高熵合金(HEA)作为一种新兴的合金材料,因其独特的高混合熵特性,展现出了超越传统合金的优异性能,包括高强度、良好的耐腐蚀性和热稳定性[10~12]。FeNiCoCr基高熵合金在海洋环境中的耐腐蚀性能表现尤为突出,这使得它们在海洋工程和深海探测等领域的应用前景备受期待[13, 14]。并且由于高熵效应,高熵合金容易形成无序的固溶体,固溶体中多种元素的随机排列导致了特定的局部无序化学环境,在抑制MIC方面有很大的研究潜力[15]。但目前高熵合金耐蚀性研究主要集中在NaCl溶液或H2SO4溶液中,关于MIC的研究还较少。目前已有的研究表明,FeCoCrNiMo0.1高熵合金在铜绿假单胞菌作用下,其表面形成的钝化膜会受到削弱,导致合金的局部腐蚀加速[16]。Yang等[17]则通过在CoCrFeMnNi高熵合金中引入N,成功提升了合金在脱硫弧菌环境下的抗点蚀能力。尽管已有研究取得了一定的进展,但关于SRB与高熵合金相互作用的详细机制,尤其是SRB对高熵合金表面钝化膜的降解和新膜形成过程的影响,目前仍然缺乏深入的理解和系统的研究。
本研究设计了一种综合性能良好的FeNiCoCrW0.2Al0.1高熵合金,旨在通过实验和理论分析,重点探究SRB对其耐腐蚀性能的影响,以进一步评估其在海洋环境中的适用性,并揭示SRB溶液中合金表面钝化膜的降解与形成机制。本研究期望为高熵合金在海洋环境中的长期稳定性和安全性提供科学依据,进而推动海洋资源开发的技术进步和经济效益的提升。
1 实验方法
在本研究中使用纯度99.9%的金属单质原材料。在称量前需要通过打磨抛光的方式去除表面的氧化层。FeCoCrNiW0.2Al0.1高熵合金(HEA)的化学成分(质量分数,%)为:Fe21.1Co22.2Cr19.6Ni22.2W13.9Al1。
合金锭的制备采用WK系列真空电弧炉进行熔炼。为了避免合金成分的不均匀性,需要考虑各组分的熔点和密度差异,将低熔点的元素单质放在铜坩埚的底部。反复熔炼3次以确保合金成分的均匀性。
熔炼结束后的合金样品采用KSL-1400X箱式电阻炉进行均匀热处理。热处理工艺为:将样品置于1150 ℃下保温2 h后进行水冷。在进行均匀化热处理时,样品被封存在真空石英管中。
细菌培养基是由山东拓普生物工程有限公司生产的,其中包括两种成分:一是脱硫弧菌培养基的基础成分,编号为M2256B;二是脱硫弧菌培养基的添加剂,编号为S2212。每1L培养基的成分(g)为:MgSO4 0.98、酵母浸粉1、NH4Cl 1、Na2SO4 1、K2HPO4 0.5、CaCl2·2H2O 0.1、刃天青1 mg、FeSO4.7H2O 0.5、C2H3NaO2S 0.1,溶液在25 ℃下的pH为7.8 ± 0.2。
将SRB菌种注入到培养基溶液中,并放置在恒温培养箱中,设置温度为37 ℃。经过一段时间的培养,直到观察到玻璃瓶底部出现了黑色沉淀物,这标志着SRB开始产生代谢产物,SRB溶液制备完成。Oguro等[9]的研究认为,SRB的数量在1 d后达到最大值,3 d后开始减少。因此,浸泡试样试管中的SRB溶液每3 d更换一次等量的新鲜溶液。
采用电解腐蚀的方法制备金相样品,并在金相显微镜下进行观察。金相样品尺寸为10 mm × 10 mm × 1 mm,使用水磨SiC砂纸逐级打磨试样,并抛光以确保表面粗糙度Ra ≤ 1.6 μm。随后进行电解腐蚀,电解液为4%硝酸溶液和10%草酸水溶液1∶1体积比的混合溶液,阳极电压设置为9~10 V直流电压,电解腐蚀时间为30 s。通过电解腐蚀制备好样品之后,通过无水酒精清洗并在烘干箱内干燥。然后将样品放置在金相显微镜下进行金相观察和拍照。
采用D2 Phaser型X射线衍射仪(XRD)测定衍射峰,对合金试样的晶体结构组成进行分析。扫描角度20°~80°,扫描速度2°/min。
使用Scientific K-Alpha 型X射线光电子能谱(XPS)对样品进行分析。样品的制备按照金相观察试样的制备工艺进行。将样品裁剪至5 mm × 5 mm大小,设置光斑大小为400 μm,工作电压为12 kV,灯丝电流为6 mA。进行全谱扫描,通能为150 eV,步长为1 eV;然后进行窄谱扫描,通能为50 eV,步长为0.1 eV。通过XPS分析,可以确定钝化膜的化学成分及其表面组成,从而深入了解样品的表面特性和组成。
使用ZSX primus型荧光显微镜(FM)观察了试样在SRB溶液中浸泡后细菌的聚集情况。观测前用0.01%吖啶橙染色细菌并用PBS缓冲液清洗。
使用GeminiSEM300型扫描电子显微镜(SEM)对试样在SRB溶液腐蚀前后的表面形貌进行观察。对于腐蚀后的试样在观察之前,使用5%戊二醛溶液对其进行浸泡,固定表面细菌并通过酒精逐级脱水。
