页岩油是继页岩气之后全球非常规油气勘探开发的又一新热点。中国页岩油资源丰富,超过132亿吨,居世界第三位。随着国内石油用量的增加,低硫、低酸和低盐的优质原油储量日渐减少,2019年我国原油对外依存度高达72.6%,页岩油对保障国家能源安全和改变能源结构具有重要的战略意义[1~3]。页岩油开发主要采用CO2压裂技术[4~6],即CO2与水基携砂压裂液交替驱,与水力压裂技术比较,具有起裂压力低,易形成复杂裂纹网格,储层伤害小,增产效果明显,施工成本低等优势,但该技术可能会带来CO2腐蚀问题,油管的腐蚀穿孔问题给页岩油的安全生产带来了巨大的挑战[7]。CO2腐蚀主要指的是CO2溶于水后形成的弱酸与金属接触时所产生的电化学腐蚀,是造成管路腐蚀失重的重要因素之一[8~11]。饱和CO2水溶液在相同pH条件下所导致的腐蚀比盐酸等强酸更加严重,造成的腐蚀速率可高达20 mm/a,井下管柱腐蚀的风险明显增加[12,13]。
页岩油压裂后的返排液中含NH+4[14],Bakken页岩油田产出水的NH+4浓度达2000~3000 mg/L。为了提高采油率而添加的压裂液黏土稳定剂、甲胺、氯化铵、二甲基二烯丙基氯化铵等化学助剂是导致采出水中形成高含NH+4的腐蚀环境的主要因素之一。氯化铵是一种强电解质,具有吸湿性,溶于水后由于铵根离子的水解作用使溶液呈酸性,常温下饱和氯化铵溶液的pH值约为5.6,对碳钢、合金钢具有较强的腐蚀性。氯化铵对炼油装置造成的严重腐蚀,是影响石化系统安全连续运行的重要因素之一[15,16],碳钢在NH4Cl环境中的腐蚀速率是其在塔顶系统酸性介质中腐蚀速率的100倍[17]。孙宝壮等[1]研究表明在炼油装置中,由于气相NH3和HCl在冷却过程中形成固体NH4Cl盐,当盐沉积在设备表面时极易形成局部高浓度的NH4Cl溶液,这将显著增加金属的应力腐蚀开裂(SCC)敏感性,NH4Cl溶度越高,腐蚀敏感性越大。段永锋等[18]的研究表明原油劣质化带来的NH4Cl结垢和腐蚀问题已由常减压装置扩展到了催化裂化、加氢裂化和延迟焦化等二次加工装置,目前常用的2205钢和316L钢等在NH4Cl水溶液中的腐蚀随着温度的升高、流速的增加和NH4Cl质量分数的增加而加剧。Wang等[17]研究碳钢在NH4Cl/CO2介质中的腐蚀行为,结果表明CO2分压的增加有利于碳钢在NH4Cl环境中形成FeCO3膜。Toba等[19]的研究显示NH4Cl会对碳钢造成显著的腐蚀,在20%和40% (质量分数)NH4Cl溶液中,碳钢的腐蚀速率分别高达25.4和60.4 mm/a。
针对NH+4对金属腐蚀的影响研究主要集中在下游炼厂设备等方面,NH+4对N80钢油管钢的腐蚀研究很少,由于炼厂的腐蚀环境及材质均与页岩油生产环境存在较大差异,在炼厂环境的腐蚀规律对页岩油采出水环境N80钢的腐蚀并不具备借鉴价值,因此,有必要开展页岩油采出环境NH4Cl对油管钢腐蚀行为研究,为页岩油的安全生产提供理论指导。
本文采用典型油管钢N80钢,利用电化学阻抗谱、动电位极化曲线等电化学方法研究N80钢在不同浓度的NH4Cl的饱和CO2环境中的腐蚀行为,并结合表面分析方法,对N80钢的表面腐蚀产物进行分析,分析NH4+浓度对N80钢的腐蚀影响规律。
1 实验方法
