甲酸盐溶液因其高比重、低成本的特点,广泛用于环空保护液。然而在超高温超高压环境中,CO2与H2S等酸性介质渗入到油套环空,甲酸盐高温分解,会进一步引发材料发生严重腐蚀。目前,针对钢材在甲酸盐中的腐蚀行为已有不少相关研究[24]。比较一致的看法是,在甲酸盐环境下CO2会加速腐蚀。研究表明,在不高于80 ℃,有CO2渗入条件下,C110管柱腐蚀速率明显升高[25]。不锈钢在甲酸盐环境下也表现出相同的特点[26~29]。在180 ℃下,超级13Cr在甲酸盐流体中外层检测到“片状”碳酸铁(FeCO3)的析出,CO2起到加速腐蚀的作用[30]。近年来的研究表明,材料的腐蚀特点通常与其表面形成的腐蚀产物有关,腐蚀性离子在不同晶型的腐蚀产物膜中的扩散迁移能力不同,从而对腐蚀造成影响不同[31]。目前对于甲酸盐环境下的腐蚀产物特征研究仍有诸多不足,因此,研究材料在甲酸盐环境下形成的腐蚀产物特征,对于进一步理解对应的腐蚀机制具有重要意义。
基于此,本文对低合金钢P110SS分别在NaCl和HCOOK溶液两种环境下的腐蚀行为及腐蚀产物特征进行研究。通过模拟高温高CO2压力完井液环境,对比低合金钢P110SS在甲酸盐与无机盐工况下的腐蚀行为差异,进而揭示低合金钢P110SS在HCOOK完井液环境中的腐蚀机理。在本研究中,通过SEM、XRD和TEM等手段观察对比分析了低合金钢在P110SS在NaCl和HCOOK溶液中形成的腐蚀产物差异,为澄清低合金钢在不同完井液中存在腐蚀差异的原因提供了新思路,也为低合金钢在油气田开发中的腐蚀防护提供了一定理论基础。
1 实验方法
实验材料为抗硫低合金钢P110SS油套管钢,其化学成分(质量分数,%)为:C 0.26,Cr 0.49,Ni 0.26,Mo 0.6,Mn 0.6,Si 0.26,S 0.003,P 0.01,Ti 0.02,Nb 0.005,V 0.005,余量为Fe。显微组织为回火索氏体。试样尺寸50 mm × 10 mm × 3 mm,距离试样一端3 mm处,开一直径6 mm的通孔,用于悬挂试样。在实验开始前,将试样分别做好标记,依次通过200#、400#、800#的耐水砂纸进行打磨处理,之后使用去离子水冲洗和乙醇脱水,最后冷风干燥。使用游标卡尺(精确度为0.01 mm)量取试样尺寸和电子天平(精确度为0.1 mg)称取样品质量。腐蚀介质为实验室配制的质量浓度1.15 g/mL NaCl溶液和质量浓度为1.25 g/mL HCOOK溶液。
