CO2地质封存是碳封存的主要方式之一。然而,在CO2封存环境下,井筒屏障材料腐蚀失效可能会引起CO2从井筒区域泄露,尤其是套管材料的腐蚀失效。CO2封存环境腐蚀性强,一般具有高温、高压、含多种杂质气体、高矿化度等特点。同时,套管由于自重等原因处于复杂的应力状态中。井筒所处应力状况、材质和腐蚀介质特点都可能对封存环境下的套管腐蚀规律产生影响。
目前,碳钢由于具有较高的性价比而广泛应用于石油行业。然而,当碳钢的服役环境较为苛刻时,其腐蚀速率可能达到20 mm·a-1 [8]。碳钢腐蚀时表面形成的FeCO3产物膜对阴离子具有选择性[9],但由于其产物膜的致密性能阻碍腐蚀性离子通过[9],从而在一定程度上抑制了钢的溶解。对于不锈钢,大多学者[10~13]认为在低CO2分压条件下,含Cr钢能有效提高钢材的耐腐蚀性。而含Cr钢的耐腐蚀性与其生成Cr(OH)3钝化膜相关[14]。超临界CO2条件下,含Cr钢的腐蚀研究较少。Wei和Gao[15]认为,低Cr钢(3Cr)不足以形成较为致密的富Cr层,而高Cr钢可以形成致密的富Cr层。致密的Cr(OH)3阻碍阴离子通过钝化膜[16],抑制了钢的溶解,使得耐腐蚀性能提高。
目前,关于CO2封存条件下的腐蚀研究主要集中在中低CO2分压情况,对于高压(大于15 MPa)富水相CO2腐蚀研究相对较少。Cui等[17,18]研究了J55、N80、P110钢在地层水环境不同温度下的腐蚀情况,结果表明随着温度从60℃增至150℃,腐蚀速率持续下降,而腐蚀速率的降低是由于试样表面致密的腐蚀产物膜造成的。Lin等[19]研究了J55、N80和P110钢在含CO2富水相中不同分压下(1.38~10 MPa)的腐蚀规律,结果表明,腐蚀产物层厚度在压力为6 MPa时达到最大值,当CO2分压继续增大的时候,腐蚀产物层厚度逐渐减小。Lin等[19]认为当压力在CO2临界压力之上时,由于CO2在水中的高溶解度,溶液中的CO
井筒套管因自重、地应力等多重复杂载荷的作用会处于以拉应力为主的特征应力状态。拉应力的出现会使得套管表面腐蚀产物膜发生破裂,继而可能失去对金属基体的保护作用。在此基础上,伴随着封存环境中多腐蚀介质、高矿化度、高温高压等特点,金属基体表面会发生严重的点蚀现象并使得应力腐蚀开裂敏感性显著提升[32~34]。Sun等[35]研究了管线钢在含碳源杂质水饱和超临界CO2相中的应力腐蚀行为:研究表明,O2杂质的存在对碳钢应力腐蚀开裂(SCC)敏感性的影响不大,而SO2和NO2的存在会大大提高碳钢的SCC敏感性。近年来的研究表明[3,36~39],由于缺乏足够的实验证据,金属在含杂密相(超临界和液相)CO2环境中是否存在SCC尚不明确。
综上所述,由于CO2封存环境的复杂性,套管钢在高温、高CO2分压环境下的腐蚀规律尚不清楚,多杂质气体(SO2、NO2、O2)耦合作用下应力对套管钢的腐蚀规律的影响也不明确。因此,本研究结合国内某油田的相关数据,拟使用N80及13Cr钢作为实验材料,模拟高温(120℃)、含多杂质气体(SO2、NO2、O2)、高矿化度地层水等多因素耦合的封存腐蚀环境,对不同CO2分压(10~20 MPa)和不同加载应力的套管钢的腐蚀规律进行研究。
1 实验方法
本文实验所用材料为N80碳钢及13Cr马氏体不锈钢。实验材料的化学成分如表1所示。N80和13Cr钢试样尺寸:50 mm × 10 mm × 5 mm、10 mm × 10 mm × 5.5 mm、67 mm × 4.5 mm × 2 mm。其中,50 mm × 10 mm × 5 mm试样用于失重法及观察腐蚀形貌、3D形貌分析;10 mm × 10 mm × 5.5 mm试样用于截面形貌及XRD分析;67 mm × 4.5 mm × 2 mm的试样用于加载应力实验。
表1 实验材料化学成分