实验材料选用N80钢,其化学成分(质量分数,%)为:C 0.285, Si 0.256,Mn 1.2515,P 0.0142,S 0.0045,Mg 0.0006,Cr 0.082,Ni 0.043,Mo 0.021,Cu 0.111,Al 0.0163,Ti 0.001,V 0.003,Nb 0.005,Zn 0.001,其余成分为Fe。试样规格为50 mm × 10 mm × 3 mm,每组实验安装5个挂片,其中3个用于失重法计算腐蚀速率,两个试样分别用于腐蚀产物分析。实验前将N80钢试样逐级打磨至1500#砂纸,之后用无水乙醇除油并干燥,置于精密天平中称重,精确至0.0001 g。电化学试样规格为10 mm × 10 mm × 3 mm,处理方式同上,背后焊铜导线,其余位置用环氧树脂密封,工作面积为1.0 cm2。经4% (体积分数)的硝酸酒精溶液侵蚀后观察金相组织,图1所示N80钢金相组织为贝氏体-马氏体组织。
图1
腐蚀失重实验的介质为添加0、150、500、1000和2000 mg/L NH4Cl的3% (质量分数)NaCl溶液。实验温度和压力分别为50℃、常压。实验前先对实验溶液通1 h CO2除去溶液中的O2,然后通过稀NaOH调节溶液至pH值6.6。将N80钢试样悬挂在实验介质中,实验周期为72 h,实验期间持续通入CO2。
电化学实验装置采用Gamry3000+型电化学工作站,采用三电极体系,工作电极为N80钢,辅助电极为铂电极,参比电极选为饱和甘汞电极,实验介质成分及条件同上。线性极化曲线测试范围为-20~+20 mV,扫描速率为0.166 mV/s,频率范围为105~10-2 Hz,阻抗测量信号幅值为10 mV正弦波,通过ZView对电化学阻抗谱进行拟合得到相关参数,72 h后进行动电位极化曲线测试,测试电位范围为-800 mV~+800 mV,扫描速率为0.5 mV/s。
腐蚀失重腐蚀实验结束后,分别采用FEI Quanta FEG250型场发射扫描电子显微镜(SEM)、Oxford IncaEnergy X-max-50 X射线能谱仪(EDS)、PANalytical X-pert 3型X射线衍射(XRD)、ThermoFisher ESCALAB 250Xi型X射线光电子能谱仪(XPS)方法表征腐蚀产物表面形貌、元素组成以及成分。
失重腐蚀试样表面的腐蚀产物膜的去除采用含六次甲基四胺的盐酸溶液,1 L的50%盐酸溶液中含3.5 g六次甲基四胺。将3个试样置于清洗溶液中超声清洗10 min,无水乙醇清洗冷风吹干后称重,精确至0.0001 g,根据
式中,W为失重率,V为腐蚀速率(mm·a-1),M1和M2分别为金属腐蚀前后质量(g),S为金属暴露在溶液中的表面积(cm2),
2 结果与讨论
2.1 失重测试结果
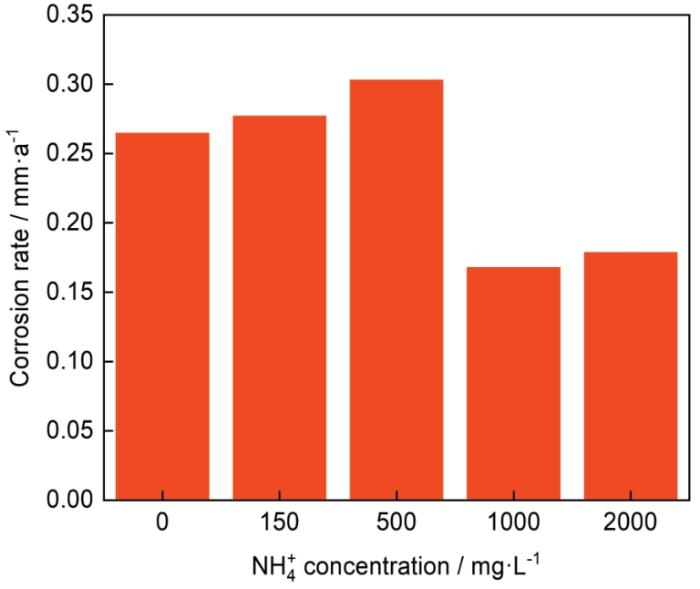
图2为不同NH+4浓度的3%NaCl溶液中N80钢的腐蚀速率图。当NH+4浓度为0~500 mg/L时,NH+4促进了N80钢的腐蚀,随着NH+4浓度的增加,N80钢的腐蚀速率逐渐增加,NH+4浓度为500 mg/L时,腐蚀速率最高,达0.3 mm/a。当NH+4浓度升至1000~2000 mg/L时,NH+4抑制了N80钢的腐蚀,N80钢的腐蚀速率总体呈现下降趋势。NH+4浓度为1000 mg/L时,腐蚀速率降至约为0.17 mm/a;2000 mg/L时,腐蚀速率略有增加,为0.18 mm/a。
图2
图2
不同NH+4浓度的3%NaCl溶液中N80钢的腐蚀速率
Fig.2
Corrosion rates of N80 steel in 3%NaCl solutions with different NH+4 concentrations
2.2 电化学结果
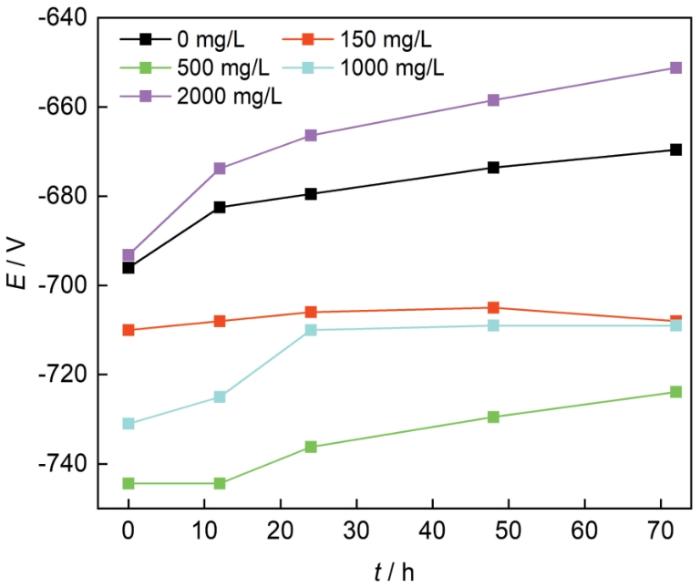
图3为N80钢的自腐蚀电位随时间的变化。一般来说,开路电位越正,腐蚀倾向越小;在腐蚀初期,各NH+4浓度下的开路电位均逐渐正移,说明随着N80钢表面腐蚀产物开始形成,腐蚀倾向逐渐减小。随着NH+4浓度的增加,N80钢的开路电位先下降后上升,表明N80钢的腐蚀倾向也存在先上升后下降的趋势,与失重法测得腐蚀速率规律相似,其中,在NH+4浓度500 mg/L时开路电位更低,具有更大的腐蚀倾向。
