高熵合金(HEAs)和中熵合金(MEAs)在工程结构中具有广泛的应用前景,引起了科学界的关注[1~4]。目前,单一面心立方(FCC)结构的CoCrNi MEA具有较高的加工硬化性能、优异的延展性和断裂韧性,是一种拥有出色力学性能的材料[5~8]。Wu等[9]研究表明,与典型的CoCrFeMnNi HEA和大多数多相合金相比,具有FCC结构的CoCrNi MEA在低温和室温下均表现出优异的力学性能。Gludovatz等[10]研究表明,具有单一FCC固溶体结构的CoCrNi MEA的断裂韧性是已知的HEA和多数多相合金都无法比拟的。Moravcik等[11]研究表明,与高强度钢和其他HEA(如FeMnCoCrNi、CoCrFeNi和Fe40Mn27Ni26Co5Cr2、Fe40.4Ni1.3Mn34.8Al7.5Cr6C1)相比,通过粉末冶金生产的CoCrNi MEA表现出优异的力学性能,且屈服强度和延展性优于传统材料。近年来,Lu等[12]研究了CoCrNi MEA和CoCrFeMnNi HEA在室温下的低周疲劳行为,结果表明前者具有更高的强度、更低的非弹性应变和更长的疲劳寿命。Laplanche等[13]揭示了CoCrNi中出现的纳米孪晶提供了高且稳定的加工硬化,这使得该MEA比CrMnFeCoNi HEA具有更好的力学性能。综上所述,CoCrNi MEA表现出优异的力学性能,因而具有广泛的工程应用前景。
对于长期的实际工程应用,MEA的耐蚀性是影响其服役寿命的关键因素。与传统不锈钢和HEAs相比,CoCrNi MEA具有更高浓度的钝化元素,如Cr和Ni,理论上拥有优异的耐腐蚀性能。然而,目前对CoCrNi MEA在不同介质中的腐蚀行为和机理的研究相对有限。Wang等[14]对比研究了CoCrNi MEA与304不锈钢在H2SO4和NaOH环境中的耐腐蚀性能和钝化膜性能。结果表明,由于高的Cr含量和厚的钝化膜,MEA在H2SO4溶液中具有良好的耐蚀性。此外,铬的快速溶解是MEA在NaOH溶液中耐蚀性差的原因。Moravcik等[15]指出具有单相FCC结构的CoCrNi MEA在0.1 mol/L H2SO4溶液中表现出比316L不锈钢更好的耐蚀性。Zhang等[16]比较了选择性激光熔化(SLM)CoCrNi MEA及其铸造合金在3.5%(质量分数) NaCl溶液中的腐蚀行为,研究表明表面缺陷是决定腐蚀性能的关键因素。由上述文献可知,关于CoCrNi MEA腐蚀行为的研究主要集中在强酸碱和中性环境中。关于CoCrNi MEA在NH4Cl环境中的耐腐蚀性能的研究尚无报道。因此,研究CoCrNi MEA在该介质中的腐蚀行为对其实际应用至关重要。
目前,在石油化工行业,随着石油的不断开采,低酸低盐的优质原油产量下降,高氯高氮原油的提取和加工量增加,许多装置的原料中氯含量超过了原设计值。因此,在高氯和高氮原油精炼设备中很容易形成高浓度的NH4Cl环境[17]。目前,炼油厂的设备在NH4Cl环境中呈现出较高的点蚀敏感性。体积小的Cl-容易穿透钝化膜并导致点蚀形核[18~25]。Duan等[26]研究了SLM 316L不锈钢在Cl-环境中的点蚀行为,表明高浓度的NaCl会导致不锈钢发生严重腐蚀。文献[27]探讨了NH+4对316L不锈钢在氯化物环境中腐蚀行为的影响。结果表明,NH+4与OH-生成H+,使溶液的pH值降低[28~30],加速了钝化膜中铬氧化物的溶解,促进了腐蚀的发生。
因此,本文通过电化学测试、统计学分析方法和浸泡腐蚀实验系统地研究了CoCrNi MEA在不同浓度NH4Cl溶液中的腐蚀行为及机理。研究结果可为CoCrNi MEA在石油化工工程中的实际应用提供必要的理论依据和数据支持。
1 实验方法
