海洋大气、飞溅、潮差、全浸区带环境对海洋工程用钢腐蚀行为的影响各有特点,导致了钢的腐蚀速率、锈层的成分和结构等方面存在显著差异[1]。不同海洋区带通过海洋环境因子的变化不同程度地影响腐蚀过程、腐蚀速率、腐蚀形态和腐蚀产物。在海洋大气区,钢表面形成一层薄液膜,在界面处发生金属的阳极溶解,阴极则发生水或氧还原反应以及锈层中氧化性组分γ-FeOOH的还原[2,3]。在飞溅区和潮差区,海工钢受到干湿交替、海水冲刷、浪花飞溅的作用力和充足的氧气供应等一系列外部因素的作用,使得钢的腐蚀速率较高且难以控制。海洋全浸区,微生物的附着将使得局部微环境酸化,加速钢的阳极溶解和局部腐蚀行为[4],而此区带充足的水分又为阴阳极反应提供了场所。
在腐蚀速率方面,Li等[5]在青岛海域对3种船用钢的腐蚀进行了研究,结果表明飞溅区钢的腐蚀最严重,腐蚀速率最高。Zhang等[6]研究表明,在干湿循环环境中,锈层较厚时,锈层的持水能力可以延长钢材表面的湿润时间,从而加速钢的腐蚀。在腐蚀形貌方面,Yu等[7]认为涨潮时海水的侵蚀与落潮时海水蒸发、离子发生浓聚的循环作用,造成潮差区钢的腐蚀形貌和腐蚀程度与大气区和全浸区有所不同。Nishimura 等[8]通过对干湿环境中腐蚀产物的研究观察到了点蚀的发生。海洋全浸区因水和氧的存在,使得阴极反应发生吸氧腐蚀常常短周期内引发局部腐蚀[9],长周期内引发均匀腐蚀。Jeffrey和Melchers [10]也通过分析典型的亚热带沿海海水腐蚀的钢表面点蚀结构的演化规律,建立了海洋全浸区由初期点蚀向后期均匀腐蚀转变的模型。在腐蚀产物方面,Gong等[11]研究表明在干湿循环条件下,碳钢表面更容易形成更厚、更松散、有气孔和裂纹的锈层,而在相同腐蚀周期内浸泡条件下形成的锈层相对较薄。Evans等[12]和Yu等[7]认为氧的扩散和Cl-的渗透在密集或厚锈层的存在下被抑制,从而减缓腐蚀的发展过程。
本文选择S420海工钢作为研究对象,通过在青岛海域现场暴露试验,同时也为了节省材料、方便投放工作操作,选择使用小片材料进行投放,研究S420钢在大气、飞溅、潮差和全浸区的腐蚀行为。对暴露时间为1 a的样品进行失重法计算腐蚀速率,评价钢的实际海洋区带环境下的腐蚀行为。结合腐蚀形貌和腐蚀产物分析,明确不同海洋区带环境对S420钢腐蚀行为和机理的影响,为海工钢的安全服役提供理论和数据支持。
1 实验方法
本文选用S420钢为研究对象,主要化学成分(质量分数,%):C 0.12,P 0.017,Si 0.50,Mn 1.70,S 0.01,V 0.12,Nb 0.05,Ti 0.05,Fe余量。将尺寸为200 mm × 100 mm × 4 mm的S420钢试样用800#砂纸打磨、清洗吹干、标记和称重后,投放到室外海域暴露在大气区、飞溅区、潮差区和全浸区。实海暴露大气区在青岛海域,距海岸线50 m左右。其环境参数包括:经纬度为36°06′ N,120°25′ E;平均温度为12.3℃;平均湿度为71%;降水量为643 mm/a;日照时间为2078 h/a;Cl-沉积速率为25.0 mg·m-2·d-1;SO2沉积速率为118.4 mg·m-2·d-1;雨水pH为6.1;湿润时间为4049 h/a。飞溅区投放的试样距离最高潮位20 cm以上,大潮时将承受浪涌。潮差区投放位置位于青岛的平均中潮位,干湿比接近1∶1。全浸区投放试样位置距离最低潮位1~1.5 m。
用除锈液(500 mL HCl + 500 mL H2O + 3.5 g六次甲基四胺)对试样进行超声除锈,记录质量变化以计算腐蚀速率,每种环境有3个平行样。计算公式如下:
其中,v表示腐蚀速率(mm/a),mt表示除锈后试样的质量(g),m0表示暴晒前试样的质量(g),S表示试样的表面积(cm2),ρ表示低合金钢的密度(约为7.86 g/cm3),t表示暴露腐蚀试验时间(a)。