腐蚀失重实验在高温高压反应釜中进行,实验参照GB10124-88进行设计。实验前,将处理好的P110SS钢试样分别与NaCl溶液和HCOOK溶液装入釜内后密封,通高纯氮气除氧2 h,升温至设定温度后,通入CO2气体到设定压力10 MPa,实验温度分别为150和180 ℃,浸泡5 d,以模拟工况环境。实验完成后,取出试样,酸洗除去试样表面腐蚀产物,酸洗液由100 mL盐酸(质量浓度1.19 g/mL),900 mL去离子水和5 g六次甲基四胺配置而成。随后用去离子水冲洗,并用酒精脱水。冷风吹干后称重,通过
式中,Vc为平均腐蚀速率,mm·a-1;m1和m2为实验前后试样的质量,g;S为试样的总面积,cm2;ρ为试样的密度,对P110SS钢取7.85 g/cm3;t为实验时间,h。采用FEI Quanta 200F型场发射环境扫描电镜(SEM)观察反应后挂片表面的腐蚀产物形貌及腐蚀产物的分层情况,使用配套能谱仪(EDS)对腐蚀产物成分进行元素分析。使用LEXT OLS4100型激光共聚焦扫描显微镜(CLSM)测量点蚀坑深,通过Bruker D8 Focus型X射线衍射仪(XRD)标定腐蚀产物的物相组成,使用JEM 2100F LaB6型透射电镜(TEM)观察腐蚀产物优势生长面。
2 结果与讨论
2.1 腐蚀速率变化规律
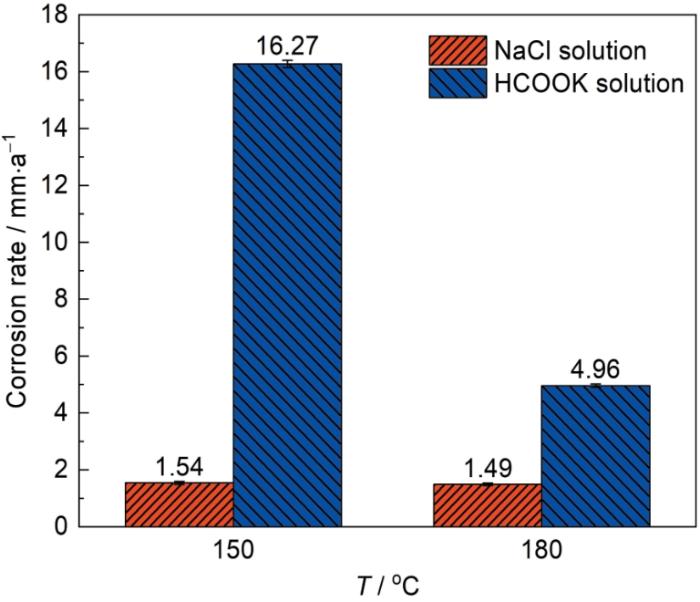
图1为P110SS钢在温度为150和180 ℃的NaCl和HCOOK溶液中的腐蚀速率柱状图。可以看出,NaCl溶液中P110SS钢的腐蚀速率为1.54 mm/a左右,这符合低合金钢在高温含CO2环境下的腐蚀特征。然而,在HCOOK溶液中P110SS钢在150 ℃时腐蚀速率达到16.27 mm/a,是相同条件下NaCl溶液的10.6倍,这说明溶液环境的改变使材料的腐蚀特点发生显著变化。温度达到180 ℃时,腐蚀速率明显降低,为4.96 mm/a,是NaCl溶液条件的3.3倍。一般而言,温度升高对反应动力学起加速作用,出现腐蚀速率随温度升高而降低的现象,一方面是因为腐蚀性气体溶解度降低,导致H+浓度降低[32],阴极去极化作用减弱。也可能是腐蚀产物对钢材保护性提高所致[33]。
图1
图1
P110SS钢在不同温度、不同溶液中浸泡5 d后的腐蚀速率
Fig.1
Corrosion rates of P110SS steel after immersion for 5 d under the different conditions of solution and temperature
2.2 微观形貌
图2
图2
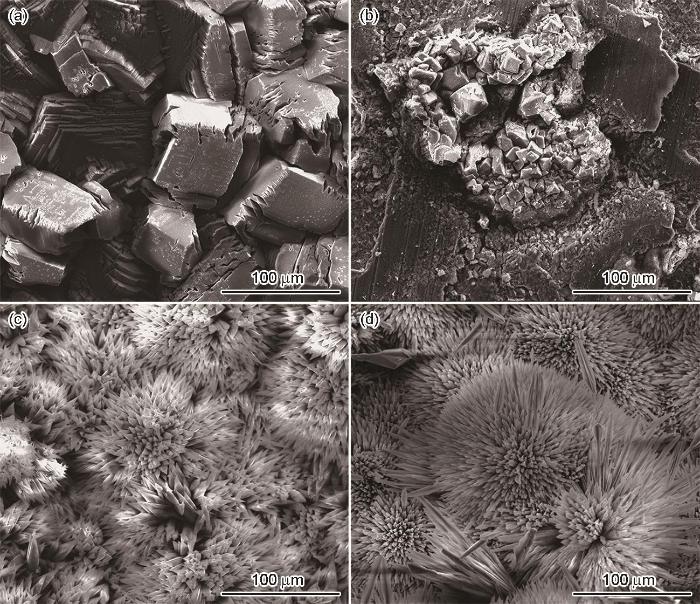
P110SS钢在不同温度、不同溶液中浸泡5 d后的SEM图
Fig.2