Table 1
| Material | C | Si | Mn | P | S | Al | Cr | Ni | V | Ti | Cu | Mo | Co |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N80 | 0.35 | 0.32 | 1.55 | 0.02 | 0.015 | 0.014 | 0.11 | 0.13 | 0.15 | 0.01 | 0.03 | 0.01 | 0.08 |
| 13Cr | 0.22 | 0.17 | 0.55 | 0.016 | 0.008 | - | 12.45 | 0.25 | 0.02 | - | 0.2 | 0.14 | - |
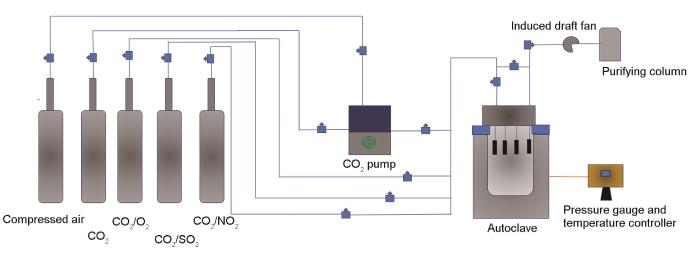
高温高压反应釜用于模拟现场CO2封存井下工况。实验所用高温高压腐蚀测试系统如图1所示。实验所用气体为99.999% CO2、99.999% N2、5.0% SO2、5.0% NO2、5.0% O2 (体积分数)和压缩空气,其中杂质气体气瓶中的平衡气为CO2。
图1
表2 压力组实验方案
Table 2
| Material | Temperature oC | Pressure MPa | Gas composition | Environment | Time h |
|---|---|---|---|---|---|
N80 | 120 | 10 | CO2 + 0.1‰ SO2 + 0.1‰ NO2 + 0.1‰ O2 | Simulation of groundwater phase | 72 |
| 15 | |||||
| 20 | |||||
13Cr | 10 | ||||
| 15 | |||||
| 20 |
表3 应力组实验方案
Table 3
| Material | Temperature ℃ | Pressure MPa | Gas composition | Environment | Loading stress | Time h |
|---|---|---|---|---|---|---|
| N80 | 120 | 20 | CO2 + 0.1‰ SO2 + 0.1‰ NO2 + 0.1‰ O2 | Simulation of groundwater phase | 0 | 72 |
| 25% σs | ||||||
| 50% σs | ||||||
| 75% σs | ||||||
| 13Cr | 0 | |||||
| 25% σs | ||||||
| 50% σs | ||||||
| 75% σs |
应力组试样,利用四点弯梁夹具对其进行应力加载。该加载方法按照GB/T 15970.2-2000(ISO 7539-2)执行。具体公式如下[41]:
式中,σ为加载应力大小,MPa;E为弹性模量,MPa;t为试样厚度,mm;y为加载挠度,mm;H为夹具外部陶瓷支点之间的距离,mm;A为夹具内外陶瓷支点之间的距离,mm。
使用克拉克溶液[42]清洗试样表面腐蚀产物,测得实验前后的质量差,通过失重法计算其平均腐蚀速率,并根据ASTM G46-94标准对腐蚀程度进行评价[43]。使用扫描电子显微镜(SEM,FEI Quanta 200F)分析腐蚀产物膜形貌;X射线衍射仪(XRD,RIGAKU Smartlab 9kW)检测试样表面腐蚀产物膜中结晶相成分;X射线光电子能谱仪(XPS,ThermoFisher Nexsa)检测分析相同元素在不同化合物中的氧化态。参照标准ASTM G46-94[43],使用3D形貌仪观察去除腐蚀产物膜后的试样表面点蚀形貌、分布以及深度,计算点蚀速率,分析点蚀敏感性。点蚀速率的计算公式如下所示:
式中,
2 结果与讨论
2.1 压力对含碳源杂质CO2 封存环境套管腐蚀规律的影响
2.1.1 腐蚀速率分析
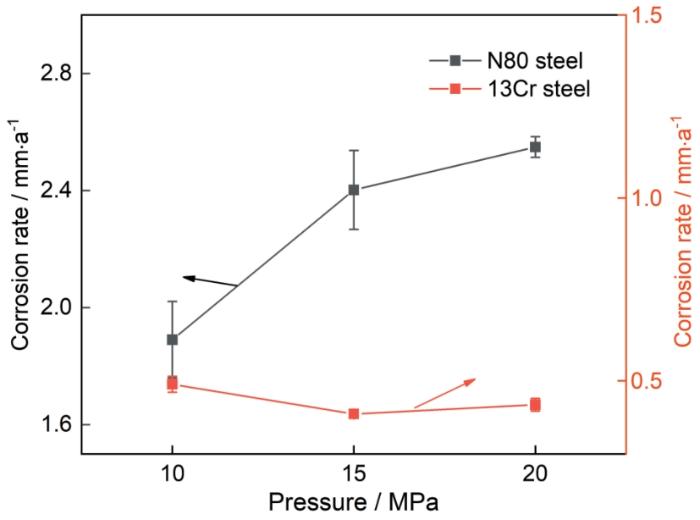
图2
图2
120℃、不同压力条件下N80及13Cr钢在富水相中腐蚀72 h后的腐蚀速率
Fig.2
Corrosion rates of N80 and 13Cr steels after 72 h of corrosion in water-rich phase at 120oC under different pressure conditions
2.1.2 腐蚀形貌分析
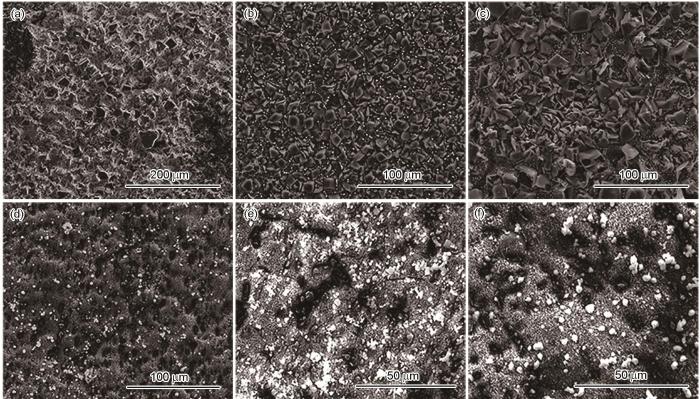
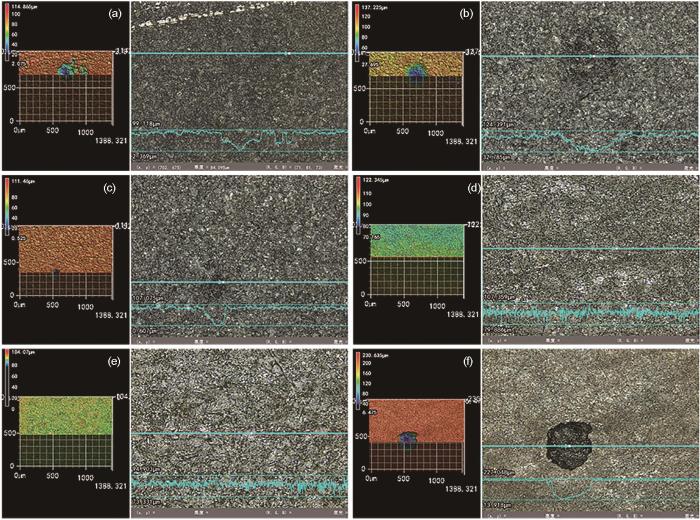
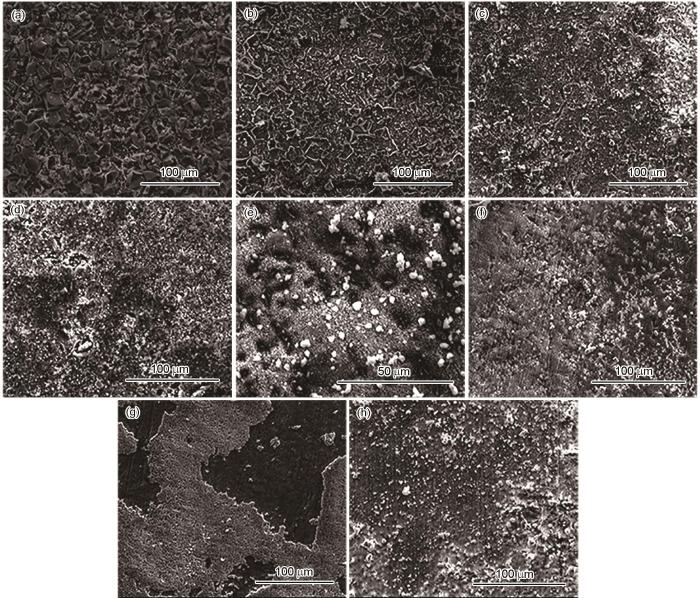
120℃、不同压力条件下N80及13Cr钢在含杂富水相中腐蚀72 h后腐蚀产物膜的表面形貌如图3所示。结果表明,所有试样表面都覆盖腐蚀产物;N80钢表面腐蚀产物颗粒随着压力的升高尺寸增大;13Cr钢表面产物分为外层白色球状腐蚀产物及内层黑色腐蚀产物,外层腐蚀产物不均匀、有孔洞。
图3
图3
120℃、不同压力条件下N80及13Cr钢在富水相中腐蚀72 h后腐蚀产物膜的表面形貌
Fig.3
SEM images of corrosion products of N80 (a-c) and 13Cr (d-f) steels after 72 h of corrosion in water-rich phase at 120oC under the pressure of 10 MPa (a, d), 15 MPa (b, e) and 20 MPa (c, f)
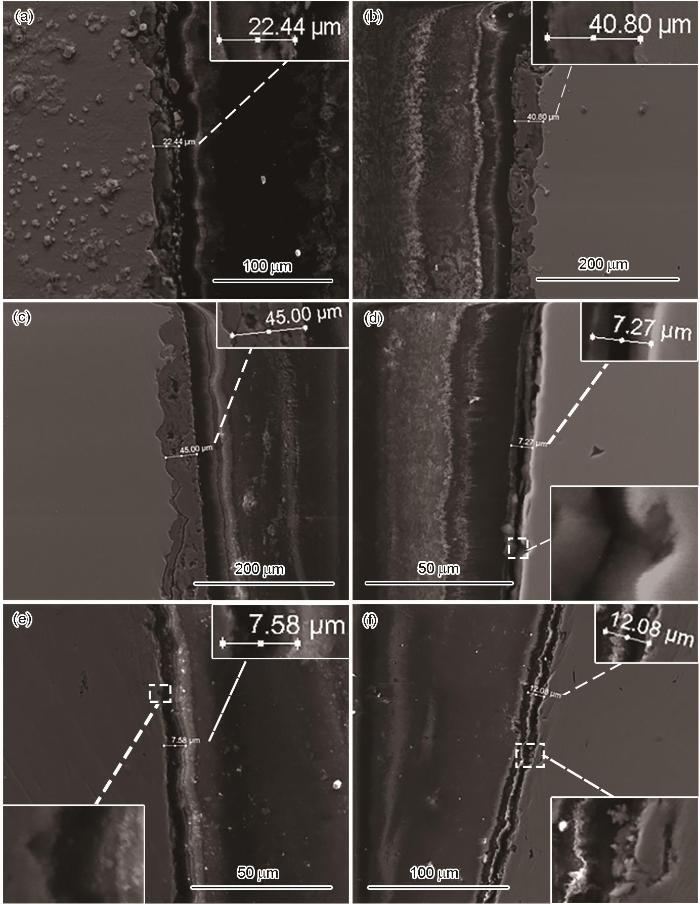
图4
图4
120℃、不同压力条件下N80及13Cr钢在富水相中腐蚀72 h后的截面形貌
Fig.4
Cross-sectional morphologies of N80 (a-c) and 13Cr (d-f) steels after 72 h of corrosion in water-rich phase at 120oC under the pressure of 10 MPa (a, d), 15 MPa (b, e) and 20 MPa (c, f)
2.1.3 点蚀速率及3D形貌分析
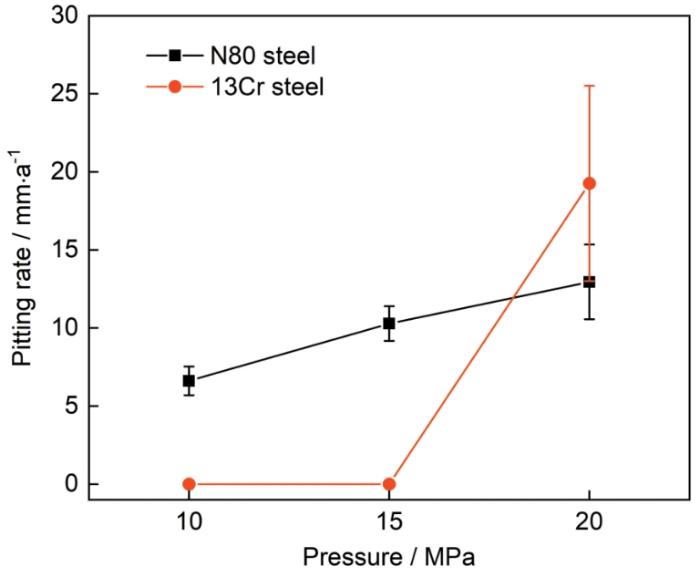
120℃、不同压力条件下N80及13Cr钢在含杂富水相中腐蚀72 h后的点蚀速率如图5所示。N80钢在10、15、20 MPa压力条件下的点蚀速率分别为6.614、10.285、12.950 mm·a-1,N80钢随压力变化的点蚀速率随着压力的升高而增大;在压力为10、15 MPa条件下,13Cr钢表面基本没有发生点蚀现象,但当压力升高到20 MPa时,13Cr钢表面发生了严重的点蚀,点蚀速率达到了19.260 mm·a-1。
图5
图5
120℃、不同压力条件下N80及13Cr钢在富水相中腐蚀72 h后的点蚀速率
Fig.5
Pitting rates of N80 and 13Cr steels after 72 h of corrosion in water-rich phase at 120oC under different pressure conditions