图3
图3
不同NH+4浓度的3%NaCl溶液中N80钢的自腐蚀电位
Fig.3
Self corrosion potentials of N80 steel in 3%NaCl solutions with different NH+4 concentrations
图4
图4
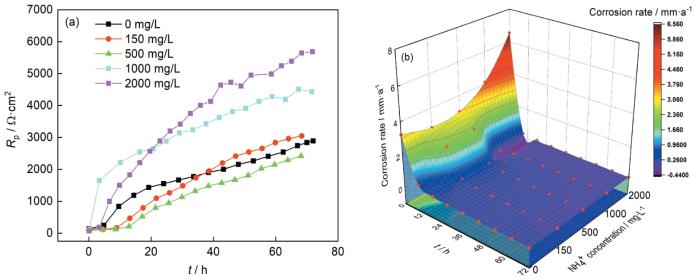
不同NH+4浓度的3%NaCl溶液中N80钢的线性极化电阻和腐蚀速率
Fig.4
Linear polarization resistances (a) and corrosion rates (b) of N80 steel in 3%NaCl solutions with different NH+4 concentrations
图4b为N80钢的瞬时腐蚀速率随NH+4浓度及实验时间的影响,在腐蚀初期0 h时,随着NH+4浓度增加腐蚀速率逐渐增加,这是由于腐蚀初期Cl-浓度的提高增加了金属发生腐蚀的倾向。随着时间的增加,不同NH+4浓度下的N80钢腐蚀速率都显著下降,并在腐蚀后期腐蚀速率逐渐平稳。
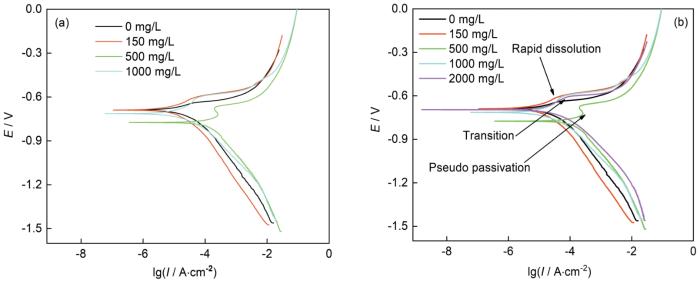
图5为不同NH+4浓度的3%NaCl溶液中N80钢的动电位极化曲线。总体来看,在NH+4浓度从0 mg/L到2000 mg/L时,N80钢的腐蚀电位先逐渐负移后正移,说明金属的腐蚀倾向先增加后减小。在NH4Cl浓度小于等于150 mg/L时,N80钢阳极极化曲线为活性溶解反应;但当NH+4浓度大于等于500 mg/L后,随着阳极电位的升高,在-0.63~-0.80 V电位范围内,阳极电流密度出现了短暂的缓慢上升甚至局部下降区间,当阳极电位大于在这个区间后,阳极曲线与NH4Cl浓度小于等于150 mg/L基本相同,呈现出活性溶解反应(图5b)。在动电位极化曲线的阳极极化曲线出现类似图5b所示的“伪钝化”[20,21]现象,即阳极曲线上呈现出类似钝化现象,但电流密度仍维持较高的水平(如超过10 μA/cm2)。在电位高于“伪钝化”区后的阳极反应速率增加可能与金属表面腐蚀产物膜层失稳有关[22,23]。Linter和Burstein[23]研究表明,在CO2环境中,电位高于“伪钝化”区后,铁溶解速率的增加是由于CO2-3的侵蚀导致钝化层不稳定造成的。
图5
图5
不同NH+4浓度的3%NaCl溶液中N80钢的动电位极化曲线
Fig.5
General (a) and detail (b) views of polarization curves of N80 steel in 3%NaCl solutions with different NH+4 concentrations
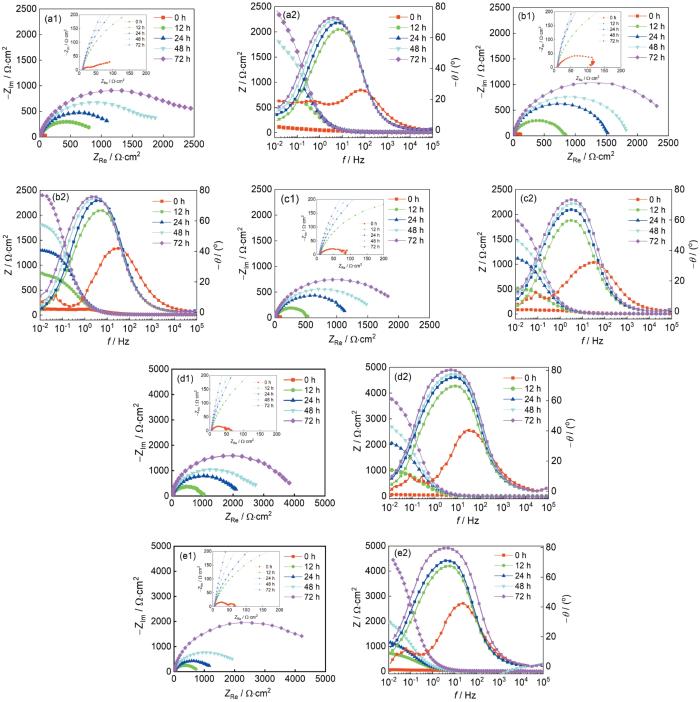
图6为N80钢在饱和CO2中的3%NaCl溶液下的电化学阻抗谱图。电化学阻抗谱能够表征金属表面的膜层的状态。N80钢在不同浓度的NH+4下的容抗弧半径均随着时间的增加而显著增大,说明腐蚀速率随时间逐渐降低,这与图4b的瞬时腐蚀速率变化规律一致。金属的膜层电阻和电荷转移电阻随时间逐渐增大,阳极溶解过程受到抑制,这可能与N80钢表面逐渐形成一层具有一定保护性质的膜有关。当NH+4浓度在0~500 mg/L容抗弧半径和阻抗膜值逐渐减小,在NH4+浓度500 mg/L时腐蚀速率最大;当NH+4浓度为1000~2000 mg/L时,容抗弧半径和阻抗膜值都逐渐增大,表明N80钢的腐蚀速率逐渐降低,氨离子浓度的增加抑制了金属的腐蚀,这与图2的腐蚀速率随NH+4浓度变化趋势相同。