SEM surface images of P110SS steel after 5 d immersion in NaCl solution (a, b) and HCOOK solution (c, d) at 150 ℃ (a, c) and 180 ℃ (b, d)
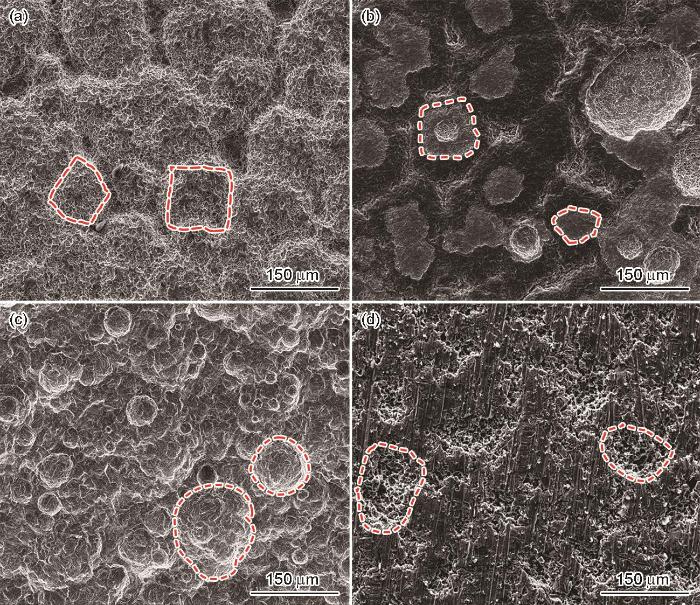
图3为试样去除腐蚀产物后的表面微观形貌,其中图3a和b为150和180 ℃条件下NaCl溶液中试样表面的微观形貌图。可以看出,试样表面出现不同程度的局部腐蚀特征。腐蚀坑大小约为50~100 μm,形状与腐蚀产物基本一致,呈菱形块状,如图3a和b中红色标注所示。图3c和d为P110SS钢在150和180 ℃下HCOOK溶液中实验后去除腐蚀产物的微观形貌图。试样表面也发生了明显的局部腐蚀,与在NaCl溶液中相似,腐蚀坑在腐蚀产物覆盖位置形成。HCOOK溶液中的腐蚀坑多呈圆形,如图3c和d中红色虚线标注所示。不同的是,NaCl溶液中形成的点蚀坑主要与腐蚀产物的脱落和小半径Cl-的穿透作用有关,而HCOOK溶液中形成的点蚀坑又产生了多个小点蚀坑,这可能是由于腐蚀过程十分剧烈,在较大“花簇”下的基体表面形成了小型“花簇”,进而继续加剧了基体的点蚀。通过对比研究,可以确定P110S钢的腐蚀特征与其腐蚀产物的生长形态存在一定相关性。
图3
图3
P110SS钢在不同温度、不同溶液中浸泡5 d后去除腐蚀产物后的SEM图
Fig.3
SEM images of P110SS steel after removal of corrosion products formed during 5 d immersion in NaCl solution (a, b) and HCOOK solution (c, d) at 150 ℃ (a, c) and 180 ℃ (b, d)
图4
图4
P110SS钢在不同温度、不同溶液中浸泡5 d后去除腐蚀产物后的CLSM图
Fig.4
CLSM surface images of P110SS steel after removal of corrosion products formed during 5 d immersion in NaCl solution (a, b) and HCOOK solution (c, d) at 150 ℃ (a, c) and 180 ℃ (b, d)
2.3 腐蚀产物截面分析
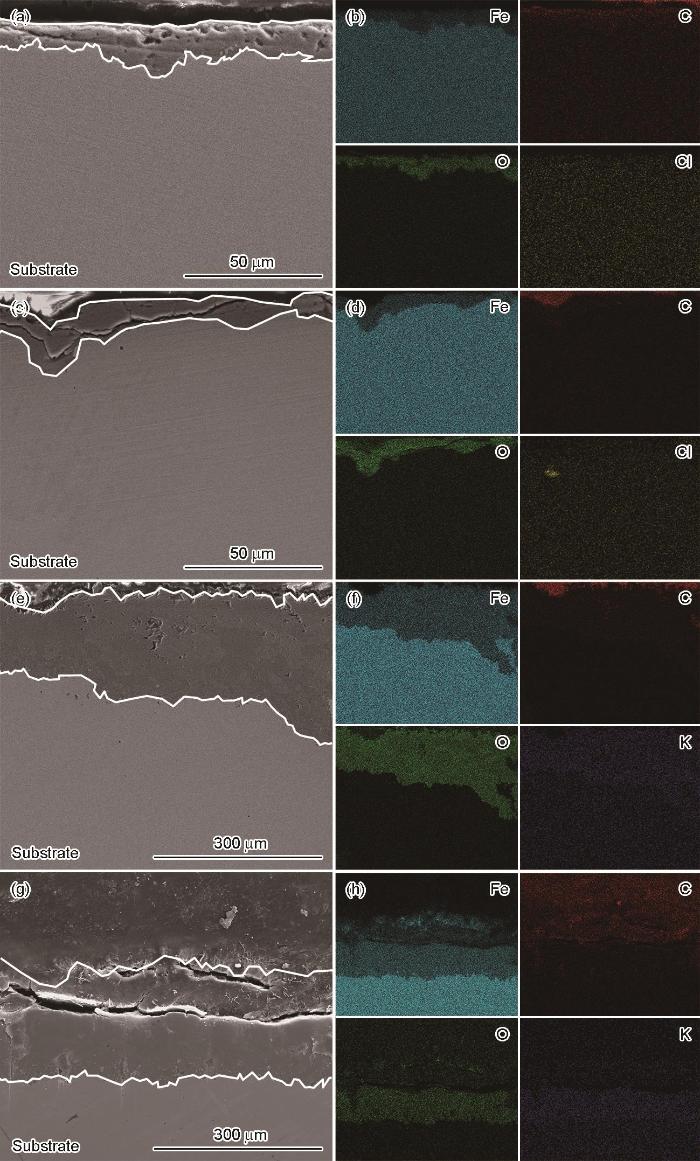
为进一步分析高温下低合金钢P110SS在NaCl溶液和HCOOK溶液中腐蚀行为存在差异的原因,对腐蚀试样的横截面进行观察,标定了腐蚀产物的元素组成和晶体类型,结果如图5所示。
图5
图5
P110SS钢在不同温度、不同溶液中浸泡5 d后的截面SEM像