120℃、不同压力条件下N80及13Cr钢在含杂富水相中腐蚀72 h后的3D形貌如图6所示。20 MPa时,N80钢试样表面点蚀坑最大深度为106.468 μm。13Cr钢试样仅在压力为20 MPa时出现明显点蚀,最大点蚀深度为213.13 μm。这些结果表明,含杂CO2在更高的压力条件下容易导致点蚀的发生。因此,普通碳钢套管材料甚至是13Cr钢在高压含杂CO2环境使用时,点蚀问题应引起重视。
图6
图6
120℃、不同压力条件下N80及13Cr钢在富水相中腐蚀72 h后的3D形貌
Fig.6
3D surface morphologies of N80 (a-c) and 13Cr (d-f) steels after 72 h of corrosion in water-rich phase at 120oC under the pressure of 10 MPa (a, d), 15 MPa (b, e), 20 MPa (c, f)
本实验中,总压力升高增加了气体在水相中的溶解度。根据CO2-H2O溶解度模型[40],压力升高使CO2溶解度增大,溶液的pH值减小,析氢反应被强化,腐蚀速率增加。同理,酸性杂质气体的体积分数一定,它们的分压会随着总压的增加而增大,这也会降低溶液pH、增大腐蚀速率。O2分压随总压的增加而增大会强化吸氧腐蚀过程,增大腐蚀速率。
2.1.4 产物膜成分分析
图7
图7
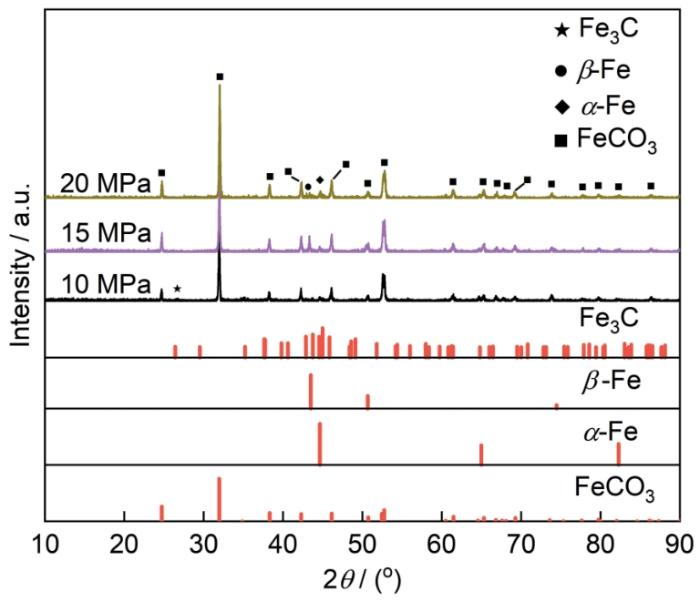
120℃、不同压力条件下N80钢在富水相中腐蚀72 h后的XRD
Fig.7
XRD patterns of N80 steel after 72 h of corrosion in water-rich phase at 120oC under different pressure conditions
图8
图8
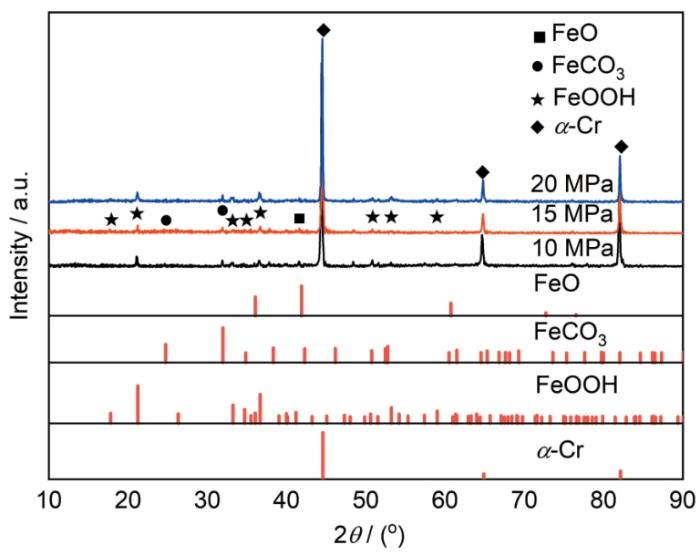
120℃、不同压力条件下13Cr钢在富水相中腐蚀72 h后的XRD
Fig.8
XRD patterns of 13Cr steel after 72 h of corrosion in water-rich phase at 120oC under different pressure conditions
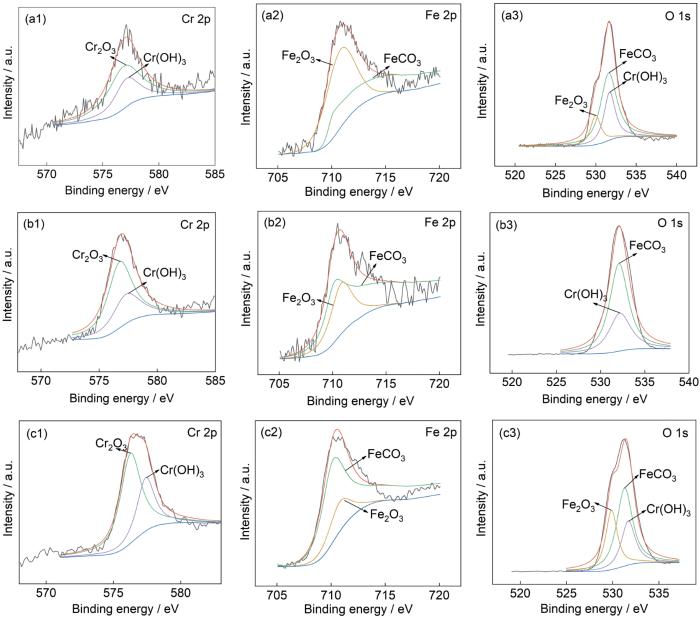
图9为120℃、不同压力条件下13Cr钢在含杂富水相中腐蚀72 h后腐蚀产物膜中Cr、Fe、O的XPS谱。根据拟合曲线结果并结合文献数据,Cr 2p1/2在577.1、577.4 eV处的峰,O 1s在531.7 eV处的峰,说明对应的物质为Cr(OH)3[46];Fe 2p在710.2 eV处的峰[47],O 1s在531.4 eV处的峰[12,48,49],说明对应的物质为FeCO3;Fe 2p在711.5 eV处的峰[49],O 1s在529.7 eV处的峰[49],说明对应的物质为FeOOH。Cr 2p、Fe 2p及O 1s在13Cr钢腐蚀产物膜中的结合能XPS拟合结果以及对应物质详见表4。
图9
图9
120℃、不同压力条件下13Cr钢在富水相中腐蚀72 h后腐蚀产物膜中Cr、Fe、O的XPS谱
Fig.9
XPS of Cr, Fe and O of 13Cr steels after 72 h of corrosion in water-rich phase at 120oC under the pressure of 10 MPa (a), 15 MPa (b) and 20 MPa (c)
表4 120℃、不同压力条件下13Cr钢在富水相中腐蚀72 h后腐蚀产物膜的结合能
Table 4
| Element | 10 MPa | 15 MPa | 20 MPa | |||||
|---|---|---|---|---|---|---|---|---|
