目前,优化Zn负极性能的策略主要包括构建人工界面层[11~14]、负极结构设计[15~17]和电解液添加剂的引入[18]。其中,电解液添加剂因其简便性和高效性而备受青睐[19]。在传统ZnSO4电解液中,Zn2+主要以六配位([Zn(H2O)6]2+)形式存在[20]。一方面,溶剂化壳中的活性H2O分子在负极界面分解产生H+,直接引发HER,同时生成的OH-与Zn2+反应形成钝化层,进一步加剧副反应[21]。另一方面,传统电解液中Zn2+的六配位结构导致其脱溶剂化能垒较高,沉积动力学较差。在电化学过程中,Zn2+倾向于在局部高电流密度区域优先沉积,形成不均匀的锌沉积层,进而诱发锌枝晶生长。其尖锐结构会进一步增大局部电流密度,进而加速HER反应。近年来,研究人员开发了有机分子、无机盐和聚合物等多种电解液添加剂,通过调控Zn2⁺溶剂化结构、优化界面双电层、抑制枝晶生长等机制提升ZIBs性能[22,23]。尽管如此,现有添加剂大多仅针对单一问题,难以全面应对锌负极所面临的多重挑战[24]。例如,枝晶抑制剂对HER反应效果有限[25],界面改性剂难以兼顾循环稳定性[26]。特别是在高电流密度和长循环条件下,单一功能添加剂往往表现出性能衰减[27,28],这严重制约了ZIBs的实际应用。
本文将瓜氨酸(Cit)作为双功能添加剂引入2 mol/L ZnSO4电解液。通过实验与理论计算相结合,揭示了Cit的协同作用机制。即:引入Cit后的电解液能够优化Zn2+的溶剂化结构并调节Zn沉积形态,减少HER反应的发生。此外,对比了添加Cit前后ZIBs的电化学性能。发现Cit的加入显著提升了ZIBs的电化学性能,表明Cit作为一种高效的双功能添加剂,在提升ZIBs性能方面具有极大潜力。
1 实验方法
称取14.4 g ZnSO4·7H2O溶解到25 mL去离子水中制备2 mol/L ZnSO4电解液。依据加入Cit添加剂含量的不同,将添加量为2 mol/L ZnSO4电解液质量的0.5%,1.0%和2.0% (质量分数) Cit分别命名为Cit05、Cit/ZnSO4和Cit20。
通过ZEISS EV0 MA15型扫描电子显微镜(SEM)表征材料的形貌,同时采用Nicolet 6700型Fourier变换红外光谱仪(FTIR)进行成分分析。此外,使用Bruker 400MHz型核磁共振仪(NMR)氢谱(2H)和DX700型X射线衍射(XRD)进行了晶体结构分析。采用OCA25型接触角分析仪测试不同电解液的润湿性,并使用S220型pH计测试了电解液的离子电导率和pH值。锌枝晶生长过程通过组装原位池并使用Yuescope光学显微镜进行原位观察。
使用CR2032型扣式电池评估电化学性能。隔膜均为玻璃纤维,分别使用直径为19 mm的Zn片、Ti片和Cu片与直径为14 mm的Zn片组装Zn||Zn对称电池、Zn||Ti对称电池和Zn||Cu不对称电池。所有电池均注入100 μL电解液。全电池以直径为14 mm的Zn片作为负极。正极以V2O5为活性物质,乙炔黑为导电剂,PVDF为粘合剂,按质量比7∶2∶1混合在NMP溶剂中研磨成浆料涂覆在抛光钛箔上。真空干燥12 h后,切割成直径为12 mm的圆片而制成的,活性物质负载量约为1.5 mg·cm-2。恒流充放电循环测试在0.2~1.6 V的电压范围内进行。使用CHI 660e型电化学工作站进行了多种电化学测试。在过电位为-150 mV时,进行了计时电流(CA)测试。Tafel、线性扫描伏安测试(LSV)和循环伏安曲线(CV)的扫速分别为10、1.0和0.1 mV·s-1。