通过真空感应熔炼技术制备等原子CoCrNi MEA,步骤为:将高纯度(≥99.99%)的Co、Cr和Ni金属作为原材料,以1700℃熔融混合,然后随炉冷却30 min至室温。在纯度为99.9%的氩气氛围中制备合金,避免杂质分子的影响。为了保证各金属组分的均匀性,MEA的熔炼次数至少为4次。从MEA铸锭上切割尺寸为10 mm × 10 mm × 3 mm的试样,用环氧树脂密封,留下1 cm2的工作表面进行测试。试样依次用180~2000号的SiC砂纸研磨,然后用无水乙醇和去离子水清洗,冷风吹干。用于微观组织观察和浸泡腐蚀实验的样品,分别用粒度为1和0.5 μm的金刚石抛光膏研磨至镜面状态。用于亚稳态点蚀研究的试样为丝状,工作面积仅为0.1 cm2,避免因电流叠加导致诱发亚稳态点蚀的困难。利用2 g FeCl3 + 5 mL HCl + 1 mL HNO3 + 10 mL H2O的侵蚀液蚀刻MEA试样表面约10 s,并通过扫描电子显微镜(SEM,FEI Quanta 250)观察其微观结构。本文选择不同浓度(1%、3%和8%)的NH4Cl溶液来模拟石化设备管道的内环境,溶液温度始终维持在30℃,避免温度波动引起的实验误差。
电化学测试是基于三电极体系,在CHI660E电化学工作站进行。其中MEA试样为工作电极,铂板为辅助电极,饱和甘汞电极为参比电极。测试前,将试样在溶液中浸泡1 h以达到预钝化状态,随后进行30 min的开路电位(OCP)测试以保证体系的稳定。电化学阻抗谱(EIS)测试是对体系施加10 mV的正弦扰动信号,在105~10-2 Hz的频率范围内进行。利用ZSimpWin软件对阻抗数据进行拟合,得到电化学特征参数。动电位极化曲线的扫描范围为-0.9~0.8 V(vs. SCE),扫描速率为1 mV/s。测试Mott-Schottky曲线前,将试样在恒定电位0.22 V (vs. SCE)下极化2 h,使表面形成稳定的钝化膜。该测试的频率为1 kHz,电位范围为-0.7~0.7 V (vs. SCE),步长为50 mV。动电位循环极化曲线是在0.33 mV/s的低扫描速率下进行的,电位范围为-0.9~0.63 V (vs. SCE)。亚稳态点蚀的研究是通过恒电位极化来进行的,实验在0.24 V下极化30 min,之后观察亚稳态点蚀的微观SEM形貌。
抛光后的试样在不同浓度NH4Cl溶液中浸泡5 d,并在浸泡腐蚀实验前后将试样充分清洗干燥,准确记录质量,利用失重法计算试样的平均腐蚀速率,并观察试样表面的腐蚀SEM形貌。
2 结果与讨论
2.1 组织结构分析
图1
2.2 开路电位分析
图2
图2
CoCrNi MEA在不同浓度NH4Cl溶液中的开路电位
Fig.2
OCP of the MEA in NH4Cl solutions with different concentrations
2.3 动电位极化曲线分析
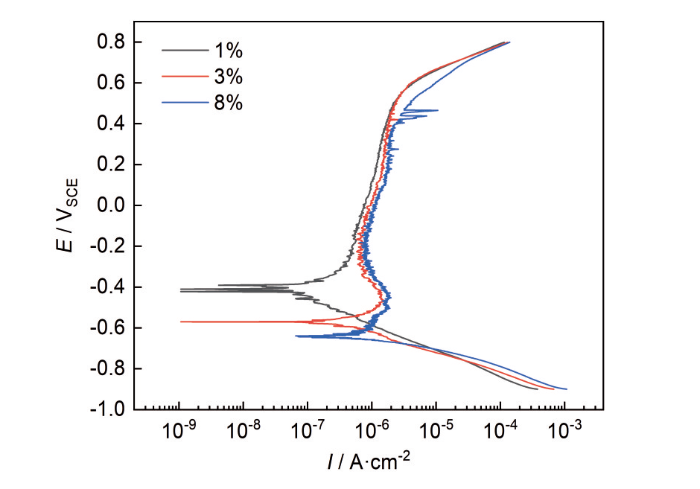
图3为MEA在不同浓度NH4Cl溶液中测得的动电位极化曲线。在电位扫描范围内,各曲线均表现出典型的钝化行为,在阳极分支存在明显的钝化区,此时钝化膜的溶解速率和生成速率处于动态平衡状态。随着NH4Cl浓度的升高(3%和8%),阳极极化曲线出现了明显的活化-钝化过渡现象,且钝化区的位置逐渐右移。这种现象表明MEA的维钝电流密度(Ip)随NH4Cl浓度的提高逐渐增大,试样的腐蚀速率加快[34]。当NH4Cl浓度从3%增加到8%时,致钝电流密度(Ipp)随之增大,这表明在高浓度NH4Cl下,MEA试样的钝性降低,不易进入钝化状态。此外,随着溶液中NH4Cl浓度的升高,腐蚀电位(Ecorr)负移,该规律与OCP一致,表明MEA的腐蚀倾向增加。点蚀电位(Epit)的大小与NH4Cl浓度的变化呈负相关,MEA的Epit值随浓度的增加而减小,点蚀敏感性增加。同时,在钝化区出现了明显的电流密度波动,表明MEA在不同浓度NH4Cl溶液中均发生了亚稳态点蚀。如表1所示,由极化曲线拟合得到的电化学特征参数可以看出,试样在浓度为8%的NH4Cl溶液中的Ip值最大,表明MEA具有最高的膜溶解速率和最差的耐腐蚀性。
图3