使用Reference 600 Plus电化学工作站,采用三电极体系,其中,参比电极为饱和甘汞电极,对电极为铂片。电解液根据实验要求的不同调整SRB溶液和无菌SRB培养基溶液。开路电位测试在-10~10 mV范围内,扫描速率为0.125 mV/s。阻抗谱(EIS)测试的频率范围为105~10-1 Hz,振幅为5 mV。极化曲线在腐蚀电位-0.5~1.5 mV范围内进行测量,扫描速率为1 mV/s。
2 结果与讨论
2.1 微观结构表征
图1
图1
FeNiCoCrW0.2Al0.1高熵合金微观形貌及XRD分析
Fig.1
Microstructural characterization (a) and XRD pattern (b) of FeNiCoCrW0.2Al0.1 HEA
2.2 细菌附着分析
用FM观察了SRB在高熵合金表面的附着行为。图2分别显示了合金试样在SRB溶液中浸泡1、2、3和5 d的生长情况。可以观察到:浸泡1 d仅有少量的SRB附着在试样表面,2 d后SRB增多,形成了一些菌落,3 d后一定数量的SRB附着在了试样表面,5 d后这个数量没有发生明显变化。因此我们认为试样在SRB溶液中浸泡3 d后,细菌数量达到了一定的峰值。FM的观测结果表明,SRB以一定数量附着在试样表面,并在3 d后达到峰值。
图2
图2
FeNiCoCrW0.2Al0.1高熵合金在SRB溶液中浸泡不同天数后的FM形貌
Fig.2
FM images of FeNiCoCrW0.2Al0.1 HEA immersed in SRB solution for 1 d (a), 2 d (b), 3 d (c) and 5 d (d)
2.3 电镜观测腐蚀形貌
图3
图3
FeNiCoCrW0.2Al0.1高熵合金试样在SRB溶液浸泡9和24 d后的SEM形貌以及去除生物膜和腐蚀产物后的SEM形貌
Fig.3
SEM surface morphologies of FeNiCoCrW0.2Al0.1 HEA after immersion in SRB solution for 9 d and 24 d (a, b), and then removal of biofilm and corrosion products (c, d), respectively
由图3的SEM观测结果可见,在SRB溶液浸泡后的试样表面形成了生物膜,这种生物膜是由细菌和其代谢产物组成的复合物。通过对比浸泡不同天数SRB溶液后的试样表面形貌可见,随着浸泡天数的增加,试样表面出现了更加严重的腐蚀凹坑,可以认为这是SRB活动和溶液环境腐蚀共同作用下的结果。
2.4 电化学极化测试
为了研究高熵合金在SRB溶液中的耐腐蚀性能并对比接种SRB后溶液环境对试样的腐蚀影响,对抛光后的试样进行了极化测试。图4表示的极化曲线分别为试样在电解液为培养基接种了SRB并培养了3 d的SRB溶液和无菌培养基溶液中测试得到的。腐蚀参数通过考虑Tafel方程进行估算。
其中,ηa/c表示阳极或阴极区域的过电位,V;ba/c表示阳极或阳极区域的Tafel斜率;I0表示腐蚀电流密度,A/cm2。
图4
图4
高熵合金试样在SRB溶液中和无菌培养基溶液中的极化曲线
Fig.4
Polarization curves of FeNiCoCrW0.2Al0.1 HEA in SRB solution and sterile medium solution
表1 电化学测试的腐蚀参数和估算腐蚀速率
Table 1
| Medium | Ecorr / mV | Icorr / A·cm-2 | Epit / mV | Ipit / A·cm-2 | Kcorr / mm·a-1 |
|---|---|---|---|---|---|
| HEA in aseptic medium | -232 | 6.30 × 10-7 | 1156 | 2.77 × 10-5 | 7.68 × 10-3 |
| HEA in SRB solution | -326 | 8.26 × 10-6 | 1064 | 4.87 × 10-5 | 1.01 × 10-1 |
| 304 stainless steel in SRB solution | -361 | 1.29 × 10-5 | - | - | 1.33 × 10-1 |
其中,Kcorr是腐蚀速率,mm/a;Icorr是腐蚀电流密度,µA/cm2;k是常数(= 3.272 mm/(µA-cm-a));EW是电极的当量质量,g;ρ是合金试样的密度(= 8.705 g/cm3)。
EW是存在的元素的原子量与交换的当量电子数(氧化数)之比的加权平均值(= 32.399 g),确定如下:
其中,f是第i种合金元素的质量分数,ni 是第i个合金元素交换的电子数,#/mol,Ai 是第i合金元素的原子量,g/mol。
2.5 电化学阻抗谱测试
图5
图5
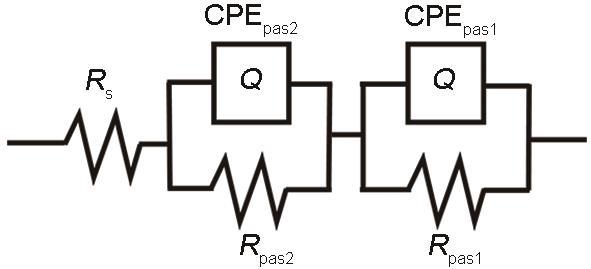