| Binding energy / eV | Peak | Binding energy / eV | Peak | Binding energy / eV | Peak | |||
| Cr 2p | 577.1 | Cr(OH)3 | 577.1 | Cr(OH)3 | 577.1 | Cr(OH)3 | ||
| O 1s | 531.4 | FeCO3 | 531.4 | FeCO3 | 531.4 | FeCO3 | ||
| Fe 2p | 529.7 | FeOOH | - | - | 529.7 | FeOOH | ||
| 531.7 | Cr(OH)3 | 531.7 | Cr(OH)3 | 531.7 | Cr(OH)3 | |||
| 710.2 | FeCO3 | 710.2 | FeCO3 | 710.2 | FeCO3 | |||
| 711.5 | FeOOH | 711.5 | FeOOH | 711.5 | FeOOH | |||
结合XRD、XPS结果分析可以得出,13Cr钢试样在各压力条件下腐蚀过后的腐蚀产物膜中均含有Cr(OH)3、FeCO3和FeOOH。相比于N80钢,13Cr钢腐蚀产物中还含有Cr2O3和Cr(OH)3,其中Cr(OH)3对阳离子的选择性以及FeCO3沉积填充富Cr层孔隙[15]是13Cr钢腐蚀速率减小的原因之一。
根据产物膜成分分析可知,13Cr钢表面生成了Cr2O3、Cr(OH)3、FeCO3、FeOOH。Lin等[50]和Hua等[24]研究指出FeOOH一般是疏松多孔的结构,在O2环境下,FeOOH的孔隙构成了一个腐蚀介质的“通道”,该“通道”可以直接通向基体表面,造成点蚀风险。图3d~f显示,13Cr钢外层腐蚀产物有很多孔洞,这些有孔洞的产物膜成分为FeCO3、FeOOH,这些孔洞可以作为“通道”使腐蚀介质与基体表面接触,这可能是为什么13Cr钢在本研究环境中的腐蚀速率远高于0.076 mm·a-1的主要原因。Wei和Gao[15]指出含Cr钢在超临界CO2腐蚀中会产生更为致密的双层腐蚀产物膜结构,耐蚀性能好,但其内部的富Cr层中有FeCO3沉积。然而在SO2-NO2-O2含杂体系中,其水溶液pH低,富Cr层中的FeCO3可能溶解;当压力升高时,水溶液pH下降,FeCO3溶解概率增大,局部腐蚀风险变高。
2.2 应力对含碳源杂质CO2 封存环境套管腐蚀规律的影响
2.2.1 腐蚀形貌分析
图10
图10
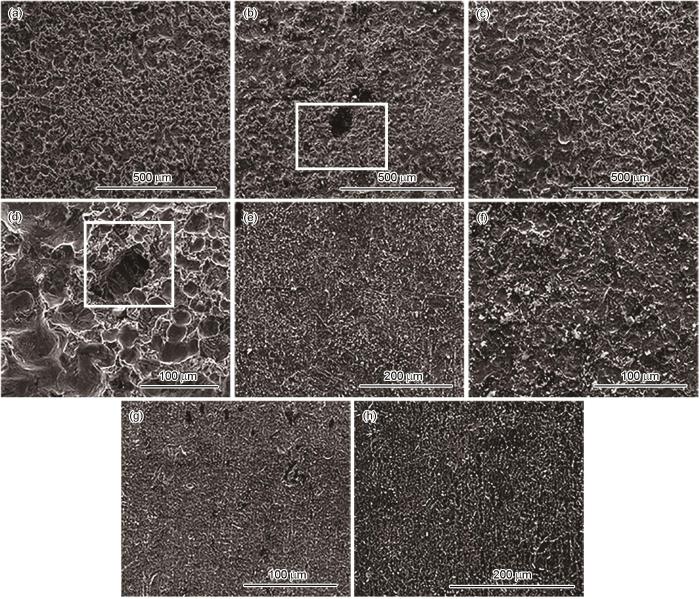
120℃、20 MPa不同加载应力条件下N80及13Cr钢在富水相中腐蚀72 h的表面形貌
Fig.10
Surface morphologies of N80 and 13Cr steels after 72 h of corrosion in water-rich phase at 120oC and 20 MPa under the loading stress of 0 (a, e), 25% σs (b, f), 50% σs (c, g) and 75% σs (d, h)
120℃、20 MPa不同加载应力条件下N80及13Cr钢在含杂富水相中腐蚀72 h去除产物膜后的表面形貌如图11所示。由图可以看出,在去除产物膜后,N80与13Cr钢在各加载应力条件下试样表面均未有裂纹出现。与无加载应力试样相比,加载应力试样表面存在明显点蚀坑。
图11
图11
120℃、20 MPa不同加载应力条件下N80及13Cr钢在富水相中腐蚀72 h去除产物膜后的表面形貌
Fig.11