理论计算使用Gaussian(G16)程序在B3LYP-D3(BJ)/6-311 + G(d, p)水平上进行计算。为了评估溶剂效应,采用了基于溶质电子密度(SMD)的隐式溶剂化模型,并以水的介电常数用于计算。结合能(EB)通过以下方程确定:
其中,Ecom是复合物的总能量,Efra是每个片段的能量。
脱溶能量(Edes)的计算公式为:
其中,Es1和Es2是解溶解前后溶解结构的总能量,Esol是溶剂的能量。
2 结果与讨论
2.1 筛选Cit最优添加比例
图1
图1
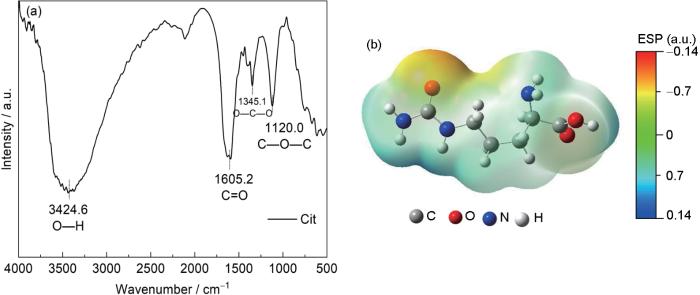
Cit结构表征与静电势分布
Fig.1
FTIR spectrum (a) and electrostatic potential diagram (b) of Cit
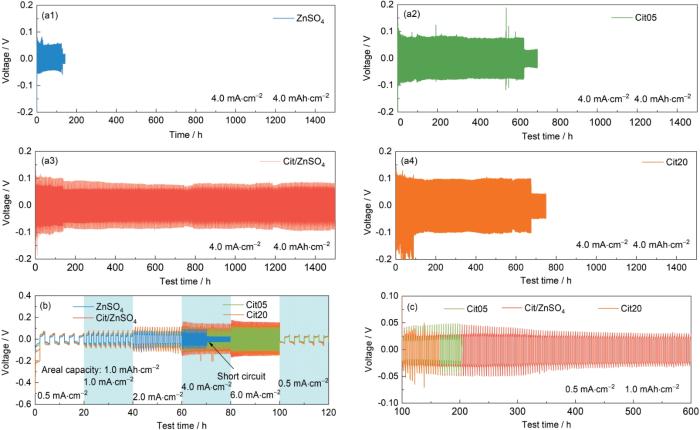
为确定Cit的最佳添加浓度,采用Zn||Zn对称电池进行恒流充放电测试,评估不同电解液中锌沉积/脱出的可逆性,如图2a所示。在4.0 mA·cm-2和4.0 mAh·cm-2条件下,采用2 mol/L ZnSO4电解液的Zn||Zn对称电池在不足200 h就发生了短路,这是由于锌枝晶刺破隔膜而引起的。而添加Cit电解液(Cit05、Cit/ZnSO4、Cit20)的电池均稳定循环超过600 h。其中,添加Cit/ZnSO4电解液的电池循环时间达到1500 h,表现出最优异的循环寿命,表明1.0%Cit为最佳添加浓度。为进一步验证Cit/ZnSO4电解液的稳定性,测试了不同电流密度下的倍率性能。如图2b所示,Cit05、Cit/ZnSO4和Cit20电解液在1.0~6.0 mA·cm-2范围内相较2 mol/L ZnSO4电解液均表现出良好的稳定性。此外,如图2c所示,在0.5~1.0 mA·cm-2条件下,Cit/ZnSO4电解液的Zn||Zn对称电池表现出更稳定的充放电过程和更长的循环寿命,且电压极化无明显增加。因此,以1.0%Cit为最佳添加浓度展开Cit对ZIBs的循环稳定性和循环寿命优化的研究。
图2
图2
通过电化学性能筛选最优Cit添加浓度
Fig.2
Electrochemical performances of Zn||Zn cells at 4.0 and 4.0 mA·cm-2 (a1-a4), rate performance at different current densities (b), and cyclic performance at 0.5 and 1.0 mA·cm-2 after the rate performance test (c)
2.2 Cit调节Zn2+ 溶剂化结构
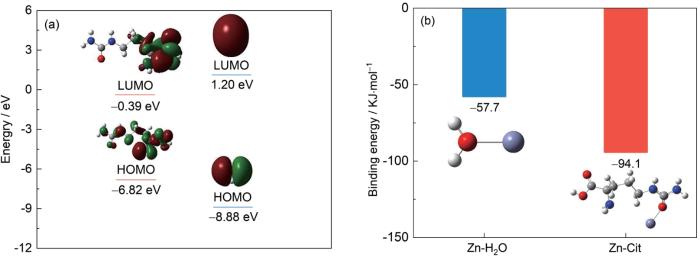
图3
图3
Cit调节Zn2+溶剂化结构相关理论计算
Fig.3
HOMO-LUMO orbital energy levels of Cit and H2O (a) and binding energies of Zn2+ with Cit and H2O (b)