用于拟合试样在SRB溶液中浸泡后的EIS的等效电路
Fig.5
Equivalent circuit model for fitting EIS data
图6
图6
高熵合金试样在SRB溶液中浸泡9和24 d后的EIS
Fig.6
Nyquist (a) and Bode (b) plots of FeNiCoCrW0.2Al0.1 HEA after immersion in SRB solution for 9 and 24 d
其中,d是膜厚度,nm;k是介电常数,ε0是真空的介电常数(= 8.85 × 10-5 F/nm),A是样品面积,nm2;C为电容,F。表2列出了EIS测试数据拟合结果的电路元件参数值和估计的钝化膜厚度。
表2 EIS拟合得到的电路元件参数以及估算的钝化膜厚度
Table 2
| Time / d | Rs / Ω·cm2 | Rpas1 / Ω·cm2 | Rpas2 / Ω·cm2 | CPEpas1 / S·s n ·cm-2 | npas1 | CPEpas2 / S·s n ·cm-2 | npas2 | dpas1 / nm | dpas2 / nm |
|---|---|---|---|---|---|---|---|---|---|
| 9 | 61.37 | 6783 | 1503 | 8.70 × 10-5 | 0.82 | 2.11 × 10-4 | 0.52 | 3.05 | 1.26 |
| 24 | 54.79 | 1334 | 7925 | 9.06 × 10-5 | 0.61 | 1.94 × 10-4 | 0.77 | 2.93 | 1.37 |
EIS测试的拟合结果表明,在SRB溶液中浸泡9及24 d后样品表面都形成了双层类型的钝化膜结构,估计分别为4.31 nm (外层1.26 nm和内层3.05 nm)和4.30 nm (外层1.37 nm和内部2.93 nm),钝化膜厚度没有随着时间天数发生明显变化。SRB溶液环境下试样形成的钝化膜很薄且没有随着时间延长发生明显变化。可以认为这可能与SRB的生物活动有关。根据Lou等[16]研究,这种生物膜会对FeCoCrNiMo0.1高熵合金的钝化元素在表面的富集产生影响,使生成的钝化膜更薄更弱。为了验证EIS测试得到的数据结果,我们需要对钝化膜进行进一步的测试分析。
2.6 XPS钝化膜元素分析
图7
图7
高熵合金在SRB溶液中浸泡24 d后表面处合金元素的XPS精细谱及峰拟合结果
Fig.7
XPS fine spectra and peak fitting results of Fe (a), Ni (b), Co (c), Cr (d), W (e), Al (f) and O (g) on the surface of FeNiCoCrW0.2Al0.1 HEA immersed for 24 d
图8
图8
高熵合金在SRB溶液中浸泡24 d后距表面4 nm处合金元素的XPS精细谱及峰拟合结果
Fig.8
XPS fine spectra and peak fitting results of Fe (a), Ni (b), Co (c), Cr (d), W (e), Al (f) and O (g) at the depth of 4 nm from the surface of FeNiCoCrW0.2Al0.1 HEA immersed for 24 d
表3 检测到的7种元素的XPS峰位置及其对应的可能氧化态
Table 3
| Oxidation state | Orbital | Energy peak | Energy peak |
|---|---|---|---|
| (0 nm) | (-4 nm) | ||
| Fe (Fe3+) | 2p3/2 | 709.36 | 709.83 |
| Fe (matel) | 2p3/2 | - | 706.88 |
| Cr (Cr(OH)3) | 2p3/2 | 576.62 | 577.45 |
| Cr (Cr2O3) | 2p3/2 | 575.39 | 576.07 |
| Cr (matel) | 2p3/2 | 572.74 | 573.83 |
| Ni (Ni2+) | 2p3/2 | 854.70 | - |
| Ni (matel) | 2p3/2 | 851.56 | 852.71 |
| Co (Co2+) | 2p3/2 | 780.18 | - |
| Co (matel) | 2p3/2 | 777.29 | 778.07 |
| W4 (W6+) | 4f7/2 | 34.35 | 35.84 |
| W4 (matel) | 4f7/2 | 29.99 | 31.23 |