Surface morphologies of N80 (a-d) and 13Cr (e-h) steels after 72 h of corrosion in water-rich phase at 120oC and 20 MPa under the loading stress of 0 (a, e), 25% σs (b, f), 50% σs (c, g) and 75% σs (d, h) after removing the corrosion products
2.2.2 应力集中区点蚀速率及3D形貌分析
在加载应力分别为0、25% σs、50% σs、75% σs的条件下,N80钢的点蚀速率分别为9.017、6.957、7.415、10.869 mm·a-1,点蚀因子分别为3.54、1.28、1.47、2.84,表明未发生明显的点蚀。当加载应力为25% σs时,N80钢试样应力集中区的点蚀速率与无加载应力条件下的试样相比略有下降。然而,随着加载应力的增加,N80钢试样的点蚀速率出现了增加趋势。
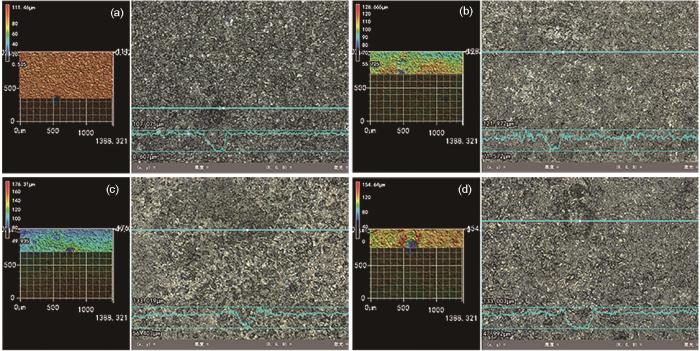
120℃、20 MPa不同加载应力条件下N80钢在含杂富水相中腐蚀72 h后的3D形貌如图12所示。N80钢在无加载应力、25%、50%、75% σs条件下的最大点蚀深度分别为106.468、68.368、76.616、104.698 μm。
图12
图12
120℃、20 MPa不同加载应力条件下N80钢在富水相中腐蚀72 h后的3D形貌
Fig.12
3D surface morphologies of N80 steel after 72 h of corrosion in water-rich phase at 120oC and 20 MPa under the loading stress of 0 (a), 25% σs (b), 50% σs (c) and 75% σs (d)
目前,关于含杂体系下超临界CO2应力腐蚀研究以富CO2相为主,富水相的研究相对较少。从实验结果来看,N80钢在120℃、20 MPa条件下应力加载对其应力腐蚀影响不明显,点蚀敏感性也无明显变化,仅仅表现为腐蚀产物层出现了裂纹。此外,Sun等[35]在研究富CO2相的应力腐蚀中提到,四点弯梁应力腐蚀的实验结果不能充分证明试样在腐蚀环境中无应力腐蚀敏感性,而慢应变拉伸实验可以对应力腐蚀敏感性进行进一步的证明。另外,四点弯梁的加载方式具有一定局限性,导致试样一侧受到拉应力作用,另一侧受到压应力作用,上述表面形貌均为腐蚀产物膜破坏更加严重的拉应力作用一侧。
3 结论
(1) 在120℃、不同压力条件下含SO2-NO2-O2杂质的超临界CO2富地层水体系中,N80钢的腐蚀速率及点蚀速率均随着压力的增大而逐渐升高。13Cr钢的腐蚀速率随压力增大无明显变化。但是,当压力为20 MPa时,13Cr钢试样出现了严重的点蚀现象。
(2)在120℃、不同压力条件下含SO2-NO2-O2杂质的超临界CO2富地层水体系中,N80钢试样表面腐蚀产物大多为FeCO3晶体,并且随着压力的升高,FeCO3腐蚀产物含量逐渐增多。13Cr钢试样在各压力条件下的腐蚀产物膜中均含有FeCO3、FeOOH和Cr(OH)3。
(3)在120℃、20 MPa不同加载应力条件下含SO2-NO2-O2杂质超临界CO2富地层水相体系中,随着加载应力的增大,N80及13Cr钢试样表面腐蚀产物层均出现了不同程度的破损现象,但是两种材料的基体表面并未观察到有裂纹生成。
参考文献
A techno-economic analysis and systematic review of carbon capture and storage (CCS) applied to the iron and steel, cement, oil refining and pulp and paper industries, as well as other high purity sources