随后,通过实验进一步研究了Cit对Zn2+溶剂化结构的调控作用。2H NMR结果显示(图4a),纯D2O的2H峰位于4.70 mg/L,加入2 mol/L ZnSO4后峰位移至4.72 mg/L,表明Zn2+与D2O形成[Zn(D2O)6]2+结构,降低了电子云密度。引入Cit后,2H峰回移至4.71 mg/L,表明Cit的极性官能团部分取代了溶剂化鞘层中的D2O分子,释放部分D2O并增加电子云密度。FTIR分析进一步显示(图4b),Cit/ZnSO4电解液中O—H伸缩振动峰(3000~3600 cm-1)蓝移,表明Cit与H2O的强相互作用削弱了原有氢键网络[30]。离子电导率测试表明(图4c),2 mol/L ZnSO4电解液的高电导率虽利于Zn2+传输,但易引发锌不均匀沉积,而Cit的引入显著降低离子电导率。接触角测试表明(图4d),加入Cit后的电解液对Zn负极的接触角从87.8°降至82.4°,这一变化降低了Zn负极界面自由能,促进Zn2+均匀分布,有效延长了ZIBs的循环寿命。
图4
图4
Cit/ ZnSO4电解液与2 mol/L ZnSO4电解液物理表征
Fig.4
NMR spectra (a), FTIR spectra (b), ionic conductivities (c) and contact angles (d) of Cit/ZnSO4 electrolyte and 2 mol/L ZnSO4 electrolyte
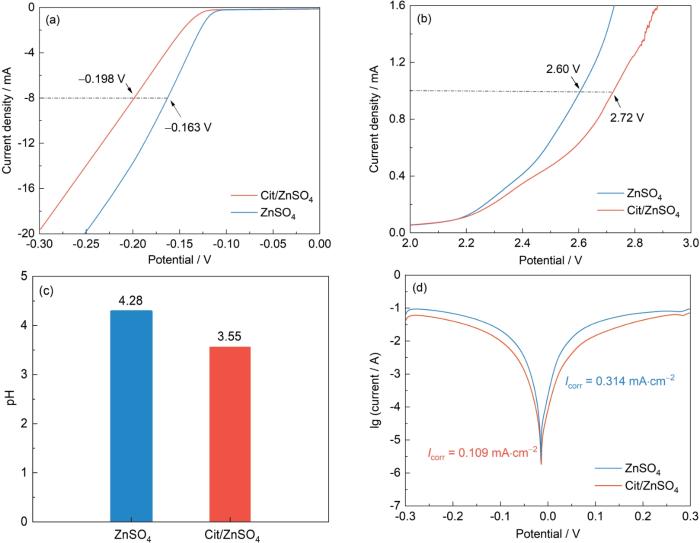
通过Zn||Ti不对称电池的LSV测试,揭示了Cit对电解液性能的多维度调控机制(图5a和b)。在-0.3~0 V区间内,相较于纯ZnSO4电解液,Cit/ZnSO4电解液在相同电流密度下的HER电位负向偏移,表明Cit的加入显著增大了HER的活化能垒,抑制了Zn负极表面的副反应。在1.9~3.0 V高电位区间,Cit的引入使析氧反应(OER)起始电位明显正移,有效拓宽了电解液的电化学反应窗口,为ZIBs的循环稳定性提供了保护[31]。pH测试显示(图5c),Cit的引入使2 mol/L ZnSO4电解液的pH从4.28降至3.55,表明游离H⁺浓度增加。尽管高H+浓度可能加速腐蚀,但适当的pH变化并未显著影响Zn负极。Tafel测试进一步证实,Cit/ZnSO4电解液的腐蚀电流从0.314 mA·cm-2显著降低至0.109 mA·cm-2 (图5d)。这些结果表明,Cit通过极性官能团重构了Zn2+溶剂化结构,优化Zn2+传输动力学,同时削弱水分子氢键网络、降低H2O活性,协同抑制HER反应并提升Zn负极稳定性。
图5
图5
组装Zn||Ti电池的性能表征
Fig.5
LSV curves (a, b), pH values (c) and Tafel curves (d) of Zn||Ti cells
2.3 Cit调节锌沉积行为
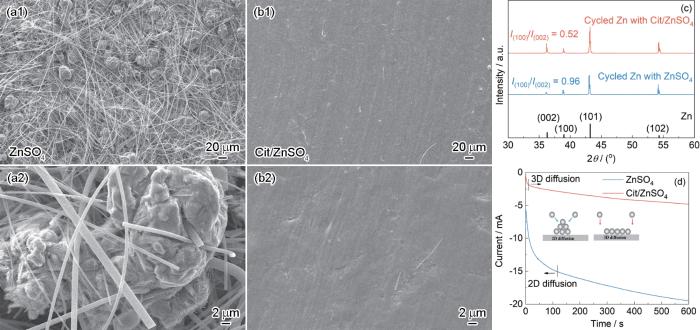
采用电化学测试探究Cit对锌沉积行为的影响,通过Zn||Zn对称电池在1.0 mA·cm-2,1.0 mAh·cm-2条件下循环120 h后的形貌观察表明(图6a,b),2 mol/L ZnSO4中锌负极表面存在大量枝晶并刺穿隔膜,而Cit/ZnSO4下锌沉积层致密光滑,无枝晶生成。从XRD图中可以观察到(图6c),Cit/ZnSO4电解液下的锌负极I(100)/I(002)峰强度比为0.96,低于2 mol/L ZnSO4电解液的0.52,证实Cit诱导了更平整的(002)晶面择优取向沉积。CA测试再一次证明了(图6d),2 mol/L ZnSO4电解液中锌沉积遵循二维扩散机制,而Cit/ZnSO4电解液则通过调控Zn2+通量,促进三维扩散,最终实现均匀沉积[32]。