| O (H2O) | 1s | 532.12 | - |
| O (O2-) | 1s | 529.29 | 530.39 |
| O (OH-) | 1s | 530.74 | 531.51 |
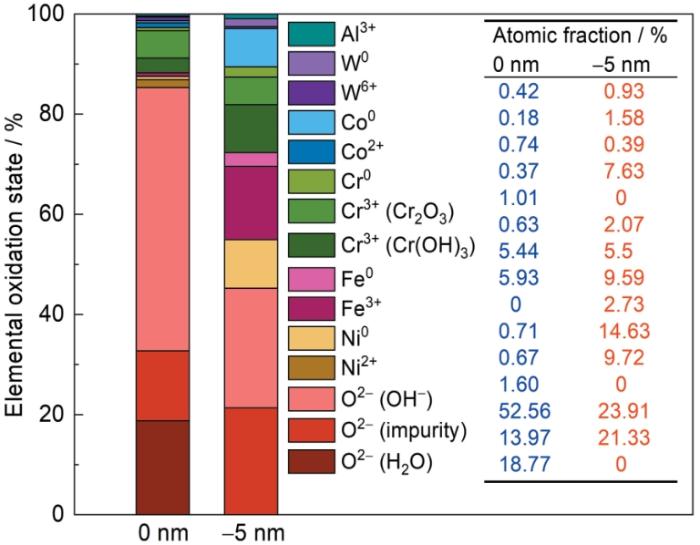
图9为试样浸泡腐蚀后钝化膜中测得的不同元素的相对分数及其对应的氧化态。根据浸泡后试样钝化膜内外层成分比例变化我们可以观察到:(1) 钝化膜内层主要由Fe、Ni、Co和Cr的金属单质及Fe和Cr的氧化物,还有少量的W、Al氧化物和W单质构成。由于内层靠近基体,金属单质比例较高,未检测到Ni2+和Co2+的明显能量峰;(2) 钝化膜外层中,各个金属元素的比例都非常低,主要由Cr的氧化物和氢氧化物构成,这可能是由于SRB溶液环境对Fe、Ni、Co元素的剧烈消耗导致的,这种成分差异证明腐蚀试样表面钝化膜发生了成分性分层,形成了双层类型的钝化膜结构,验证了EIS测试中得出的结论。
图9
图9
高熵合金试样在SRB溶液中浸泡时形成的表面钝化膜的组成元素及其相对分数与对应的氧化态
Fig.9
Relative fractions and corresponding oxidation states of various elements detected in the passivation film formed on FeNiCoCrW0.2Al0.1 HEA during immersion in SRB solution
2.7 腐蚀机理分析
通过EIS测试可见,试样在SRB溶液环境下生成的钝化膜为双层且不会随着在溶液中的暴露时间增加而变厚。而XPS测试结果印证了这一结构,并且钝化膜中元素成分比例的变化反映了试样在SRB溶液环境下钝化膜外层的Fe、Co和Ni会优先消耗,钝化膜发生了结构性分层,形成了一种双层的钝化膜结构。基于上述结论,我们提出了FeNiCoCrW0.2Al0.1高熵合金在SRB溶液中形成钝化膜的可能机制,如图10所示。钝化膜可按以下步骤形成:
图10
图10
FeNiCoCrW0.2Al0.1高熵合金在SRB溶液中形成钝化膜的机理示意图
Fig.10
Schematic diagram of the formation of passivation film on FeNiCoCrW0.2Al0.1 HEA during immersion in SRB solution
(1) 高熵合金在空气中自然钝化,形成含有Fe、Ni、Cr和Co氧化物的钝化膜,同时存在少量的W和Al氧化物。
(2) 试样在SRB溶液的暴露过程中,由于SRB代谢活动产生的H2S溶解在水中,合金外层钝化膜中的Fe、Ni、Co氧化物优先发生阴极去极化反应,造成钝化膜局部腐蚀。
(3) 随着腐蚀的进行,钝化膜外层的元素消耗加剧。同时SRB与其代谢产物在试样表面富集形成生物膜,一定程度上隔绝了O2-和OH-,削弱了钝化膜的进一步生成。钝化膜在成分上分层为两个不同的层,内层成分与自然钝化膜类似,接近基体,而外层由Cr2O3构成,与生物膜直接接触。
钝化膜的双层结构可以使本高熵合金在SRB溶液中具有相对较高的耐腐蚀性。这是由于外层Cr2O3在金属表面上形成了一个化学稳定的界面,不容易与腐蚀介质发生化学反应,抑制了SRB胞外电子转移的过程,阻碍了金属离子的释放。内层直接附着在金属表面,提供进一步的保护。即使外层受损,内层可以防止金属基体与腐蚀环境的直接接触。但由于生物膜阻碍了钝化元素的富集,使得样品表面生成的钝化膜更薄更弱,削弱了钝化膜的保护作用,使得试样在SRB溶液环境下更易受到腐蚀,这印证了SEM中观测到的结果,即试样在SRB溶液中浸泡后出现了较为严重的腐蚀凹坑。
3 结论
(1) 提出了FeNiCoCrW0.2Al0.1高熵合金在SRB溶液中钝化膜形成机制:合金表面在空气中自然钝化,形成含有Fe、Ni、Co和Cr氧化物的单钝化层。随着在SRB溶液中的暴露时间的延长,钝化层脱落成两层。内层的成分与最初的单层相同。然而,由于Fe、Ni和Co的消耗,外层的主要成分转变为Cr2O3,这两层的组合构成了双层钝化,有很强的保护性能。
(2) SRB的生物活动使得SRB及其代谢产物吸附在试样表面形成生物膜,这层生物膜妨碍了钝化元素在试样表面的富集,削弱了双层钝化膜的保护作用,导致了FeNiCoCrW0.2Al0.1高熵合金在SRB溶液环境下耐蚀性能的下降。
参考文献
Review on modelling of corrosion under droplet electrolyte for predicting atmospheric corrosion rate