[J].
Determining the corrosive potential of CO2 transport pipeline in high pCO2–water environments
[J].
Internal corrosion of carbon steel pipelines for dense-phase CO2 transport in carbon capture and storage (CCS)-a review
[J].
Corrosion behaviour of X65 carbon steel in supercritical-CO2 containing H2O and O2 in carbon capture and storage (CCS) technology
[J].
Corrosion behaviour of N80 carbon steel in formation water under dynamic supercritical CO2 condition
[J].
Corrosion and failure assessment for CO2 EOR and associated storage in the Weyburn Field
[J].
Foam assisted CO2-EOR
concepts, challenges and applications [A].
Corrosion behavior of carbon steel in supercritical CO2-water environments
[A].
Effect of chromium on the pitting resistance of oil tube steel in a carbon dioxide corrosion system
[J].
Observation and analysis of pseudopassive film on 6.5%Cr steel in CO2 corrosion environment
[J].
Effect of free Cr content on corrosion behavior of 3Cr steels in a CO2 environment
[J].
Characterization of corrosion scale formed on 3Cr steel in CO2-saturated formation water
[J].
Effect of Cr content on the corrosion performance of low-Cr alloy steel in a CO2 environment
[J].
Essential criterion for evaluating the corrosion resistance of 3Cr steel in CO2 environments: prepassivation
[J].
Understanding the general and localized corrosion mechanisms of Cr-containing steels in supercritical CO2-saturated aqueous environments
[J].
Corrosion behaviour of 13Cr in supercritical CO2 Environment
[J].
超临界CO2环境13Cr材质腐蚀行为研究
[J].