图6
图6
Zn||Zn电池循环过程表征
Fig.6
SEM images of Zn anodes of Zn||Zn cells after 120 h cycling at 1.0 mA·cm-2 with 1.0 mAh·cm-2 (a, b), XRD patterns (c), and CA curves (d)
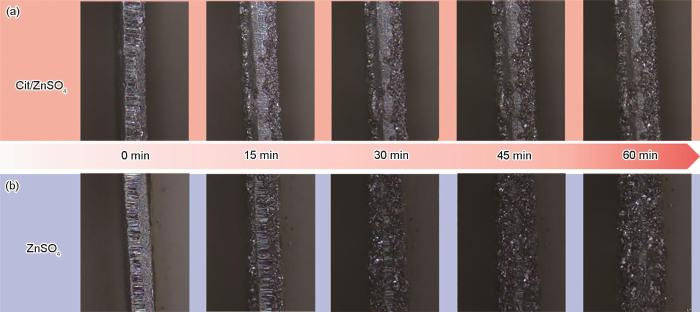
通过原位光学显微镜实时成像捕捉了锌沉积的动态过程,来揭示Cit的引入对锌箔形貌演变的影响。如图7所示,在2 mol/L ZnSO4电解液中,锌箔表面仅15 min便出现不均匀沉积,形成了孤立的沉积点并演变为电荷集中区域,Zn在局部高电流密度区域优先沉积,形成不均匀的锌沉积层,进而诱发锌枝晶生长。而在Cit/ZnSO4电解液中,锌沉积始终保持平坦均匀,未观察到孤立沉积点或枝晶结构。这得益于Cit分子通过其极性官能团与Zn2+配位,抑制了局部电荷积累,优化了Zn2+在锌箔表面的分布与沉积过程。这一结果不仅直观展示了Cit在锌沉积行为调控中的优越性,也为优化电解液配方、提升锌负极稳定性提供了强有力的实验支持。
图7
图7
锌沉积过程的原位光学显微镜图
Fig.7
In situ optical microscopy images showing the deposition of Cit/ZnSO4 (a) and ZnSO4 (b) at 5.0 mA·cm-2
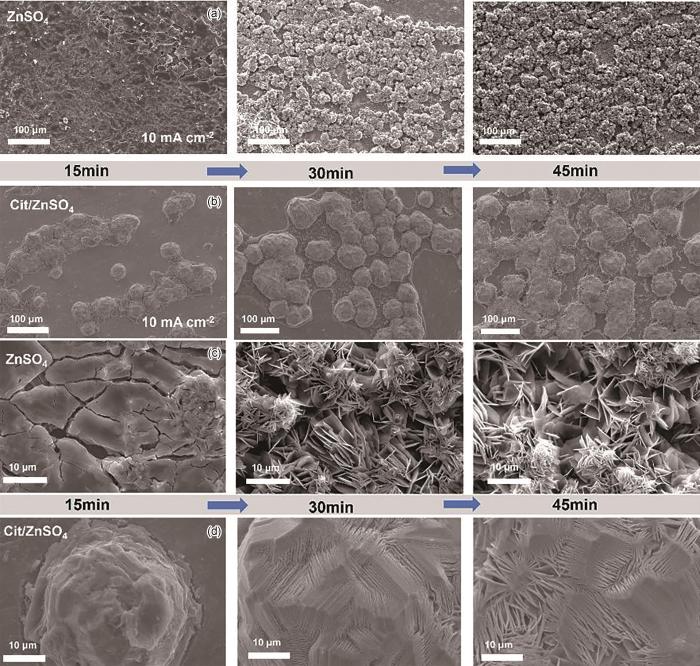
为了更加清楚的观察锌沉积形貌,对电镀不同时间的锌箔进行了SEM测试。如图8a和b所示,在10.0 mA·cm-2电流密度下,2 mol/L ZnSO4电解液中锌沉积行为是先在锌箔表面形成较为粗糙的锌床,随着电镀时间的增加,在锌床上形成大量的随机分布的Zn凸起物。通过其放大图(图8c,d),可见Zn凸起物是生长在锌床上的尖锐的锌沉积物,并且随着时间的增加,这些锌沉积物变得更加尖锐。与2 mol/L ZnSO4电解液中锌沉积行为不同的是,Cit/ZnSO4电解液中锌负极的沉积物顶部是层层堆叠的致密结构,这避免了Zn枝晶形成刺破隔膜而导致电池短路。这些结果表明,Cit通过调控Zn2⁺沉积行为,有效抑制了Zn枝晶的不可控生长。并缓解了因局部电流密度集中而加剧的HER反应,从而实现了Zn负极的稳定防护。
图8
图8
电镀后Zn箔的不同倍率SEM图
Fig.8
Low-magnification (a, b) and high-magnification (c, d) SEM images of ZnSO4 (a, c) and Cit/ZnSO4 (b, d) electroplating at 10.0 mA·cm-2
2.4 Cit/ZnSO4 电解液电化学性能
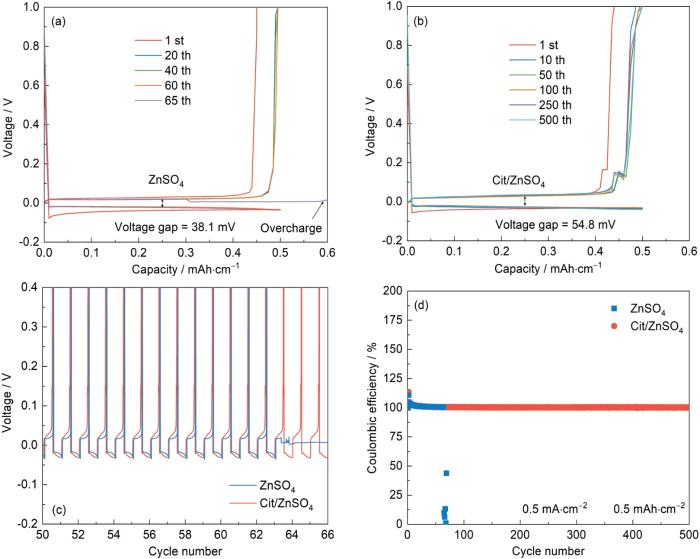