[J].Atmospheric corrosion of metals is the most common type of corrosion which has a significant impact on the environment and operational safety in various situations of everyday life. Some of the common examples can be observed in land, water and air transportation systems, electronic circuit boards, urban and offshore infrastructures. The dew drops formed on metal surface due to condensation of atmospheric moisture facilitates corrosion as an electrolyte. The corrosion mechanisms under these droplets are different from classically known bulk electrolyte corrosion. Due to thin and non-uniform geometric thickness of the droplet electrolyte, the atmospheric oxygen requires a shorter diffusion path to reach the metal surface. The corrosion under a droplet is driven by the depletion of oxygen in the center of the droplet compared to the edge, known as differential aeration. In case of a larger droplet, differential aeration leads to preferential cathodic activity at the edge and is controlled by the droplet geometry. Whereas, for a smaller droplet, the oxygen concentration remains uniform and hence cathodic activity is not controlled by droplet geometry. The geometry of condensed droplets varies dynamically with changing environmental parameters, influencing corrosion mechanisms as the droplets evolve in size. In this review, various modelling approaches used to simulate the corrosion under droplet electrolytes are presented. In the efforts of developing a comprehensive model to estimate corrosion rates, it has been noted from this review that the influence of geometric evolution of the droplet due to condensation/evaporation processes on corrosion mechanisms are yet to be modelled. Dynamically varying external factors like environmental temperature, relative humidity, presence of hygroscopic salts and pollutants influence the evolution of droplet electrolyte, making it a complex phenomenon to investigate. Therefore, an overview of available dropwise condensation and evaporation models which describes the formation and the evolution of droplet geometry are also presented from an atmospheric corrosion viewpoint.