Corrosion behavior of oil tube steels under conditions of multiphase flow saturated with super-critical carbon dioxide
[J].
Study on corrosion properties of pipelines in simulated produced water saturated with supercritical CO2
[J].
Effect of temperature and pressure on the morphology of carbon dioxide corrosion scales
[J].
Discussion of the CO2 corrosion mechanism between low partial pressure and supercritical condition
[J].
Corrosion behavior of deep water oil production tubing material under supercritical CO2 environment: Part 1. effect of pressure and temperature
[J].
Corrosion of mild steel in high CO2 environment: Effect of the FeCO3 layer
[A].
Characterization of 13Cr steel corrosion in simulated EOR-CCUS environment with flue gas impurities
[J].
The effect of O2 content on the corrosion behaviour of X65 and 5Cr in water-containing supercritical CO2 environments
[J].
Effects of O2 and SO2 on water chemistry characteristics and corrosion behavior of X70 pipeline steel in supercritical CO2 transport system
[J].
Synergistic effect of O2, H2S and SO2 impurities on the corrosion behavior of X65 steel in water-saturated supercritical CO2 system
[J].
Exploring the influence of flue gas impurities on the electrochemical corrosion mechanism of X80 steel in a supercritical CO2-saturated aqueous environment
[J].
Initial corrosion mechanism for API 5L X80 steel in CO2/SO2-saturated aqueous solution within a CCUS system: Inhibition effect of SO2 impurity
[J].
Effect of impurity on the corrosion behavior of X65 steel in water-saturated supercritical CO2 system
[J].
Protectiveness, morphology and composition of corrosion products formed on carbon steel in the presence of Cl-, Ca2+ and Mg2+ in high pressure CO2 environments
[J].
State-of-the-art overview of pipeline steel corrosion in impure dense CO2 for CCS transportation: mechanisms and models
[J].
Research progress of stress corrosion
[J].
应力腐蚀研究进展
[J].
Electrochemical noise of stress corrosion cracking of P110 tubing steel in sulphur-containing downhole annular fluid
[J].Stress corrosion cracking (SCC) is considered as the main risk of tubing steels during the exploitation of oil and gas fields, which could result in sudden and catastrophic failures of downhole tubing. Especially in annular downhole environment, P110 tubing steel is prone to sulfide stress corrosion cracking and hydrogen embrittlement (HE) where S2- could be originated from bio-reduction of SO42- inspired by sulfate-reducing bacteria (SRB). Currently, extensive work have been performed to investigate the influence factors on SCC and mechanism of tubing steels, but limited researches have been conducted on the SCC of P110 tubing steel in annular downhole environment, particularly, on the early detection of SCC. In this work, the SCC behavior of P110 low alloy steel in simulating sulphur-containing annular fluid (SAF) and the effect of S2- concentration on the initiation and propagation of crack were investigated by slow stress rate test (SSRT), non-destructive electrochemical noise (ECN), SEM and EIS techniques. The results showed that, during the elastic stress stage, the addition of S2- accelerated the breakdown of passivation film on the surface of P110 steel tensile specimen. There are many short duration current transients caused by metastable pits on ECN curves. The transformation time of metastable to stable pits is shortened significantly by the addition of S2-, which not only promotes the growth of pits but the initiation of cracks from the stable pits under the action of tensile stress. Compared with the ECN spikes from metastable pits, the spikes associated to the advance of cracks are featured by longer average duration (about 400 s), stronger amplitude (40 μA), and higher charge (about 4000 μC). As a result, the susceptibility of P110 steel to SCC increases with S2- concentration, and the propagation of SCC is dominated by anodic dissolution characteristic of discontinuous advance.
井下含硫环空液中P110油管钢应力腐蚀开裂的电化学噪声特征
[J].
Effect of Cl-concentration on the stress corrosion sensitivity of martensitic stainless steel in saturated CO2 solution
[J].
饱和CO2溶液中Cl-浓度对马氏体不锈钢应力腐蚀敏感性的影响
[J].
Unraveling the effect of O2, NO2 and SO2 impurities on the stress corrosion behavior of X65 steel in water-saturated supercritical CO2 streams
[J].
Influence of SO2 on the corrosion and stress corrosion cracking susceptibility of supercritical CO2 transportation pipelines
[J].
Transport of gaseous and dense carbon dioxide in pipelines: is there an internal stress corrosion cracking risk?
[A].
A systematic review of key challenges of CO2 transport via pipelines
[J].
Should supercritical CO2 pipelines comply with ANSI/NACE MR0175/ISO 15156?
[J].
CO2-H2O mixtures in the geological sequestration of CO2. I. Assessment and calculation of mutual solubilities from 12 to 100 °C and up to 600 bar
[J].
Impact of surface condition on sulphide stress corrosion cracking of 316L stainless steel
[J].
The point defect model for the passive state
[J].
Preparation of metal-polymer dispersions by plasma techniques. An ESCA investigation
[J].
An XPS characterization of FeCO3 films from CO2 corrosion
[J].
X-ray photoelectron spectroscopic studies of iron oxides
[J].
X-ray photoelectron spectroscopy studies of solvated metal atom dispersed catalysts. Monometallic iron and bimetallic iron-cobalt particles on alumina
[J].
Effect of O2 on corrosion of 3Cr steel in high temperature and high pressure CO2–O2 environment
[J].