为探究Cit对ZIBs可逆性和稳定性的影响,组装了Zn||Cu不对称电池进行了电化学测试。在0.5 mA·cm-2和0.5 mAh·cm-2条件下,2 mol/L ZnSO4电解液的Zn||Cu电池在循环65 cyc后发生过充现象(图9a),而Cit/ZnSO4电解液的Zn||Cu电池稳定循环超过500cyc (图9b)。从电压-循环曲线圈数可以观察到,相较于Cit/ZnSO4电解液稳定的电压,采用2 mol/L ZnSO4电解液组装的Zn||Cu不对称电池在65cyc后发生了点压不稳定现象(图9c)。此外,采用Cit/ZnSO4电解液的Zn||Cu电池具有2 mol/L ZnSO4电解液更为稳定的Coulomb效率(图9d)。这证明了Cit的引入有效提升了Zn2+沉积/脱出过程的高效性和稳定性,大幅提升了Zn||Cu不对称电池的电化学性能。
图9
图9
组装Zn||Cu电池电化学性能
Fig.9
Voltage vs. capacity curves (a, b), voltage vs. cycle number curves (c) and coulombic efficiency plots (d) of Zn||Cu cells
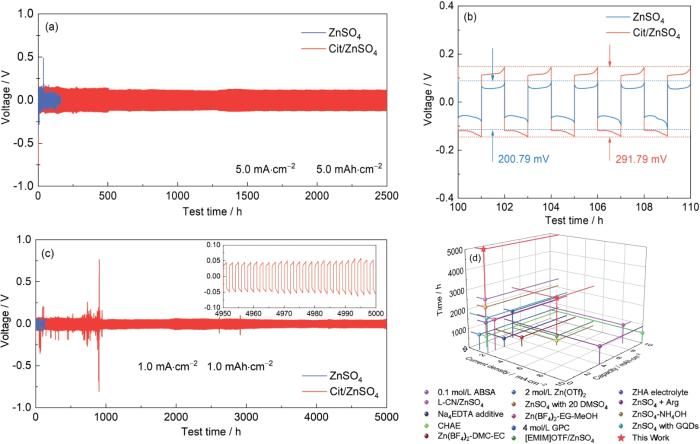
在5.0 mA·cm-2和5.0 mAh·cm-2条件下,Zn||Zn对称电池采用Cit/ZnSO4电解液的循环时间达到2500 h,远超2 mol/L ZnSO4电解液的171 h (图10a)。通过进一步放大时间-电压曲线的特定片段(图10b),可见Cit的引入显著增加了过电位,过电位从291.79 mV升高至200.79 mV。通过这一变化可以推测,Cit与锌负极表面之间的强相互作用增加了Zn2+沉积/脱出的能量阻碍,进而有效地防止不利的Zn2+沉积。在1.0 mA·cm-2和1.0 mAh·cm-2条件下,Cit/ZnSO4电解液的Zn||Zn电池稳定循环超过5000 h (图10c),展现了优异的循环稳定性。与其它ZIBs电解液优化策略相比(图10d,表1),Cit/ZnSO4电解液在循环时间和稳定性方面表现突出,证明了Cit作为添加剂的优越性,显著提升了ZIBs的循环寿命。
图10
图10
组装Zn||Zn电池的电化学性能和循环寿命对比
Fig.10
Cycling performances of Zn||Zn cells at 5.0 mA·cm-2 and 5.0 mAh·cm-2 (a, b) and at 1.0 mA·cm-2 and 1.0 mAh·cm-2 (c), and cycle life comparison plot (d)
表1 Zn||Zn电池循环寿命对比
Table 1
| Electrolyte | Current density / mAh·cm-2 | Capacity / mAh·cm-2 | Cycle time / h | Ref. |
|---|---|---|---|---|
| 0.1 mol/L ABSA | 1 | 1 | 500 | [33] |
| 4 mol/L GPC | 1 | 4 | 1450 | [34] |
| L-CN/ZnSO4 | 8.85 | 8.85 | ≈1000 | [35] |
| [EMIM]OTF/ZnSO4 | 5 | 5 | 500 | [36] |
| Na4EDTA additive | 2 | 2 | 450 | [37] |