图3
CoCrNi MEA在NH4Cl溶液中的动电位极化曲线
Fig.3
Potentiodynamic polarization curves of the MEA in NH4Cl solutions with different concentrations
表 1 动电位极化曲线拟合结果
Table 1
| Mass fracton of NH4Cl / % | Ecorr / V | Ip / 10-7 A⋅cm-2 | Epit / V | Epp / V | Ipp / 10-6 A⋅cm-2 |
|---|---|---|---|---|---|
| 1 | -0.410 | 6.135 | 0.538 | - | - |
| 3 | -0.570 | 7.457 | 0.527 | -0.471 | 1.523 |
| 8 | -0.6430 | 8.87 | 0.468 | -0.452 | 1.990 |
2.4 电化学阻抗谱分析
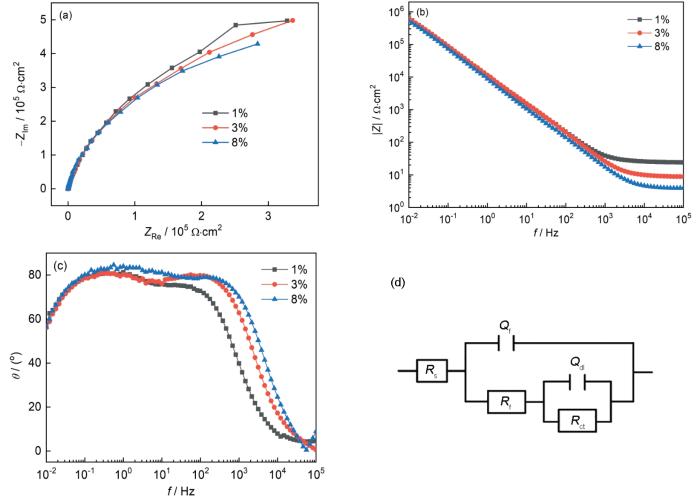
图4
图4
CoCrNi MEA在NH4Cl溶液中的EIS曲线及等效拟合电路
Fig.4
Nyquist (a) and Bode (b, c) plots of the MEA in NH4Cl solutions, and equivalent circuit for fitting EIS data (d)
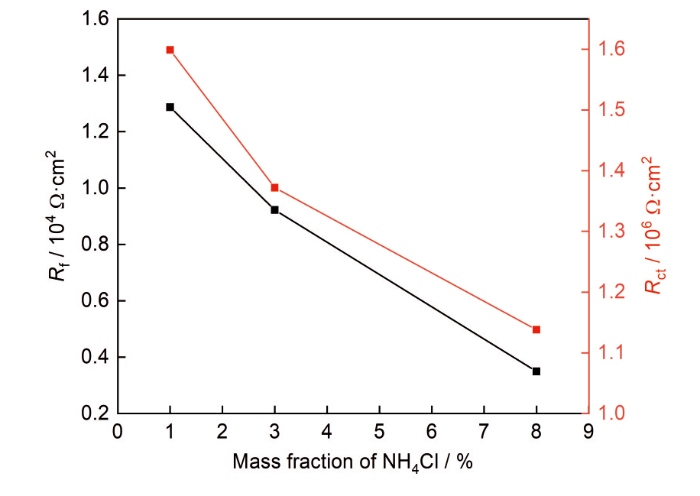
根据电化学阻抗谱的特征,选用图4d所示的等效电路模型拟合EIS数据。其中,Rs为溶液电阻,Rf和Qf为钝化膜电阻和电容,Rct和Qdl分别为电荷转移电阻和非理想双电层电容,拟合结果见表2。Rf和Rct一般用于评估合金的耐腐蚀性能[37,38]。Rf值越大,说明材料表面形成的钝化膜致密,保护性能好;Rct反映了电荷在金属/溶液界面转移过程中受到的阻力,Rct值越大,说明电荷转移过程困难,反应速率减慢,腐蚀速率降低。通过比较,随着NH4Cl浓度的升高,Rf和Rct值逐渐减小(如图5所示),MEA表面钝化膜的保护作用逐渐降低。高NH4Cl浓度降低了钝化膜的稳定性,严重破坏了其致密性,使腐蚀性离子更容易穿透钝化膜与基体直接接触,大大削弱了膜的保护能力。因此,NH4Cl浓度的增加降低了MEA的耐腐蚀性能。
表2 电化学阻抗谱拟合结果
Table 2
| Mass fraction of NH4Cl / % | Rs / Ω·cm2 | Rf / 104 Ω·cm2 | Qf / 10-5 F·cm-2 | Qdl / 10-5 F·cm-2 | Rct / 106 Ω·cm2 |
|---|---|---|---|---|---|
| 1 | 24.88 | 1.287 | 1.569 | 1.759 | 1.599 |
| 3 | 9.035 | 0.9225 | 1.27 | 4.295 | 1.372 |
| 8 | 4.014 | 0.3494 | 1.63 | 4.589 | 1.138 |
图5
图5
CoCrNi MEA在NH4Cl溶液中的Rf和Rct值
Fig.5
Rf and Rct values of the MEA in NH4Cl solutions
2.5 Mott-Schottky曲线分析
为了进一步分析钝化膜对合金基体的保护性能,利用Mott-Schottky曲线分析了MEA在不同浓度NH4Cl溶液中形成的钝化膜的半导体性质。基于Mott-Schottky理论,
其中,“±”表示n型和p型半导体,ε为钝化膜的介电常数,对于MEA,其值为12[40],ε0为真空介电常数(8.854 × 10-12 F·m-1),k为Boltzmann常数(1.38 × 1023 J/K),T为绝对温度。EFB为平带电势(V),e为电子电荷(1.602189 × 10-19 C)。N为载流子密度(cm-3)。E为电极电势(V)。