Corrosion resistances of metallic materials in environments containing chloride ions: A review
[J].
Ultimate strength and mechano-electrochemical investigations of steel marine structures subject to corrosion
[D].
Corrosion mechanisms of carbon steel- and stainless steel-bolt fasteners in marine environments
J.
海洋环境中碳钢和不锈钢螺栓紧固件的腐蚀机制差异研究
[J].通过对碳钢和不锈钢紧固件在海洋环境中的腐蚀特征、腐蚀产物和电偶极化等内容的比较性研究,提出了两种不同的腐蚀机制。对于碳钢紧固件,锈层增加了额外的IR降,削弱了阴极区对阳极缝隙区的极化作用,供氧的差异导致螺纹曝露部位的腐蚀更为严重,腐蚀形式以均匀腐蚀为主。对于不锈钢紧固件,缺氧的环境导致螺纹缝隙部位的钝化膜性能劣化,螺杆曝露区对螺纹缝隙区的电偶极化作用促使缝隙区腐蚀更为严重,腐蚀形式以点蚀为主。针对碳钢和不锈钢紧固件在海洋环境中的不同腐蚀机制,提出了差异化的腐蚀防护技术思路。
Corrosion resistance of Q690 high strength steel in simulated corrosive environment of ocean splash zone
[J].
Q690高强钢在模拟海洋浪溅区环境下耐蚀性能研究
[J].为研究海洋浪溅区Q690高强钢材腐蚀行为和表面形貌分布特征,通过盐水周浸-湿热循环试验得到不同周期锈蚀试件,利用激光共聚焦显微镜 (LSCM) 采集表面的坐标参数,分析了微观形貌尺寸随着腐蚀周期的变化规律。结果表明:在腐蚀初始阶段,表面产生少量针状点蚀产物,金属色泽逐渐丧失,随着腐蚀损伤程度增加,材料表面点蚀形貌逐渐向坑蚀发展,处于腐蚀后期时,分布有大量层状产物,局部区域存在剥落现象。此外,根据微观扫描分析可知,表面堆积产物对基体内部具有较好保护作用,腐蚀行为沿着水平方向快速延伸,最终完全包裹试件表面,当腐蚀周期为100 d时,体积损失率和表面腐蚀高度分别为1.38%和840 μm。
An overview of mechanisms by which sulphate-reducing bacteria influence corrosion of steel in marine environments
[J].This communication provides an overview of the literature on the biocorrosion of steel in marine media, influenced by the presence of sulphate-reducing bacteria (SRB). Electrochemical aspects, microbial interactions within biofilms, the significance of medium composition and the role of iron sulphides, and hydrogen effects are discussed. A brief description of recent experiments involving the use of electrochemical techniques for corrosion assessment, surface studies employing energy dispersive X-ray analysis (EDAX), X-ray photoelectron spectroscopy (XPS), X-ray diffraction (XRD) and electron microprobe complemented with electron microscopy observations, as well as the application of novel techniques, such as micro sensors and atomic force microscopy, is given. The growth of SRB in marine environments causes significant modifications of many physicochemical parameters at the steel/seawater interface, including local changes in pH and redox potential values, variations in anion and cation concentrations and alteration of the composition and structure of corrosion products. Complex chemical and biological reactions and equilibria are also markedly altered during bacterial proliferation. These effects, which are absent in abiotic media, often lead to significant changes in the corrosion behaviour of steel. The complicated nature of the local environment at the steel/seawater interface is enhanced in the presence of microorganisms and their extracellular polymeric substances (EPS). As a consequence of biofilm heterogeneity, areas with different ion concentrations are formed and the development of corrosion product layers of dissimilar protective characteristics occurs.