| ZHA electrolyte | 1 | 1 | 1300 | [38] |
| CHAE | 1 | 1 | 580 | [39] |
| ZnSO4 + Arg | 10 | 4 | ≈900 | [40] |
| Zn(BF4)2-DMC-EC | 3 | 3 | 480 | [23] |
| ZnSO4-NH4OH | 5 | 5 | 250 | [41] |
| 2 mol/L Zn(OTf)2 | 1 | 0.25 | 800 | [42] |
| ZnSO4 with GQDs | 2 | 0.2 | 1800 | [43] |
| ZnSO4 with 20 DMSO | 1 | 1 | 2100 | [44] |
| Zn(BF4)2-EG-MeOH | 2 | 1 | 1600 | [45] |
| Cit/ZnSO4 | 1 | 1 | 5000 | This work |
| 5 | 5 | 2500 |
2.5 Cit/ZnSO4 电解液全电池性能
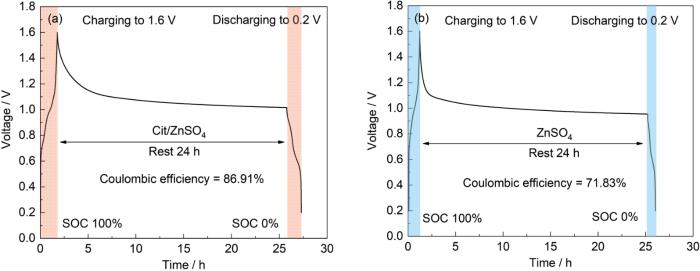
为评估Cit/ZnSO4电解液的实际应用潜力,组装了Zn||V2O5全电池并测试其性能。为评估Cit/ZnSO4电解液的实际应用潜力,组装了Zn||V2O5全电池并测试其性能。通过完全充电后静置24 h的自放电测试(图11a,b),发现使用Cit电解液组装的电池静置后库伦效率达86.91%,明显优于2 mol/L ZnSO4电解液71.83%的库伦效率。这表明,使用纯2 mol/L ZnSO4电解液容易引起正极材料的溶解和结构畸变,导致容量迅速下降,而Cit的引入有效抑制了这一过程。
图11
图11
Zn||V2O5全电池自放电曲线
Fig.11
Self-discharge curves of Zn||V2O5 full cells with Cit/ZnSO4 electrolyte (a) and 2 mol/L ZnSO4 electrolyte (b)
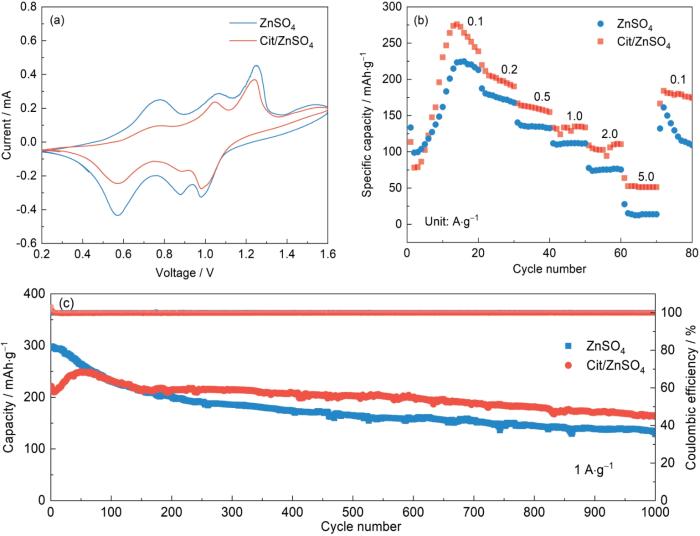
图12
图12
Zn||V2O5全电池电化学性能
Fig.12
CV curves (a), rate performance (b), and long-cycle performance (c) of Zn||V2O5 full cells
3 结论
(1) 引入Cit的电解液能够降低Zn2+溶剂化鞘层中H2O分子的活性,优化Zn2+的溶剂化结构,减缓HER反应速率。
(2) Cit的引入可以降低Zn2+溶剂化的能垒,实现锌的均匀沉积,避免了因局部电流密度过大造成的HER反应加速。
(3) 基于Cit/ZnSO4电解液的Zn||Zn对称电池在5.0 mA·cm-2和5.0 mAh·cm-2条件下仍能稳定循环2500 h。此外,Zn||V2O5全电池在1.0 A·g-1电流密度下循环1000次后仍保持较高的容量保持率。这些性能都显著优于纯2 mol/L ZnSO4电解液的电化学性能。表明Cit加入实现了电解液-电极界面的协同调控,增强了对Zn负极的防护,显著提升ZIBs的循环稳定性和容量保持率。
参考文献
Progress in interface structure and modification of zinc anode for aqueous batteries
[J].