图6
图6
CoCrNi MEA在NH4Cl溶液中形成钝化膜的Mott-Schottky曲线,载流子浓度及钝化膜厚度
Fig.6
Mott-Schottky plots (a), carrier densities (b) and thickness (c) of the passive films formed on the MEA in NH4Cl solutions
钝化膜厚度的计算结果如图6c所示,MEA试样表面钝化膜随NH4Cl浓度的升高而变薄。当NH4Cl浓度为8%时,MEA表面较薄的钝化膜不利于对合金基体起到良好的屏障作用,腐蚀性离子容易穿透钝化膜造成合金发生腐蚀。该结果与电化学测试的结论一致,从钝化膜角度解释了MEA耐腐蚀性能随NH4Cl浓度增加而降低的原因。
2.6 亚稳态点蚀分析
一般来说,点蚀的发展过程经历了3个阶段:点蚀的形核、亚稳态点蚀的生长、稳态点蚀的扩展[45]。在循环动电位极化曲线中,当反向扫描阳极极化曲线的电流密度大于正向扫描时的电流密度时,这种情形称为正滞后,此时试样表面发生点蚀[46]。由正向和反向扫描曲线形成的闭合区域称为滞后环。滞后环的面积越大表明点蚀形成和发展速度越快[47,48]。图7a给出了MEA试样在不同浓度NH4Cl溶液中的循环动电位极化曲线。随着NH4Cl浓度的增加,曲线闭合区域的面积明显增加,表明点蚀的形核速率增大。图7b为循环极化曲线中滞后环面积的拟合值,该值的变化与溶液中NH4Cl浓度呈正相关,表明NH4Cl浓度的增加促进了亚稳态点蚀的发展,增大了稳态点蚀形成的概率。换言之,高浓度的NH4Cl溶液严重损坏了钝化膜并降低了MEA的耐腐蚀性能。为了进一步研究亚稳态点蚀的发展过程,根据循环极化曲线选取0.24 V作为恒电位极化测试的外加电位。
图7
图7
CoCrNi MEA在NH4Cl溶液中的循环动电位极化曲线和滞回环面积
Fig.7
Cyclic-polarization curves (a) and hysteresis ring areas (b) of the MEA in NH4Cl solutions
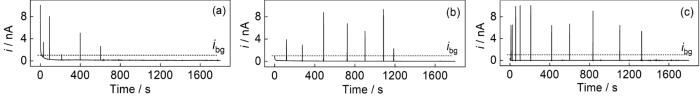
使用针状试样在该电位下进行恒电位极化测试。图8显示了MEA试样在不同浓度NH4Cl溶液中测得的电流-时间的瞬态曲线。显然,随着NH4Cl浓度的增加,曲线的波动加剧,亚稳态点蚀电流峰的数量和幅度均增加,表明较高的NH4Cl浓度促进了亚稳态点蚀的形核和发展[49]。如图9所示,亚稳态点蚀电流峰呈现出两种形态,分别为单峰和重叠峰。在图9a中,电流随时间的变化趋势分为:缓慢增大、急速升高和急剧下降3个阶段,这分别对应亚稳态点蚀的形核、生长和再钝化过程。在亚稳态点蚀的形核和生长过程中(tgrow),电流密度不断增加。在这个阶段,试样表面的钝化膜与高活性区域形成微电偶腐蚀电池,合金基体元素溶解形成的腐蚀产物覆盖在亚稳态点蚀表面,从而延缓了亚稳态点蚀的进一步发展[50]。在这之后,电流密度急剧下降(trep)并恢复到稳定状态,这表明亚稳态点蚀未进一步扩展,这可能是由于亚稳态点蚀发生再钝化恢复到初始钝化状态[51]。
图8
图8
CoCrNi MEA在NH4Cl溶液中的电流-时间瞬态曲线
Fig.8
Current-time transient curves of the MEA in the solutions containing 1% (a), 3% (b) and 8% (c) NH4Cl
图9
图9
CoCrNi MEA的亚稳态点蚀电流峰
Fig.9
Single peak (a) and overlapped peak (b) of metastable pitting of the MEA