Fig.5
SEM cross-sectional images and EDS element mappings for P110SS steel immersed for 5 d in NaCl solution (a-d) and HCOOK solution (e-h) at 150 ℃ (a, b, e, f) and 180 ℃ (c, d, g, h)
图5a和b分别为NaCl溶液中150和180 ℃下腐蚀产物膜的横截面图。可以看出,腐蚀产物层均有Fe、C和O元素,推测腐蚀产物主要为FeCO3。NaCl溶液中腐蚀产物层的平均厚度约为19.1 μm,两种温度下测量厚度差别不大。图5c和d分别为低合金钢P110SS钢在150和180 ℃下HCOOK溶液中的腐蚀产物膜横截面图。腐蚀产物层同样为Fe、C和O元素,但腐蚀产物层明显厚于NaCl溶液,150 ℃腐蚀产物层平均厚度约为200.5 μm,180 ℃条件下为183.1 μm。结合腐蚀速率的结果(图1),说明在HCOOK溶液中形成的腐蚀产物对基体保护性较差。同时,在NaCl溶液腐蚀环境下的点蚀坑底部观察到有Cl-富集,而在HCOOK溶液中则未观察到相应特征,这也解释了图4中NaCl溶液腐蚀环境下局部腐蚀更严重的原因。
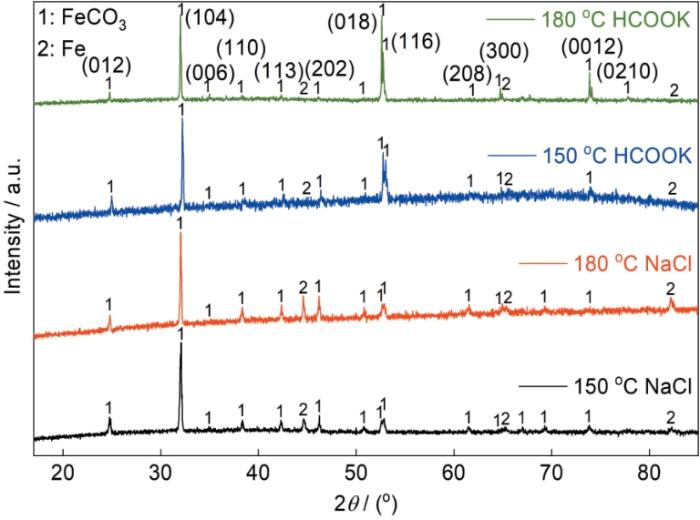
2.4 腐蚀产物XRD分析
图6
图6
P110SS钢在不同温度、不同溶液中浸泡5 d后腐蚀产物的XRD谱
Fig.6
XRD patterns of P110SS steel after 5 d immersion under the different conditions of solution and temperature
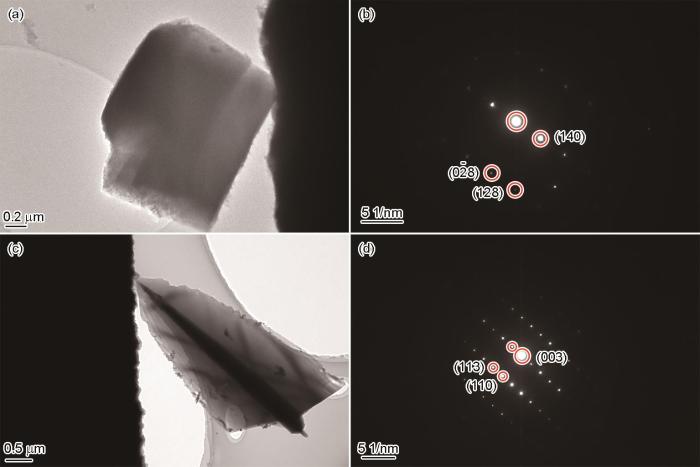
2.5 腐蚀产物TEM分析
在150 ℃ NaCl溶液和180 ℃ HCOOK溶液中形成的腐蚀产物TEM分析结果如图7所示。图7a为NaCl溶液环境下生成的FeCO3明场像,可以看出,NaCl溶液中形成的FeCO3在透射电镜下呈现菱形块状,与图2中扫描电镜观察到的形态一致。图7b的为电子束从腐蚀产物FeCO3晶体六方结构菱铁矿[16
图7
图7
P110SS钢在150 ℃下NaCl、180 ℃下HCOOK溶液中浸泡5 d后腐蚀产物的TEM图和选区电子衍射
Fig.7
TEM images and selected-area diffraction patterns of the corrosion products formed on P110SS steel after 5 d immersion at 150 ℃ in NaCl solution (a, b) and at 180 ℃ in HCOOK solution (c, d)