图6
图6
不同NH+4浓度的3%NaCl溶液中N80钢的电化学阻抗图
Fig.6
Nyquist (a1~e1) and Bode (a2~e2) diagrams of N80 steel in 3%NaCl solutions containing 0 mg/L (a), 150 mg/L (b), 500 mg/L (c), 1000 mg/L (d) and 2000 mg/L (e) NH+4
图7
图7
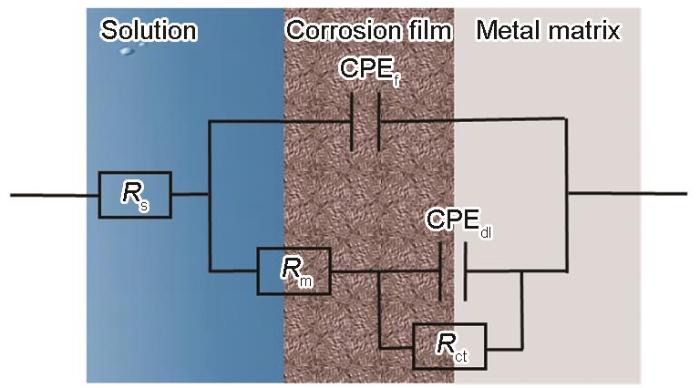
符合EIS数据的等效电路图
Fig.7
Equivalent circuit diagram used for EIS fitting analysis
表1 N80钢的EIS拟合数据
Table 1
| NH+4 concentration mg·L-1 | Time h | Rs Ω·cm2 | CPEf F·cm-2 | n1 | Rm Ω·cm2 | CPEdl F·cm-2 | n2 | Rct Ω·cm2 |
|---|---|---|---|---|---|---|---|---|
| 0 | 72 | 9.241 | 4.649 × 10-4 | 0.8960 | 1681 | 2.009 × 10-3 | 0.3911 | 2197 |
| 150 | 72 | 9.577 | 6.201 × 10-4 | 0.9148 | 2260 | 8.881 × 10-3 | 1 | 264.8 |
| 500 | 72 | 7.670 | 6.670 × 10-4 | 0.9144 | 1014 | 1.008 × 10-3 | 0.4964 | 1206 |
| 1000 | 72 | 4.846 | 3.522 × 10-4 | 0.9382 | 3240 | 3.261 × 10-3 | 0.7541 | 784.8 |
| 2000 | 72 | 4.976 | 3.888 × 10-4 | 0.9268 | 3936 | 2.684 × 10-3 | 0.6916 | 2024 |
图8
图8
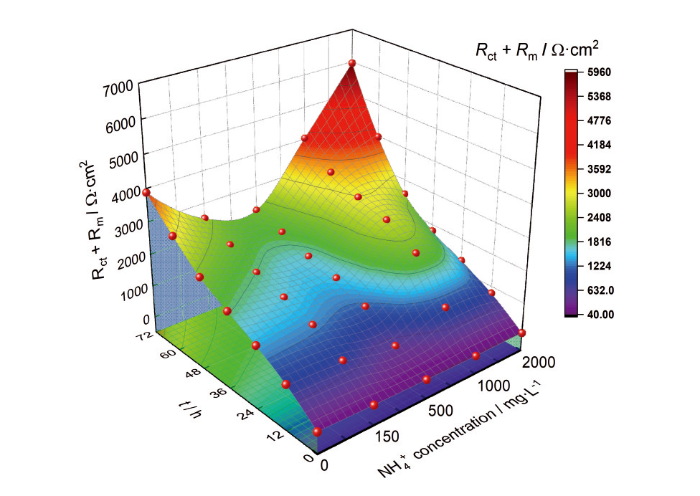
不同NH+4浓度的3%NaCl溶液中N80钢的(Rm + Rct)值的变化
Fig.8
(Rm + Rct) values of N80 steel in 3%NaCl solutions with different NH+4 concentrations
2.3 腐蚀形貌表征和成分分析
2.3.1 腐蚀形貌观察
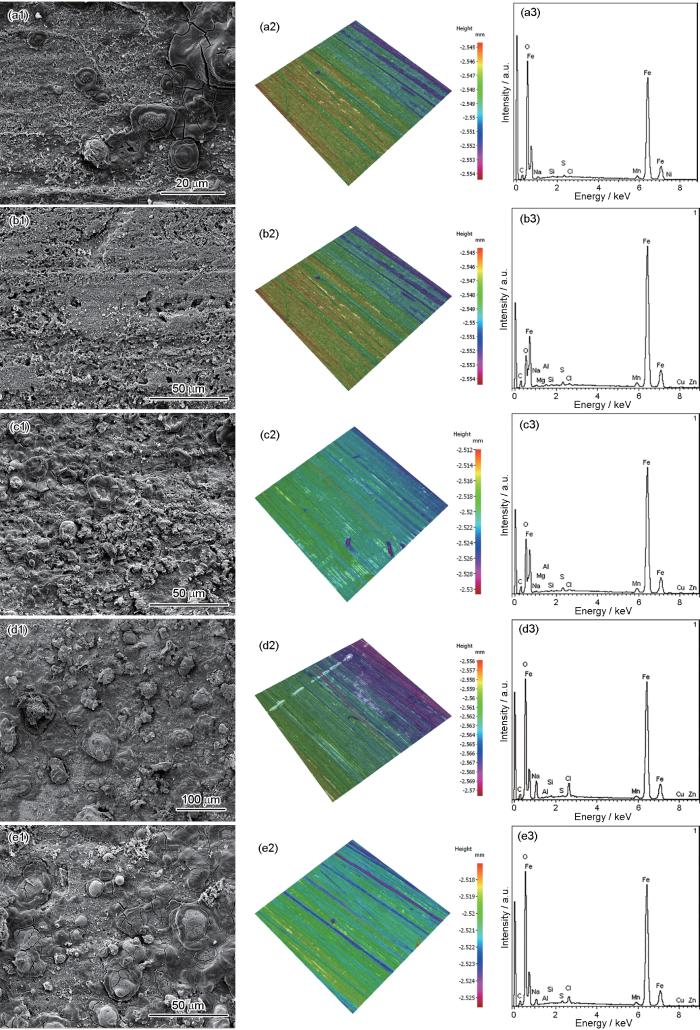
图9为在不同NH+4浓度的3%NaCl溶液中N80钢的腐蚀形貌,3D轮廓图和腐蚀产物元素。与0 mg/L相比,当NH+4浓度为150和500 mg/L时,N80钢表面腐蚀产物疏松多孔;当NH+4浓度为1000和2000 mg/L时,N80钢表面的腐蚀产物较为致密,紧紧的粘附在金属表面,对金属基体具有较好的保护性,表面的皲裂细纹可能是实验后吹干干燥脱水所致。这说明图中NH+4浓度较低时对N80钢腐蚀的促进作用及高浓度NH+4时对腐蚀的抑制作用与金属表面腐蚀产物膜的状态有关。
图9
图9
在不同NH+4浓度的3%NaCl溶液中N80钢的腐蚀形貌、3D轮廓图和腐蚀产物元素组成
Fig.9