图10统计了亚稳态点蚀的平均数量密度(Navg)、瞬态电流峰值(Ipeak)以及寿命。其中,Navg值对应单位面积的亚稳态点蚀数量,Ipeak值表征亚稳态点蚀的发展程度,寿命代表亚稳态点蚀的生长和再钝化率。随着NH4Cl浓度的提高,以上数值均逐渐增大,因而图10中的曲线均呈现上升趋势。这反映出在低浓度NH4Cl溶液中,亚稳态点蚀难以活化并形核,且容易恢复至初始钝化状态。当NH4Cl浓度增加到8%后,样品表面的电化学活性增加,亚稳态点蚀更易被激发,因而数量明显增加[50]。同时,亚稳态点蚀的寿命增加表明其演变为稳态点蚀的概率提高[54]。此外,Ipeak值的变化规律也表明,当NH4Cl浓度为8%时,合金表面形成的亚稳态点蚀的发展程度更大,难以再钝化。有害离子聚集在亚稳态点蚀周围,从而促进了稳态点蚀的形成和扩张。因此,高浓度NH4Cl溶液促进了亚稳态点蚀的萌生和发展,并促使其演变为稳态点蚀。图11为MEA在浓度为8%的NH4Cl溶液中观察到的亚稳态点蚀形貌及尺寸,在一些文献中也出现了类似的亚稳态点蚀[55,56]。
图10
图10
CoCrNi MEA在NH4Cl溶液中的亚稳态点蚀的平均数量密度,电流瞬态峰值和寿命
Fig.10
Average pit number density (a), peak value of current transient (b) and lifetime of metastable pitting (c) of the MEA in NH4Cl solutions
图11
图11
CoCrNi MEA在8%NH4Cl溶液中浸泡后的亚稳态点蚀形貌
Fig.11
Metastable pitting morphology of the MEA immersed in 8% NH4Cl solution
2.7 浸泡腐蚀实验分析
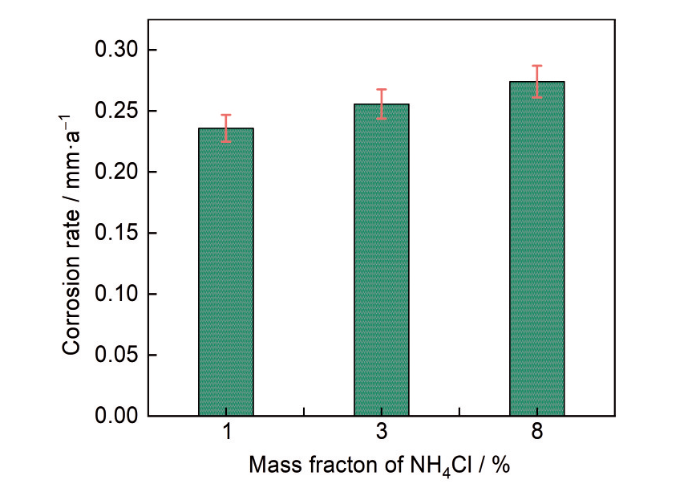
MEA在不同浓度NH4Cl溶液中的平均腐蚀速率如图12所示,随着NH4Cl浓度的升高,MEA的腐蚀速率逐渐增大,这进一步说明MEA的耐腐蚀性能随着NH4Cl浓度的增加而降低。图13所示,通过SEM观察到MEA试样在不同浓度NH4Cl溶液中浸泡5 d后的腐蚀形态主要为点蚀,且点蚀数量随着NH4Cl浓度的升高而增加。如图13a1,a2所示,在1%的低浓度NH4Cl溶液中,MEA试样表面零星分布着极少量的细小点蚀,腐蚀程度非常轻微。当浓度增加到8%时,MEA试样表面出现的点蚀坑明显增加(图13c1,c2),腐蚀加剧。在高浓度的NH4Cl溶液中,NH4+发生水解反应产生大量的H+(
图12
图12
MEA在NH4Cl溶液中的平均腐蚀速率
Fig.12
Average corrosion rates of the MEA in NH4Cl solutions with different concentrations
图13
图13
CoCrNi MEA在不同浓度NH4Cl溶液中浸泡5 d后的表面腐蚀形貌
Fig.13
Corrosion morphologies of the MEA immersed for 5 d in the solutions containing 1% (a1, a2), 3% (b1, b2) and 8% (c1, c2) NH4Cl
3 结论
(1) 随着NH4Cl浓度的升高,MEA的Ip值增大,Ecorr值负移,活化-钝化过渡区出现。此外,合金钝化膜内的缺陷密度增加,膜的保护性能下降,导致MEA的耐腐蚀性能降低。
(2) 在高浓度的NH4Cl溶液中,NH+4与Cl-的共同作用一方面加速了亚稳态点蚀的形核和生长速度,提高了钝化膜的溶解速率;另一方面延长了亚稳态点蚀的寿命,抑制了其再钝化的过程。
(3) MEA呈现出局部腐蚀的特征,腐蚀形态主要是点蚀。随着NH4Cl浓度的增加,MEA的腐蚀速率加快,腐蚀程度变严重。
参考文献
Microstructural development in equiatomic multicomponent alloys
[J].