2.6 讨论
腐蚀的阳极反应为Fe氧化为Fe2+,随后与CO32-结合生成FeCO3,附着在基体表面,从而对腐蚀反应起到隔绝阻碍的作用,其反应过程式如
尽管P110SS钢在NaCl溶液和HCOOK溶液中腐蚀的阴阳极反应相似,但在不同溶液下的腐蚀速率及腐蚀产物形态上存在显著差异。这是因为HCOOK属于强碱弱酸盐,其水溶液呈弱碱性。H2CO3在碱性环境下的电离加剧,促进了CO2的进一步溶解。与在NaCl溶液中Fe2+与CO
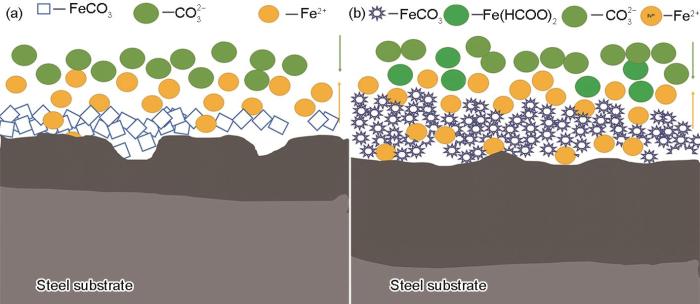
P110SS钢在NaCl和HCOOK溶液中的腐蚀机理示意图如图8a和b所示。根据腐蚀产物截面形貌(图5)可以看出,HCOOK溶液腐蚀环境下形成的内层腐蚀产物膜更厚,然而腐蚀速率却显著升高。且TEM观测结果表明,这种以中间产物置换形成的FeCO3沿[001]方向定向生长,形成以(003)晶面为优势生长晶面的三棱柱结构。相比于在NaCl溶液中形成的无择优取向的FeCO3,这种结构的腐蚀产物有利于Fe2+、HCOO-、CO
图8
图8
P110SS钢在含CO2的NaCl溶液和HCOOK溶液中的腐蚀产物膜结构示意图
Fig.8
Schematic diagrams of the microstructures of corrosion product films formed on P110SS steel during immersion in CO2-containing NaCl solution (a) and HCOOK solution (b)
3 结论
(1) 高温高压CO2条件下,P110SS钢在HCOOK溶液中的腐蚀速率是NaCl溶液中的3~10倍,两种环境中形成的腐蚀产物形态存在显著差异。
(2) 与NaCl溶液中形成的腐蚀产物相比,材料在HCOOK溶液中形成的FeCO3在元素组合和晶体类型上无显著差异,但XRD结果表明,在(018)、(116)和(0012)3个晶面上的衍射峰强度明显增强。
(3) NaCl溶液中形成的FeCO3腐蚀产物呈菱形块状,无明显的优势生长面和生长方向。HCOOK溶液中形成的腐蚀产物则呈“羽毛”状堆积在基体表面,沿[001]方向定向生长,其优势生长面为(003)晶面。不同环境下腐蚀速率的差异可能与腐蚀产物对传质过程阻碍能力不同有关。
参考文献
Advances in research of CO2 corrosion in oil and gas industry
[J].