Corrosion morphologies (a1~e1), 3D contour graphics (a2~e2) and elemental compositions (a3~e3) of N80 steel after immersion in 3%NaCl solutions containing 0 mg/L (a), 150 mg/L (b), 500 mg/L (c), 1000 mg/L (d) and 2000 mg/L (e) NH+4
N80钢在不同NH+4浓度3%NaCl溶液中的腐蚀产物主要为Fe、C、O。此外,EDS能谱显示还有少量Mn、Al、Cu等,这些元素来源于N80钢的组分。
将N80钢表面酸洗去除腐蚀产物后进行3D轮廓仪观察,结果表明,在不同NH+4浓度溶液中,N80钢表面仍存在打磨痕迹,但均存在不同程度的点蚀。N80钢在0,150,500,1000和2000 mg/L NH+4浓度溶液中的最大点蚀深度和最大点蚀速率分别为10.66,10.58,12.09,9.62和7.31 μm及1.29,1.29,1.47,1.17和0.89 mm/a,如表2所示,点蚀深度随NH+4浓度基本上呈现先增加后降低的趋势,500 mg/L时点蚀深度最深为12.09 μm,最大点蚀速率为1.47 mm/a。
2.3.2 腐蚀产物分析
图10
图10
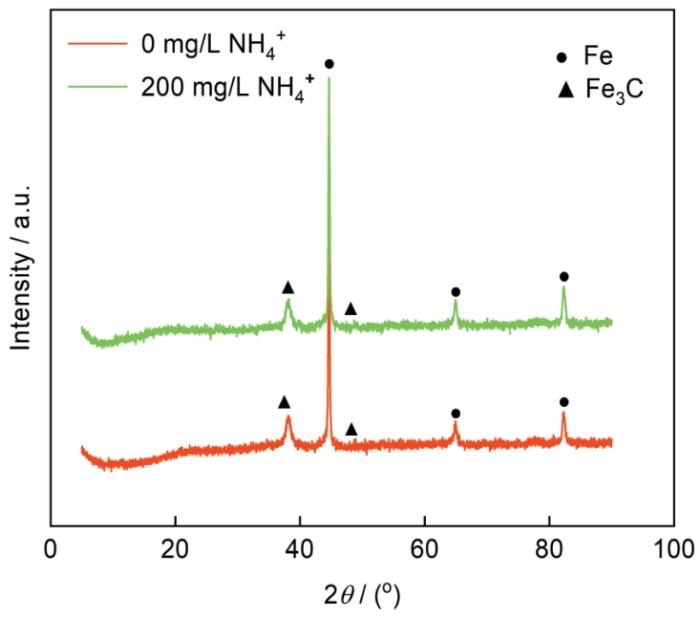
在不同NH+4浓度的3%NaCl溶液中N80钢腐蚀产物的XRD谱图
Fig.10
XRD patterns of N80 steel after immersion in 3%NaCl solutions containing 0 and 2000 mg/L NH+4
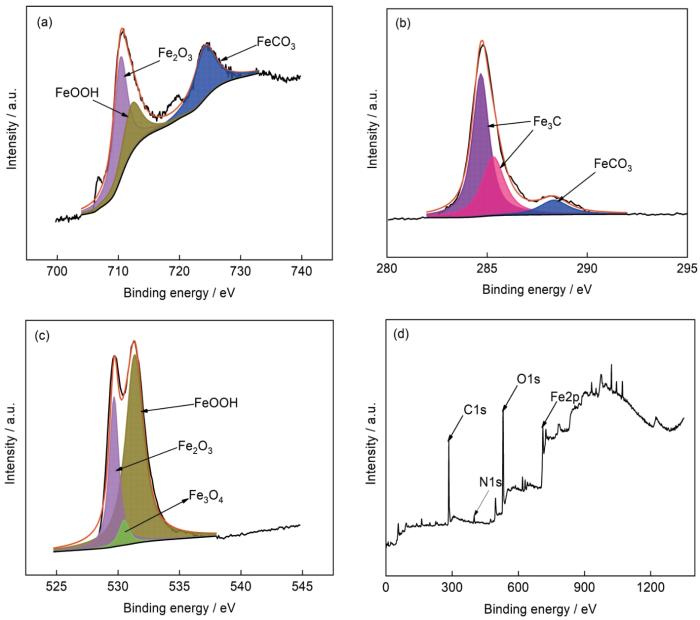
图11是NH+4为2000 mg/L时N80钢表面腐蚀产物膜的XPS图谱,可以看出:表面腐蚀产物含FeCO3、Fe2O3、FeOOH、Fe3C和少量Fe3O4。其中,FeCO3结合能峰位置为724.0 和288.3 eV,Fe2O3结合能峰位置为710.9和529.8 eV,FeOOH结合能峰位置为713.4和533.1 eV,Fe3C结合能峰位置为284.65 和285.45 eV,Fe3O4结合能峰位置为531.15 eV。FeOOH是一种高电阻率化合物[25],FeOOH和FeCO3对N80钢的腐蚀起一定的保护作用。XPS分峰拟合结果中N 1s峰的出现,说明在高NH+4浓度时,金属表面存在N的吸附[26]。
图11
图11
在不同NH+4浓度3%NaCl溶液中N80钢腐蚀产物的XPS谱图
Fig.11
XPS fine peaks of Fe 2p (a), C 1s (b) and O 1s (c), and survey spectrum (d) of N80 steel after immersion in 3%NaCl solutions with different NH+4 concentrations
2.4 N80钢在不同NH+4浓度下饱和CO2 的3%NaCl溶液中的腐蚀机理
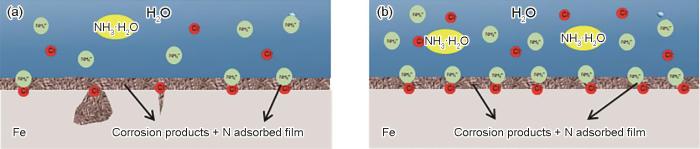