In-situ Mo nanoparticles strengthened CoCrNi medium entropy alloy
[J].
Nanostructured high-entropy alloys with multiple principal elements: novel alloy design concepts and outcomes
[J].
Microstructures and properties of high-entropy alloys
[J].
Microstructure and properties of CoCrNi medium-entropy alloy produced by gas atomization and spark plasma sintering
[J].
Temperature dependence of the mechanical properties of equiatomic solid solution alloys with face-centered cubic crystal structures
[J].
Yield strength increase of a CoCrNi medium entropy alloy by interstitial nitrogen doping at maintained ductility
[J].
High-entropy alloys
[J].Alloying has long been used to confer desirable properties to materials. Typically, it involves the addition of relatively small amounts of secondary elements to a primary element. For the past decade and a half, however, a new alloying strategy that involves the combination of multiple principal elements in high concentrations to create new materials called high-entropy alloys has been in vogue. The multi-dimensional compositional space that can be tackled with this approach is practically limitless, and only tiny regions have been investigated so far. Nevertheless, a few high-entropy alloys have already been shown to possess exceptional properties, exceeding those of conventional alloys, and other outstanding high-entropy alloys are likely to be discovered in the future. Here, we review recent progress in understanding the salient features of high-entropy alloys. Model alloys whose behaviour has been carefully investigated are highlighted and their fundamental properties and underlying elementary mechanisms discussed. We also address the vast compositional space that remains to be explored and outline fruitful ways to identify regions within this space where high-entropy alloys with potentially interesting properties may be lurking.
Recovery, recrystallization, grain growth and phase stability of a family of FCC-structured multi-component equiatomic solid solution alloys
[J].
Exceptional damage-tolerance of a medium-entropy alloy CrCoNi at cryogenic temperatures
[J].High-entropy alloys are an intriguing new class of metallic materials that derive their properties from being multi-element systems that can crystallize as a single phase, despite containing high concentrations of five or more elements with different crystal structures. Here we examine an equiatomic medium-entropy alloy containing only three elements, CrCoNi, as a single-phase face-centred cubic solid solution, which displays strength-toughness properties that exceed those of all high-entropy alloys and most multi-phase alloys. At room temperature, the alloy shows tensile strengths of almost 1 GPa, failure strains of similar to 70% and K-JIc fracture-toughness values above 200 MPa m(1/2); at cryogenic temperatures strength, ductility and toughness of the CrCoNi alloy improve to strength levels above 1.3 GPa, failure strains up to 90% and K-JIc values of 275 MPa m(1/2). Such properties appear to result from continuous steady strain hardening, which acts to suppress plastic instability, resulting from pronounced dislocation activity and deformation-induced nano-twinning.
Mechanical and microstructural characterization of powder metallurgy CoCrNi medium entropy alloy
[J].
Superior low-cycle fatigue properties of CoCrNi compared to CoCrFeMnNi
[J].
Reasons for the superior mechanical properties of medium-entropy CrCoNi compared to high-entropy CrMnFeCoNi
[J].
Corrosion behavior of CoCrNi medium-entropy alloy compared with 304 stainless steel in H2SO4 and NaOH solutions
[J].
Interstitial nitrogen enhances corrosion resistance of an equiatomic CoCrNi medium-entropy alloy in sulfuric acid solution
[J].
Corrosion performance of selective laser-melted equimolar CrCoNi medium-entropy alloy vs its cast counterpart in 3.5 wt% NaCl
[J].
Effect of relative humidity on ammonium chloride corrosion in refineries
[J].
Long-term corrosion monitoring of carbon steels and environmental correlation analysis via the random forest method
[J].In this work, the atmospheric corrosion of carbon steels was monitored at six different sites (and hence, atmospheric conditions) using Fe/Cu-type atmospheric corrosion monitoring technology over a period of 12 months. After analyzing over 3 million data points, the sensor data were interpretable as the instantaneous corrosion rate, and the atmospheric “corrosivity” for each exposure environment showed highly dynamic changes from the C1 to CX level (according to the ISO 9223 standard). A random forest model was developed to predict the corrosion rate and investigate the impacts of ten “corrosive factors” in dynamic atmospheres. The results reveal rust layer, wind speed, rainfall rate, RH, and chloride concentration, played a significant role in the corrosion process.
The electrochemical behaviour of 316L austenitic stainless steel in Cl- containing environment under different H2S partial pressures
[J].
Pit growth studies in stainless steel foils. II. Effect of temperature, chloride concentration and sulphate addition
[J].