石油天然气工业中CO2腐蚀的研究进展
[J].
Key issues related to modelling of internal corrosion of oil and gas pipelines-A review
[J].
Carbonic acid corrosion of steel
[J].
Research on electrochemical behavior and corrosion scale characteristics of CO2 corrosion for tubing and casing steel
[D].
油套管钢CO2腐蚀电化学行为与腐蚀产物膜特性研究
[D].
Corrosion behavior of P110S oil casing steel in sulfur containing environment
[J].
P110S油套管在微含硫环境中的腐蚀行为研究
[J].采用不同含量的Na<sub>2</sub>S来模拟不同的含硫环境,利用挂片浸泡实验研究P110S钢级油套管在含硫体系中的腐蚀行为;利用电化学测试研究了其在不同含硫浓度中电化学特征;利用扫描电镜、激光共聚焦、XRD以及拉曼光谱对样品表面的腐蚀产物和形貌进行了表征。结果表明,P110S钢级油套管钢在含硫体系中发生严重的腐蚀,腐蚀速率和腐蚀电流密度均随含硫浓度增大而增大,腐蚀类型由均匀腐蚀转变为点蚀,腐蚀产物疏松。
Corrosion characteristics of P110SS casing steel for ultra-deep well in artificial formation water with low H2S and high CO2 content
[J].
低H2S和高CO2分压下超深井用P110SS油套管钢腐蚀特征研究
[J].研究了P110SS钢在含低硫化氢油气井下腐蚀规律及特征。通过高温高压反应釜模拟超深井的腐蚀工况,对P110SS钢在不同温度、H<sub>2</sub>S、CO<sub>2</sub>分压条件下进行浸泡实验,通过腐蚀失重计算其腐蚀速率,辅以SEM、EDS和XRD等手段对腐蚀产物的形貌和成分进行表征。结果表明,H<sub>2</sub>S、CO<sub>2</sub>分压增大均会导致P110SS的腐蚀速率增大;然而温度升高却降低其腐蚀速率。分析腐蚀产物可见,H<sub>2</sub>S、CO<sub>2</sub>浓度和温度的变化均会导致腐蚀产物成分和结构发生转变。说明在高温高压条件下,H<sub>2</sub>S腐蚀起主导作用,Fe<sub>7</sub>S<sub>8</sub>腐蚀产物对基体的保护作用较差,腐蚀速率高;低H<sub>2</sub>S分压下,CO<sub>2</sub>腐蚀起主导作用,腐蚀速率的大小取决于腐蚀产物膜的致密性;相比于CO<sub>2</sub>,温度对腐蚀速率的影响更显著。
Corrosion mechanism of 13Cr stainless steel in completion fluid of high temperature and high concentration bromine salt
[J].
Corrosion inhibitor for heavy brines
[P].
Failure analysis of a fracture tubing used in the formate annulus protection fluid
[J].
Stress corrosion cracking behavior of super 13Cr stainless steel in CO2-containing CaCl2 completion fluid
[J].
超级13Cr钢在含CO2的CaCl2完井液中应力腐蚀开裂行为
[J].
Study on corrosion behavior of P110S steel in CO2-H2S-saturated solution
[J].
The evolution and application of formate brines in high-temperature/high-pressure operations
[A].
Physical and chemical properties of cesium and other formate brines as drilling/completion fluids
[J].
甲酸铯及其他甲酸盐水溶液的物理化学特性
[J].
Formate drilling fluid system application
[D].