一般来说,随着NH4Cl浓度的提高,金属的腐蚀将会逐渐加重,这是因为NH+4在水中发生水解:NH+4 + H2O ⇋ NH3·H2O + H+,导致溶液酸性增强。但是,从以上实验结果可以看出,在相同pH的实验条件下,随NH+4浓度增加,低NH4Cl浓度条件下促进了腐蚀,而高NH4Cl浓度条件下却抑制了金属的腐蚀。Wang等[26]对低碳钢在NH4Cl溶液中腐蚀行为研究表明,低浓度的NH4Cl溶液会促进碳钢的腐蚀,高浓度的NH4Cl溶液会抑制碳钢的腐蚀,这种腐蚀现象被解释为:低NH4Cl浓度条件下NH+4和Cl-在金属表面的竞争吸附,而高NH4Cl浓度条件下以NH+4的吸附有关。图12为NH+4原子在N80钢表面吸附机理示意图。在不同NH4Cl浓度的3%NaCl溶液中,由于Cl-的特性吸附使电极相界区由带正电荷转向带负电荷,有利于带正电荷的NH+4吸附在电极表面的负电荷区[27]。当NH+4浓度在0~500 mg/L时,如图12a示,当NH+4浓度低时,Cl-和NH+4竞相吸附在N80钢表面[26],同时NH+4吸附膜层并不能完全覆盖N80钢表面,未被NH+4吸附膜层覆盖的暴露的金属表面更容易被Cl-侵蚀,造成N80钢的溶解;当NH+4浓度1000~2000 mg/L,如图12b所示,NH4+中N原子吸附在N80钢表面,这层NH+4吸附层与腐蚀产物组成的混合膜层阻碍了金属基体与腐蚀介质之间的接触,从而减小了腐蚀速率。
图12
图12
N原子在N80钢表面吸附机理示意图
Fig.12
Adsorption mechanism schematic of N atoms on N80 steel surface in 3%NaCl solutions with low concentration (a) and high concentration (b) of NH+4
3 结论
(1) NH+4浓度小于等于500 mg/L时,N80钢的腐蚀速率随NH+4浓度增加而增加;当NH+4浓度大于500 mg/L后,N80钢的腐蚀速率随NH+4浓度增加而降低。
(2) 由于N原子在N80钢表面的吸附,当NH+4浓度大于等于500 mg/L后,N80钢的阳极极化曲线出现了“伪钝化”区。
(3) 各NH+4浓度条件下,N80钢表面均存在点蚀现象,最大点蚀速率与均匀腐蚀速率随NH+4浓度变化趋势基本相同,在500 mg/L时均匀腐蚀和点蚀速率最大。
参考文献
Stress corrosion cracking behavior of 316L stainless steel with varying microstructure in ammonium chloride environment
[J].
不同组织的316L不锈钢在NH4Cl环境下应力腐蚀行为与机理
[J].采用动电位极化曲线、电化学阻抗谱以及U形弯试样浸泡实验研究了不同组织 (原始组织、固溶组织与敏化组织) 的316L不锈钢在NH<sub>4</sub>Cl环境下的应力腐蚀开裂 (SCC) 行为与机理,分析了NH<sub>4</sub>Cl浓度对不同热处理状态的316L不锈钢应力腐蚀行为和机理的影响。结果表明:不同组织的316L不锈钢在NH<sub>4</sub>Cl环境中均具有明显的SCC敏感性,原始组织、固溶组织、敏化组织的SCC敏感性依次升高;随着NH<sub>4</sub>Cl溶液浓度升高,316L不锈钢不同组织的钝化膜稳定性降低,原始组织、固溶组织及敏化组织在NH<sub>4</sub>Cl环境下,破钝电位依次降低,维钝电流密度依次升高,阻抗值依次减小,钝化膜更加活泼易破坏,饱和NH<sub>4</sub>Cl浓度下极易发生点蚀导致SCC的萌生。316L不锈钢在NH<sub>4</sub>Cl环境中的SCC裂纹扩展机制主要为穿晶型阳极溶解机制。
Effect of temperature on corrosion behavior of pipeline steels N80 and TP125V in artificial CO2-saturated fracturing fluid of shale gas
[J].