Pitting corrosion dynamics and mechanisms of 304 stainless steel in 3.5%NaCl solution
[J].Pitting rate of a single pit and pitting mechanisms of the 304 stainless steel in 3.5%NaCl solution were investigated by utilizing electronic speckle pattern interferometer (ESPI), electrochemical noise (EN) and three-dimensional video microscope. The results show that under 0.05 V polarization, the pitting corrosion process can be divided into four stages: drastic fluctuations of the current noise occured at 740 s, which means the passivation film was breaking, thus it can be concluded that the span of pitting incubation period is 740 s; a speckle occurred on the ESPI image at 750 s, thus the span of initiation period of the steady pitting is about 10 s; the growth rate of the pit increased during 750-780 s, which indicates that the pit corrosion is in active dissolution period; since then, the growth rate of the pit declined rapidly which means the pit was repassivated. After\linebreak 793 s, the growth rate of the pit raised again as secondary pits emerged. The pit image was observed and its volume was measured by three-dimensional video microscope, and the results were in agreement with those which were obtained by corrosion product concentration analysis. Some secondary pits were found in the bottom of the pit in three-dimensional reconstruction images.
304不锈钢在3.5%NaCl溶液中的点蚀动力学及机理
[J].
Experimental design to study the influence of temperature, pH, and chloride concentration on the pitting and crevice corrosion of UNS S30403 stainless steel
[J].
Electrochemical study on the corrosion behaviour of a new low-nickel stainless steel in carbonated alkaline solution in the presence of chlorides
[J].
Influence of the cold working induced martensite on the electrochemical behavior of AISI 304 stainless steel surfaces
[J].It is clear that the corrosion resistance of carbon steels decreases as cold working amount increases, but for austenitic stainless steels, the relation between cold-working and corrosion performance is not clear. The electrochemical behavior of AISI 304 stainless steel with 3 different cold working amounts is characterized by Mott-Schottky analysis, OCP records, EIS and cyclic polarization curves. An innovative cell with gel electrolyte is used for an easy study of the deformed surfaces without modifying them. After the polarization tests, the influence of the deformation on the amount of pits and on their morphological characteristics is also analyzed. The microstructural changes caused by cold rolling are studied, and the residual stresses are determined by XRD using the sin(2) psi method. It is proved that AISI 304 stainless steel decreases its pitting resistance in a medium with chlorides when it is subjected to moderate cold rolling, but heavy thickness reduction causes a subsequent recovery of corrosion resistance. The results obtained suggest that this trend is related to changes in the magnitude and type of the stresses (tensile or compressive) on the surface of the material. (C) 2018 Published by Elsevier Editora Ltda. on behalf of Brazilian Metallurgical, Materials and Mining Association.
Evaluation of microstructure, mechanical properties and pitting corrosion in dissimilar of alternative low cost stainless steel grade 204Cu and 304 by GTA welding joint
[J].
Pitting behavior of SLM 316L stainless steel exposed to chloride environments with different aggressiveness: pitting mechanism induced by gas pores
[J].
Effect of NH4+ on the pitting corrosion behavior of 316 stainless steel in the chloride environment
[J].
Transpassive dissolution of alloy 625, chromium, nickel, and molybdenum in high-temperature solutions containing hydrochloric acid and oxygen
[J].
Corrosion behavior of AZ31 magnesium alloy in the chloride solution containing ammonium nitrate
[J].
Mechanistic study of ammonium-induced corrosion of AZ31 magnesium alloy in sulfate solution
[J].The influence of NH4+ ions on the corrosion behavior of AZ31 magnesium alloy was investigated by immersion test, hydrogen evolution, electrochemical methods and morphology observation. The results demonstrate the acceleration effect of NH4+ on corrosion of AZ31 magnesium alloy due to the disruption of protective MgO film in NH4+-containing solution. The loose and cracked corrosion products of AZ31 magnesium alloy in NH4+-containing solutions are mainly composed of (Mg0.833Al0.167)(OH)2(CO3)0.083·0.75H2O and Mg5(CO3)4(OH)2·5H2O. When the NH4+ concentration is lower than 0.01 M, knife-cut like corrosion occurs in some active area of the surface due to the partial dissolution of MgO layer. As the NH4+ concentration is increased to 0.1 M, the MgO layer is completely disrupted, resulting in the occurrence of uniform corrosion. Both cathodic and anodic reactions are accelerated by NH4+ ions, while the effect of original pH values on the reaction kinetics can be neglected in NH4+-containing solutions.
A comparative study on the corrosion behavior of CoCrNi medium-entropy alloy and 316L stainless steel in simulated marine environment
[J].
The corrosion behavior of CoCrNi medium entropy alloy with alternating current interference in carbonate/bicarbonate solution
[J].
Pitting corrosion of Ni-Cr-Fe alloys at open circuit potential in chloride plus thiosulfate solutions
[J].
Passivation behavior and surface chemistry of 2507 super duplex stainless steel in artificial seawater: Influence of dissolved oxygen and pH
[J].
Mechanisms of plastic deformation, hardening, and fracture in single crystals of nitrogen-containing austenitic stainless steels
[J].
Passive and transpassive behaviour of Alloy 31 in a heavy brine LiBr solution
[J].