甲酸盐钻井液体系的应用
[D].
Research on stress corrosion sensitivity of C110 casing in wellbore protection fluid
[J].
Failure analysis of P110 tubing string in the ultra-deep oil well
[J].
Corrosion failure analysis of high strength grade super 13Cr-110 tubing string
[J].
Influence of a small velocity variation on the evolution of the corrosion products and corrosion behaviour of super 13Cr SS in a geothermal CO2 containing environment
[J].
Effect of the flow velocity on the corrosion behavior of UNS S41426 stainless steel in the extremely aggressive oilfield environment for the Tarim area
[J].
Effect of the micro-plasma arc welding technique on the microstructure and pitting corrosion of AISI 316L stainless steels in heavy LiBr brines
[J].
Understanding the interaction mechanism of chloride ions and carbon dioxide towards corrosion of 3Cr steel
[J].
Corrosion and corrosion fatigue of AISI 420C (X46Cr13) at 60 ℃ in CO2-saturated artificial geothermal brine
[J].
Study on the corrosion behavior of P110S in high-temperature CaCl2 completion fluid
[J].
P110S在高温CaCl2完井液中的腐蚀规律研究
[J].
CO2 corrosion of steel in formate brines for well applications
[A].
Effect of CO2 on the corrosion behavior of C110 carbon steel in formate solution environment
[J].
CO2渗入对C110管柱在甲酸盐完井液中腐蚀行为的影响
[J].
Localized corrosion resistance of super 13Cr stainless steel in formate completion fluid containing CO2
[J].
含CO2甲酸盐完井液中超级13Cr不锈钢的局部腐蚀性能
[J].
Corrosion behavior of high strength 15Cr martensitic stainless steel in organic salt completion fluid
[J].
高强15Cr马氏体不锈钢在有机盐完井液中的腐蚀行为
[J].
Corrosion behavior of 2507 super duplex stainless in potassium formate completion fluid
[J].
2507超级双相不锈钢在甲酸盐完井液中的腐蚀行为
[J].
Anti-corrosion behavior of TC4 alloy in organic salt completion fluid
[J].
TC4钛合金在有机盐完井液中的腐蚀性能
[J].
Formation and evolution of the corrosion scales on super 13Cr stainless steel in a formate completion fluid with aggressive substances
[J].
Ion-selectivity of iron sulfides and their effect on H2S corrosion
[J].
Discussion on corrosion of ground equipment in oil and gas field from carbon dioxide
[J].
二氧化碳对油气田地面设备的腐蚀探讨
[J].
Behavior pattern and research progress of carbon dioxide corrosion in oil well
[J].
油井二氧化碳腐蚀行为规律及研究进展
[J].
Corrosion behavior of casing steels 13Cr and N80 during sequestration in an impure carbon dioxide environment
[J].
含杂CO2封存条件下13Cr和N80套管钢腐蚀规律研究
[J].井筒屏障金属材料腐蚀失效是影响碳封存安全性的关键问题。针对高温高压含杂CO<sub>2</sub>封存环境下井筒套管腐蚀规律问题,利用高温高压反应釜模拟封存条件下的井下工况,分别研究了N80及13Cr钢在不同压力、应力条件下含杂质(SO<sub>2</sub>、NO<sub>2</sub>和O<sub>2</sub>)的超临界CO<sub>2</sub>富水相中的腐蚀规律。本研究利用失重法得到腐蚀速率,并利用扫描电镜(SEM)、X射线衍射仪(XRD)和X射线光电子能谱仪(XPS)等对产物膜进行了表征分析。结果表明,N80钢的均匀腐蚀与点蚀速率均随着压力的升高而增大;压力对13Cr钢的均匀腐蚀影响不明显,但在压力为20 MPa时出现严重的点蚀现象;给试样施加拉应力后,随着应力的增加,N80及13Cr钢的腐蚀产物层均出现了不同程度的破损,但是基体表面未观察到裂纹生成。
Fundamental aspects of the corrosion of N80 steel in a formation water system under high CO2 partial pressure at 100 ℃
[J].
Effect of extremely aggressive environment on the nature of corrosion scales of HP-13Cr stainless steel
[J].
On the fundamentals of electrochemical corrosion of X65 steel in CO2-containing formation water in the presence of acetic acid in petroleum production
[J].
Effect of thiosulphate/H2S on crevice corrosion behaviour of P110 carbon steel in CO2-saturated solution
[J].