温度对CO2饱和页岩气压裂液环境中N80和TP125V钢腐蚀行为影响研究
[J].采用失重法、扫描电镜、X射线衍射仪、三维超景深显微镜结合电化学测量,着重研究了N80和TP125V钢在页岩气环境中60~120 ℃范围内的腐蚀速率的变化规律。结果表明,N80和TP125V钢腐蚀速率随温度的增加先增加后减小,100 ℃时达到最高值,分别为 (0.169±0.014) 和 (0.198±0.007) mm/a;局部腐蚀速率也达到最大值,分别为1.13和2.47 mm/a,点蚀坑密度分别是2.0×10<sup>3</sup>和2.6×10<sup>3</sup> pits/cm<sup>2</sup>。在60和120 ℃时,N80钢的腐蚀速率要高于TP125V钢的;而在90、100和110 ℃时,TP125V钢的腐蚀速率要高于N80钢的。表面分析结果表明,温度同时也会显著影响腐蚀产物膜的结构和组成。
Prospective prospect of shale oil exploration and development
[N].
页岩油勘探开发前景可期
[N].
CO2 mass transfer and oil replacement capacity in fractured shale oil reservoirs: from laboratory to field
[J].CO2-based fracturing is widely introduced to stimulate shale oil reservoirs for its multiple advantages. However, the range of CO2 entering the matrix around fractures and CO2-oil replacement capacity between matrix and fractures cannot be fully explained. To address this issue, a radial constant volume diffusion experiment on shale cores was designed in this study, and the pressure drop curve history was matched through numerical model to determine the composition effective diffusion coefficient. A field-scale numerical model was established, in which a series of certain grids were used to explicitly characterize fracture and quantify the prosess of CO2 mass transfer and oil replacement. Based on the field-scale numerical model, the process of shut-in, flow back, and oil production was simulated. The distribution of CO2 in fractured shale oil formation and its impact on crude oil during shut-in stage and flow back stage were investigated. This study concludes that CO2 gradually exchanges the oil in matrix into fractures and improve the fluidity of oil in matrix until the component concentrations of the whole reservoir reaches equilibrium during the shut-in process. Finally, about 30∼35 mole % of CO2 in fractures exchanges for oil in matrix. The range of CO2 entering the matrix around fractures is only 1.5 m, and oil in matrix beyond this distance will not be affected by CO2. During the process of flow back and production, the CO2 in fracture flows back quickly, but the CO2 in matrix is keeping dissolved in oil and will not be quickly produced. It is conclued that the longest possible shut-in time is conducive to making full use of the CO2-EOR mechanism in fractured shale oil reservoirs. However, due to the pursuit of economic value, a shut-in time of 10 days is the more suitable choice. This work can provide a better understanding of CO2 mass transfer mechanism in fractured shale oil reservoirs. It also provides a reference for the evaluation of the shut-in time and production management after CO2 fracturing.
A review of microscopic seepage mechanism for shale gas extracted by supercritical CO2 flooding
[J].Shale gas exploitation with supercritical CO2 is a promising technology that not only improve gas recovery but also achieve geological storage of CO2. This paper presents the research status of shale gas seepage mechanism under supercritical CO2 conditions from several aspects including the adsorption and desorption of gas, the competitive adsorption of CO2 and CH4, the multi-scale spatial gas mass transfer process and the shale gas seepage mechanism under multi-field coupling. Adsorption potential theory provides a method for predicting the real shale reservoir adsorption capacity under supercritical conditions. The desorption model needs to be considered simultaneously due to the existence of hysteresis. The adsorption selectivity of CO2/CH4 is always greater than 1, and there is an optimal selectivity coefficient to optimize the in-situ process conditions. For the competitive adsorption mechanism, the reason why CO2 adsorption capacity of shale is stronger than that of CH4 is explained in terms of kinetic diameter, critical temperature, molecular polarity and diffusion rate. The interaction between CO2 and CH4 will make the shale gas seepage mechanism more complex, so further research is needed on the mass transfer process involving multi-component gas. The changes in reservoir physical properties after supercritical CO2 fracturing, the mathematical model of multi-field fully coupled gas microscopic seepage and the universal stress-sensitive model are the focus of future research.
Experimental study on the supercritical CO2 fracturing of shale considering anisotropic effects
[J].
The study of under deposit corrosion of carbon steel in the flowback water during shale gas production
[J].
Influence mechanism of H2S/CO2-charging on corrosion of J55 steel in an artificial solution
[J].
H2S/CO2对J55钢腐蚀的影响机制
[J].通过浸泡腐蚀实验与高温高压电化学测试,研究了J55钢在1.0 MPa CO<sub>2</sub>、0.3 MPa H<sub>2</sub>S及1.0 MPa CO<sub>2</sub>+0.3 MPa H<sub>2</sub>S气体组分下的腐蚀特征,并采用XRD、SEM和EDS分析腐蚀产物膜组成与形貌。结果显示,溶液中气体组分为H<sub>2</sub>S及CO<sub>2</sub>+H<sub>2</sub>S下的腐蚀速率相近,其表面产物为FeS。在只含H<sub>2</sub>S气体时,J55钢的表面FeS产物膜致密;而在CO<sub>2</sub>氛围下J55钢的腐蚀速率最高,其产物为疏松、覆盖率较低的FeCO<sub>3</sub>。高温高压原位电化学测试显示,不含腐蚀气体时溶液介质对J55钢的腐蚀表现为阴极控制;加入H<sub>2</sub>S使腐蚀转为阳极控制,腐蚀电位明显升高;而CO<sub>2</sub>的加入能强化阴极控制效果,同时降低腐蚀电位;溶液中CO<sub>2</sub>与H<sub>2</sub>S共存时,CO<sub>2</sub>使FeS的成膜电位增加。EIS图显示,J55钢在不含腐蚀性气体时极化电阻最大,仅含CO<sub>2</sub>时极化电阻最小,仅含H<sub>2</sub>S时出现高频容抗弧与低频容抗弧两个时间常数。
Analysis on corrosion-induced failure of shale gas gathering pipelines in the southern Sichuan Basin of China
[J].
