夫碱膜对铜的缓蚀行为
[J].
Characterization of the deposition of n-octanohydroxamate on copper surfaces
[J].
Direct electroless plating of iron-boron on copper
[J].
The discussion of descriptors for the QSAR model and molecular dynamics simulation of benzimidazole derivatives as corrosion inhibitors
[J].
Experimental and theoretical surface enhanced raman scattering study of 2-amino-4-methylbenzothiazole adsorbed on colloidal silver particles
[J].
An exploration about the interaction of mild steel with hydrochloric acid in the presence of N-(Benzo[d]thiazole-2-yl)-1-phenylethan-1-imines
[J].
Quantum chemical and experimental investigations on inhibitory behavior of amino-imino tautomeric equilibrium of 2-aminobenzothiazole on steel corrosion in H2SO4 solution
[J].
Inhibition action of benzotriazole and tolytriazole on corrosion of copper in deionized water
[J].
苯并三氮唑及其甲基衍生物在去离子水中对铜的缓蚀作用
[J].
Molecular dynamics simulation of interaction between cuprous oxide crystal and benzotriazole derivatives
[J].
苯并三氮唑及其衍生物与氧化亚铜晶体相互作用的MD模拟
[J].用分子动力学(MD)方法,模拟计算了4种铜缓蚀剂[苯并三氮唑(BTA)、5-羧甲基苯并三氮唑(CBTAH-ME)、5-羧丁基苯并三氮唑(CBTAH-BU)、5-羧辛基苯并三氮唑(CBTAH-OC)]与Cu2O晶体的相互作用。结果发现,缓蚀剂分子与Cu2O晶体的结合能排序为CBTAH-OC>CBTAH-BU>CBTAH-ME>BTA。对体系各种相互作用以及对关联函数g(r)的分析表明,体系结合能主要来自库仑作用的贡献。在与Cu2O(001)晶面结合过程中,BTA及其衍生物分子发生了扭曲变形,且分子中的N原子与Cu2O晶体中的Cu原子之间形成了配位键。
Correlation between electronic parameters and corrosion inhibition of benzothiazole derivatives-NMR parameters as important and neglected descriptors
[J].
Benzotriazole as a volatile corrosion inhibitor during the early stage of copper corrosion under adsorbed thin electrolyte layers
[J].
Study of corrosion behavior of copper in 3.5wt.%NaCl solution containing extracellular polymeric substances of an aerotolerant sulphate-reducing bacteria
[J].
Evaluation of an organic corrosion inhibitor on abiotic corrosion and microbiologically influenced corrosion of mild steel
[J].
Corrosion inhibition of carbon steel in hydrochloric acid by furan derivatives
[J].
The inhibition of mild steel corrosion in 1 M hydrochloric acid solutions by triazole derivative
[J].
Electrochemical behavior of copper passivated by BTA and MBT in NaCl solution
[J].
铜经BTA和MBT钝化处理后在NaCl溶液中电化学行为分析
[J].
SERS studies of corrosion inhibition of BTA and its derivative on copper electrodes in NaCl solution
[J].
苯并三氮唑及其衍生物在NaCl溶液中对铜缓蚀作用的表面增强拉曼光谱
[J].
Corrosion inhibition of BTA and its derivative 4CBTA on copper electrode in 3%NaCl solution
[J].
苯并三氮唑与4-羧基苯并三氮唑在氯化钠溶液中对铜的缓蚀作用
[J].
Ground structure and excited state proton transfer reaction of 2-aminobenzothiazole
[J].
2-氨基苯并噻唑的结构及激发态质子转移动力学
[J].
Vibrational, nuclear magnetic resonance and electronic spectra, quantum chemical investigations of 2-amino-6-fluorobenzothiazole
[J].
FTIR, FT-Raman, FT-NMR, UV-visible and quantum chemical investigations of 2-amino-4-methylbenzothiazole
[J].
Structural, vibrational, electronic investigations and quantum chemical studies of 2-amino-4-methoxybenzothiazole
[J].