Ultimate strength analysis of aged steel-plated structures exposed to marine corrosion damage: A review
[J].
The effect of Srb, a homologue of the mammalian SRP receptor α-subunit, on Bacillus subtilis growth and protein translocation
[J].To determine the signal recognition particle (SRP)-SRP receptor (Srb) system in Bacillus subtilis (Bs), we cloned the Bs srb gene, which encodes a homologue of the mammalian SRP receptor alpha-subunit [Oguro et al., DNA Res. 2 (1995) 95-100]. We sequenced a 6098-bp DNA containing srb and analyzed the gene organization. Primer extension experiment and Northern blot analysis revealed that srb constitutes an operon with two additional ORFs. A database search of known proteins revealed that one encodes a homologue of Escherichia coli RNase III [36.0% identical amino acids (aa)] and the other encodes a homologue of yeast Smc1 (26.6% identical aa). We then constructed a Bs mutant in which srb expression was induced by IPTG. The depletion of Srb caused a defect in the cell growth and the cells became filamentous and twisted. Furthermore, pulse-chase experiments using this mutant revealed that the 17% of the beta-lactamase precursor accumulated in the cell after a 4-min chase in the absence of IPTG, although almost all of the precursors were converted into the mature from after a 1-min chase in the presence of IPTG.
Microstructures and properties of high-entropy alloys
[J].
Microstructural development in equiatomic multicomponent alloys
[J].
Nanostructured high-entropy alloys with multiple principal elements: novel alloy design concepts and outcomes
[J].
Outstanding performance of FeNiCoCr-based high entropy alloys: The role of grain orientation and microsegregation
[J].
Cavitation resistance of NiCoCrFe-Nb0.45 eutectic high entropy alloy for hydraulic machinery
[J].
NiCoCrFeNb0.45共晶高熵合金在水力机械中的抗空蚀性能研究
[J].
Local structures of high-entropy alloys (HEAs) on atomic scales: an overview
[J].
Microbiologically influenced corrosion of FeCoCrNiMo0.1 high-entropy alloys by marine Pseudomonas aeruginosa
[J].
Enhanced pitting corrosion resistance of CoCrFeMnNi high entropy alloy in the presence of Desulfovibrio vulgaris via nitrogen doping
[J].
Surface chemistry and corrosion behaviour of 304 stainless steel in simulated seawater containing inorganic sulphide and sulphate-reducing bacteria
[J].
Corrosion behaviour of X60 steel in the presence of sulphate-reducing bacteria (SRB) and iron-reducing bacteria (IRB) in seawater
[J].In this study, the corrosion behavior of X60 was investigated in the presence of a mixed culture of sulfate-reducing bacteria (SRB) and iron-reducing bacteria (IRB) via weight loss, electrochemical impedance spectroscopy (EIS), and Tafel polarization techniques. Moreover, field emission scanning electron microscope (FESEM) images and X-ray diffraction spectrometry (XRD) were used to study the morphological changes of the surface of X60 and the composition of corrosion products after exposing X60 to the abiotic and biotic systems. EIS result confirmed the occurrence of microbial corrosion in the presence of SRB and IRB. In the exposed coupon to the mixture of bacteria, the result illustrated that the existence of IRB promoted the SRB growth, so corrosion rate increased compared to the exposed coupon to SRB and IRB, respectively. The mixture of SRB and IRB showed a synergistic effect and enhanced the pitting corrosion on the surface of X60.
Corrosion behavior of P110S oil casing steel in sulfur containing environment
[J].
P110S油套管在微含硫环境中的腐蚀行为研究
[J].采用不同含量的Na<sub>2</sub>S来模拟不同的含硫环境,利用挂片浸泡实验研究P110S钢级油套管在含硫体系中的腐蚀行为;利用电化学测试研究了其在不同含硫浓度中电化学特征;利用扫描电镜、激光共聚焦、XRD以及拉曼光谱对样品表面的腐蚀产物和形貌进行了表征。结果表明,P110S钢级油套管钢在含硫体系中发生严重的腐蚀,腐蚀速率和腐蚀电流密度均随含硫浓度增大而增大,腐蚀类型由均匀腐蚀转变为点蚀,腐蚀产物疏松。
Understanding the nature of passivation film formed during corrosion of Fe39Mn20Co20Cr15Si5Al1 high entropy alloy in 3.5wt%NaCl solution
[J].