Characteristics of CO2 corrosion scale formed on N80 steel in stratum water with saturated CO2
[J].
Corrosion characteristics of P110SS casing steel for ultra-deep well in artificial formation water with low H2S and high CO2 content
[J].
低H2S和高CO2分压下超深井用P110SS油套管钢腐蚀特征研究
[J].研究了P110SS钢在含低硫化氢油气井下腐蚀规律及特征。通过高温高压反应釜模拟超深井的腐蚀工况,对P110SS钢在不同温度、H<sub>2</sub>S、CO<sub>2</sub>分压条件下进行浸泡实验,通过腐蚀失重计算其腐蚀速率,辅以SEM、EDS和XRD等手段对腐蚀产物的形貌和成分进行表征。结果表明,H<sub>2</sub>S、CO<sub>2</sub>分压增大均会导致P110SS的腐蚀速率增大;然而温度升高却降低其腐蚀速率。分析腐蚀产物可见,H<sub>2</sub>S、CO<sub>2</sub>浓度和温度的变化均会导致腐蚀产物成分和结构发生转变。说明在高温高压条件下,H<sub>2</sub>S腐蚀起主导作用,Fe<sub>7</sub>S<sub>8</sub>腐蚀产物对基体的保护作用较差,腐蚀速率高;低H<sub>2</sub>S分压下,CO<sub>2</sub>腐蚀起主导作用,腐蚀速率的大小取决于腐蚀产物膜的致密性;相比于CO<sub>2</sub>,温度对腐蚀速率的影响更显著。
The corrosion behavior of X65 steel in CO2/oil/water environment of gathering pipeline
[J].The purpose of this paper was to investigate the corrosion behavior of X65 steel in the CO2/oil/water environment using mass loss method, potentiodynamic polarization technique and characterization of the corroded surface techniques.
Unveiling corrosion behavior of pipeline steels in CO2-containing oilfield produced water: towards combating the corrosion curse
[J].
Isotopic geochemical characteristics and identification indexes of shale gas hydraulic fracturing flowback water/produced water
[J].
页岩气压裂返排液/采出水同位素地球化学特征与鉴别指标
[J].
Corrosion behavior of several kinds of steel in NH4Cl environment
[J].
几种钢材在NH4Cl环境中的腐蚀行为
[J].
Effect of ammonium salt on corrosion of pipelines and components in a crude oil distillation column: Electrochemical and AIMD studies
[J].
Investigation of the corrosion behavior of mild steel in the NH4Cl/CO2 corrosive medium
[J].
Study on corrosion of carbon steel and alloy steel in ammonium chloride solution
[J].
碳钢及合金钢在氯化铵溶液中腐蚀规律研究
[J].
Corrosion of carbon steel and alloys in concentrated ammonium chloride solutions
[J].Corrosion resistance and the behavior of carbon steel and alloys in high concentration ammonium chloride (NH4Cl) solutions were investigated. This study was conducted to better understand material performance in severe NH4Cl corrosive environments in refineries. Immersion tests were performed under boiling 20 wt% and 40 wt% NH4Cl solutions. Sixteen materials commonly used in refineries and two new alloys were examined, including carbon steel; aluminized carbon steel; aluminum brass; Types 405, 410, 304L, 316L, and 321 stainless steels (UNS S40500, S41000, S30403, S31603, and S32100); 300-series stainless steel with 6% Mo; two types of super duplex stainless steels; Alloys 800, 625, and C-276 (UNS N08800, N06625, and N10276); Grade 2 and Grade 19 titanium (UNS R50400 and R53530); Alloy A (equivalent to a modified Type 317L [UNS S31703] stainless steel); and a modified Alloy 825 (UNS N06845). Significant corrosion was observed in carbon steel in both solutions. The corrosion rates of carbon steel were as high as 25.4 mm/y and 60.4 mm/y in 20 wt% and 40 wt% NH4Cl solutions, respectively. However, neither general corrosion nor pitting was observed on titanium alloys and alloys with a pitting resistant equivalent number (PREN) of 40 and higher. The maximum pit depth had a close relationship with the PREN. In addition, an electrochemical study was used to investigate the corrosion behavior of carbon steel. The effects of the temperature and NH4Cl concentration were determined from polarization curves and the corrosion potential.
Research on the corrosion behavior and corrosion resistance mechanism of low C medium Cr steel in CO2 EOR gathering & transportation environment
[D].
低C中Cr钢在CO2驱集输环境中的腐蚀行为及耐蚀机制研究
[D].
Electrochemical behaviour of tungsten-carbide hardmetals
[J].
Electrochemistry of CO2 corrosion of mild steel: Effect of CO2 on iron dissolution reaction
[J].
Reactions of pipeline steels in carbon dioxide solutions
[J].
Fe3C influence on the corrosion rate of mild steel in aqueous CO2 systems under turbulent flow conditions
[J].
Corrosion and galvanic compatibility studies of a high-strength copper-nickel alloy
[J].
Electrochemical investigation of corrosion of mild steel in NH4Cl solution
[J].