Current status and future directions of multivalent metal-ion batteries
[J].
Conductive biomaterials as bioactive wound dressing for wound healing and skin tissue engineering
[J].
Multi-ion engineering strategies toward high performance aqueous zinc-based batteries
[J].
Electrolyte strategies toward better zinc-ion batteries
[J].
Reversible aqueous zinc/manganese oxide energy storage from conversion reactions
[J].
Antifreezing hydrogel electrolyte with ternary hydrogen bonding for high-performance zinc-ion batteries
[J].
Eutectic electrolyte with unique solvation structure for high-performance zinc-ion batteries
[J].
Recent advances in Zn‐Ion batteries
[J].
Research progress of zinc ion batteries in zinc metal electrodes and electrolytes
[J].
锌离子电池的锌金属负极和电解液的研究进展
[J].
Charge-enriched strategy based on MXene-based polypyrrole layers toward dendrite-free zinc metal anodes
[J].
Ultrathin surface coating of nitrogen-doped graphene enables stable zinc anodes for aqueous Zinc-Ion Batteries
[J].
High-yield carbon dots interlayer for ultra-stable zinc batteries
[J].
Degradation behavior of pure zinc and Zn-xLi alloy in artificial urine
[J].
Zn及锌锂合金在人工尿液中的腐蚀行为
[J].通过体外浸泡实验及电化学测试的方法,研究了Zn-xLi (x=0,0.5%,0.8%) 在人工尿液 (AU) 中长达28 d的腐蚀行为。结果表明,尽管浸泡过程中,样品依然存在结壳现象,但对比前人研究过的几种金属,Zn/Zn-xLi结壳现象有所缓解,这在输尿管植入应用中是十分可喜的现象。样品在人工尿液中的腐蚀产物为CaZn<sub>2</sub>(PO<sub>4</sub>)<sub>2</sub>·2H<sub>2</sub>O,电化学测定浸泡28 d后样品的腐蚀速率为0.21~0.34 mm·a<sup>-1</sup>。
Encapsulation of metallic Zn in a hybrid MXene/graphene aerogel as a stable Zn anode for foldable Zn-Ion batteries
[J].
Localizing concentrated electrolyte in pore geometry for highly reversible aqueous Zn metal batteries
[J].
A chemically polished zinc metal electrode with a ridge-like structure for cycle-stable aqueous batteries
[J].
A universal additive strategy to reshape electrolyte solvation structure toward reversible Zn storage (Adv. Energy Mater. 15/2022)
[J].
Corrosion inhibitor for Zn anode of neutral aqueous zinc ion batteries
[J].
中性水系锌离子电池负极缓蚀剂研究进展
[J].围绕锌负极在中性电解质中腐蚀产生枝晶、钝化、析氢等关键问题,综述了锌负极缓蚀剂防护方法的研究进展,旨在从溶剂化结构调控、静电屏蔽、吸附作用、原位固态电解质界面4种防护机理,揭示不同类型缓蚀剂的性能、稳定性及实际应用的可行性,进而为锌负极保护提供依据,展望缓蚀剂防护方法未来的发展方向。
Strategies of regulating Zn2+ solvation structures for dendrite-free and side reaction-suppressed zinc-ion batteries
[J].This review gives a comprehensive introduction of the solvation structure regulation strategies for dendrite-free and side reaction-suppressed zinc-ion batteries, and further proposes the potential directions and perspectives for further research.
Corrosion behavior of galvanized steel in a simulated marine atmospheric environment
[J].