Passivation and electrochemical behavior of 316L stainless steel in chlorinated simulated concrete pore solution
[J].
Corrosion behavior of an equiatomic CoCrFeMnNi high-entropy alloy compared with 304 stainless steel in sulfuric acid solution
[J].
Passivity breakdown on copper: influence of chloride ion
[J].
Effect of nitrogen on corrosion behaviour of a novel high nitrogen medium-entropy alloy CrCoNiN manufactured by pressurized metallurgy
[J].A novel high nitrogen medium-entropy alloy CrCoNiN, which had higher strength and slightly lower ductility than CrCoNi alloy, was successfully manufactured by pressurized metallurgy. The microstructure and corrosion behaviour were investigated by microscopic, electrochemical and spectroscopic methods. The results indicated that nitrogen existed in the form of Cr2N precipitates and uniformly distributed N atoms, and nitrogen alloying significantly refined the grain size. Besides, nitrogen enriched on the outmost surface of passive film and metal/film interface as ammonia (NH3 and NH4+) and CrN, respectively. The significant improvement of corrosion resistance of CrCoNiN was attributed to the lower metastable pitting susceptibility together with thicker, less defective and more compact passive film.
The history of the point defect model for the passive state: a brief review of film growth aspects
[J].
Role of chloride ion in passivity breakdown on iron and nickel
[J].
Comparison of corrosion resistance of CoCrFeMnNi high entropy alloys with pipeline steels in an artificial alkaline soil solution
[J].
CoCrFeMnNi高熵合金和管线钢在碱性土壤环境中的耐蚀性对比研究
[J].采用电化学技术、X射线光电子能谱、原子力显微镜测试和浸泡实验研究了有/无热处理的等原子比CoCrFeMnNi高熵合金和管线钢在碱性土壤模拟溶液中的耐蚀性差异。结果表明:高熵合金呈现出局部腐蚀特征,腐蚀形态为零星分布的针孔状点蚀,而X80和X100管线钢表面发生了全面腐蚀,并有大尺寸腐蚀坑存在。高熵合金热处理后在碱性土壤模拟溶液中形成的钝化膜结构致密稳定,含有较多Cr的氧化物和水以及更少的FeO,有利于其钝化膜的保护性;而管线钢钝化膜薄且含有缺陷,对基体的保护性较差。有/无热处理高熵合金的耐蚀性均优于X80和X100管线钢,且热处理可提升高熵合金的耐蚀性能。
Semiconducting and passive film properties of nitrogen-containing type 316LN stainless steels
[J].
Effects of boron on microstructure and metastable pitting corrosion behavior of super 304H austenitic stainless steel
[J].
Effects of pH and chloride concentration on pitting corrosion of AA6061 aluminum alloy
[J].
Effects of pH and Cl- concentration on corrosion behavior of the galvanized steel in simulated rust layer solution
[J].
Characterization of pitting corrosion behavior of AZ91 Mg-alloy without and with MAO coating
[J].
AZ91镁合金和MAO涂层的点蚀行为研究
[J].采用循环极化曲线研究了AZ91镁合金及其表面微弧氧化 (MAO) 涂层在3.5%NaCl溶液中的点蚀行为。采用光学显微镜和扫描电镜观察循环极化不同阶段的点蚀形貌,探讨了点蚀在AZ91镁合金和MAO涂层上的萌生和扩展机制。结果表明,合金上点蚀倾向于在α-Mg相上萌生,而涂层上点蚀在多孔结构和裂纹处萌生,合金和涂层上点蚀的初始形态均为开口的“火山口”形貌。合金上点蚀坑中沉积一层腐蚀产物层对点蚀产生一定钝化效果,导致了点蚀在合金上横向扩展。而涂层上点蚀造成涂层的剥离和腐蚀产物的溶解,无法对点蚀形成钝化效果,导致点蚀在涂层上向纵深扩展。点蚀在循环极化过程中持续扩展的微观形貌验证了合金和涂层的循环极化曲线上出现正的滞后环,而不同的点蚀扩展现象也验证了涂层上较大的滞后环面积。
The metastable pitting of mild steel in bicarbonate solutions
[J].
Growth of corrosion pits on stainless steel in chloride solution containing dilute sulphate
[J].
Detailed resolution of microscopic depassivation events on stainless steel in chloride solution leading to pitting
[J].
Impedance of metastable pitting corrosion
[J].
The effect of dichromate ion on the pitting corrosion of AISI 316 stainless steel part II: pit initiation and transition to stability
[J].
The nucleation and growth of corrosion pits on stainless steel
[J].
Critical pitting temperature dependence of 2205 duplex stainless steel on dichromate ion concentration in chloride medium
[J].
Proposed stability product criterion for open hemispherical metastable pits formed in the crevices of different aspect ratios (l/d) on 316L stainless steel in 3.5% NaCl solution
[J].
Characterization of the passive film and corrosion of martensitic AM355 stainless steel
[J].