模拟海洋大气环境中镀锌钢的腐蚀行为和机理
[J].
Reversible Zn metal anodes enabled by trace amounts of underpotential deposition initiators
[J].
Zincophobic electrolyte achieves highly reversible zinc-ion batteries
[J].
Electrolyte additive for interfacial engineering of lithium and zinc metal anodes
[J].
A dual-functional rare earth halide additive for high-performance aqueous zinc ion batteries
[J].
15-Crown-5 ether as efficient electrolyte additive for performance enhancement of aqueous Zn-ion batteries
[J].
A zwitterionic-type multifunctional electrolyte additive of trigonelline hydrochloride stabilizes zinc anodes for advanced aqueous zinc-ion batteries
[J].
Zincophilic armor: phytate ammonium as a multifunctional additive for enhanced performance in aqueous zinc-ion batteries
[J].
A polysulfide-immobilizing polymer retards the shuttling of polysulfide intermediates in lithium-sulfur batteries
[J].
Volumetric behavior of glycine in aqueous succinic acid and sodium succinate buffer at different temperatures
[J].
Monosodium glutamate, an effective electrolyte additive to enhance cycling performance of Zn anode in aqueous battery
[J].
Stabilizing zinc anode for high-performance aqueous zinc ion batteries via employing a novel inositol additive
[J].
Self-assembled protection layer induced by bifunctional additive for reversible aqueous zinc metal battery
[J].
Electric double layer oriented eutectic additive design toward stable Zn anodes with a high depth of discharge
[J].
Reversible adsorption with oriented arrangement of a zwitterionic additive stabilizes electrodes for ultralong-life Zn-ion batteries
[J].A zwitterionic additive (l-CN) with a positively charged quaternary ammonium site and multifunctional polar groups was employed to achieve ultralong-life Zn-ion batteries.
Enhancing Zn-Metal anode stability: Key effects of electrolyte additives on ion-shield-like electrical double layer and stable solid electrolyte interphase
[J].
Dual-function electrolyte additive for highly reversible Zn anode
[J].
Critical solvation structures arrested active molecules for reversible Zn electrochemistry
[J].Aqueous Zn-ion batteries (AZIBs) have attracted increasing attention in next-generation energy storage systems due to their high safety and economic. Unfortunately, the side reactions, dendrites and hydrogen evolution effects at the zinc anode interface in aqueous electrolytes seriously hinder the application of aqueous zinc-ion batteries. Here, we report a critical solvation strategy to achieve reversible zinc electrochemistry by introducing a small polar molecule acetonitrile to form a "catcher" to arrest active molecules (bound water molecules). The stable solvation structure of [Zn(HO)] is capable of maintaining and completely inhibiting free water molecules. When [Zn(HO)] is partially desolvated in the Helmholtz outer layer, the separated active molecules will be arrested by the "catcher" formed by the strong hydrogen bond N-H bond, ensuring the stable desolvation of Zn. The Zn||Zn symmetric battery can stably cycle for 2250 h at 1 mAh cm, Zn||VO full battery achieved a capacity retention rate of 99.2% after 10,000 cycles at 10 A g. This paper proposes a novel critical solvation strategy that paves the route for the construction of high-performance AZIBs.© 2024. The Author(s).
Highly reversible Zn metal anode stabilized by dense and anion-derived passivation layer obtained from concentrated hybrid aqueous electrolyte
[J].
Amino acid-induced interface charge engineering enables highly reversible Zn anode
[J].
Regulating the water molecular in the solvation structure for stable zinc metal batteries
[J].
Anion texturing towards dendrite‐free Zn anode for aqueous rechargeable batteries
[J].The reversibility of metal anode is a fundamental challenge to the lifetime of rechargeable batteries. Though being widely employed in aqueous energy storage systems, metallic zinc suffers from dendrite formation that severely hinders its applications. Here we report texturing Zn as an effective way to address the issue of zinc dendrite. An in-plane oriented Zn texture with preferentially exposed (002) basal plane is demonstrated via a sulfonate anion-induced electrodeposition, noting no solid report on (002) textured Zn till now. Anion-induced reconstruction of zinc coordination is revealed to be responsible for the texture formation. Benchmarking against its (101) textured-counterpart by the conventional sulphate-based electrolyte, the Zn (002) texture enables highly reversible stripping/plating at a high current density of 10 mA cm, showing its dendrite-free characteristics. The Zn (002) texture-based aqueous zinc battery exhibits excellent cycling stability. The developed anion texturing approach provides a pathway towards exploring zinc chemistry and prospering aqueous rechargeable batteries.© 2020 Wiley-VCH GmbH.
Graphene quantum dots enable dendrite-free zinc ion battery
[J].
Immunizing aqueous Zn Batteries against dendrite formation and side reactions at various temperatures via electrolyte additives
[J].