为了观察海工钢在不同海洋区带环境下的腐蚀形貌,采用数码相机(Canon 6D MarkII)获得宏观腐蚀产物的形貌。采用扫描电子显微镜(SEM, Gemini 300)获得微观腐蚀产物形貌和截面形貌,采用能谱仪(EDS, Ultimmax 40)分析腐蚀产物元素分布,X射线衍射仪(XRD, D8 Advance X)和Raman光谱(Renishawin Via)分析腐蚀产物的成分和相组成,采用激光共聚焦显微镜(CLSM, KEYENCE VK-X250)观察去除腐蚀产物后的腐蚀形貌。蚀坑深度和蚀坑体积参数统计用CLSM进行分析,每个视场的分析面积为0.06 mm2,每个试样采集4个区域。
2 实验结果
2.1 宏观腐蚀形貌
图1
图1
S420钢的宏观腐蚀形貌
Fig.1
Macro corrosion morphologies of S420 steel after exposed corrosion in atmospheric zone (a), splash zone (b), tidal zone (c) and immersion zone (d)
2.2 腐蚀速率测试
图2为S420钢在大气区、飞溅区、潮差区和全浸区暴露1 a以后试样的腐蚀速率。大气区、飞溅区、潮差区和全浸区的腐蚀速率分别为0.25、1.07、1.12和0.54 mm/a,其中,大气环境下S420钢腐蚀速率最低,潮差环境下S420钢腐蚀速率最高。
图2
图2
S420钢在大气、飞溅、潮差和全浸环境中暴露1 a后的腐蚀速率
Fig.2
Corrosion rate of S420 steel after 1 a exposure to atmospheric, splash, tidal, and immersion zone
2.3 腐蚀产物的形貌分析
图3
图3
S420钢在大气区、飞溅区、潮差区和全浸区中腐蚀1 a后腐蚀产物的腐蚀形貌
Fig.3
Corrosion morphologies of S420 steel after 1 a exposure to atmospheric (a, b), splash (c, d), tidal (e, f) and immersion (g, h) zone
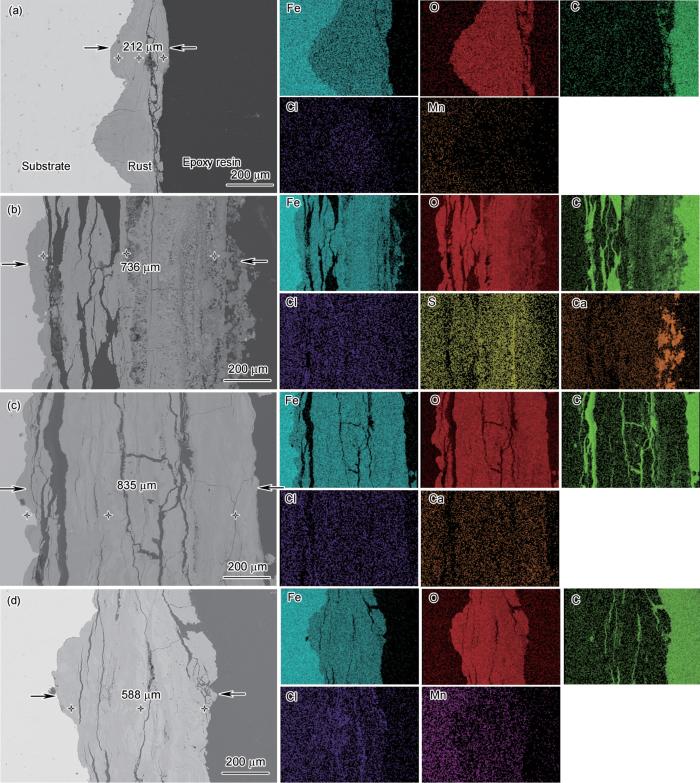
图4为S420钢在4种区带环境下暴露1 a后腐蚀产物的截面形貌及EDS结果。可以看出,钢的锈层均呈现3层结构,内锈层与钢基体连接,中间锈层有明显裂纹,外锈层与环境接触。在4个区带环境下腐蚀的试样截面形貌图中,均可以观察到附着良好的内锈层和中间的平行裂纹。在潮差区暴露的试样中,垂直于界面的裂纹更为明显,甚至在大气区环境下,可以看到有裂纹贯穿到金属/锈层界面。在潮差区和全浸区,垂直裂纹存在于外层,这更易导致局部腐蚀产物的剥落。在全浸环境下形成的锈层比其他3种情况下更致密。整体来看,4种区带环境下的锈层下表面皆不平整,存在局部凹坑。
图4
图4
S420钢在大气区、飞溅区、潮差区和全浸区4种环境下暴露1 a后的腐蚀产物截面形貌及EDS结果
Fig.4
Cross-sectional morphologies and EDS results of the corrosion products of S420 steel after 1 a exposure to atmospheric (a), splash (b), tidal (c) and immersion (d) zone
从形貌上看,4种区带下钢的锈层厚度也有所不同。通过对比分析看出,飞溅区和潮差区形成的锈层相比于大气区和全浸区形成的锈层要明显较厚,说明S420钢暴露在这两个区带的腐蚀更为严重,这也证实了失重法计算得到的腐蚀速率最高的结果。S420钢暴露在大气区的锈层厚度最薄,但也达到了212 μm左右。但是正如图4所示,锈层的结构并不致密,可以观察到许多裂纹和孔洞。垂直界面的裂纹比横向裂纹更危险,因为垂直界面的裂纹为Cl-、O2和H2O等腐蚀性物质的传输提供了通道。与外层锈层相比,暴露在飞溅区和潮差区的内锈层存在明显的裂纹和较大的孔洞,这使得腐蚀介质更容易与钢基体接触,从而加速腐蚀。
图5为S420钢腐蚀1 a后去除腐蚀产物后试样的表面形貌和CLSM图。在大气、飞溅和潮差环境中腐蚀1 a后,可以看到表面出现了大量的点蚀坑。在放大图中,观察到大气区的表面形貌更为粗糙,凹坑分布比较密集。全浸区环境下部分凹坑相互连通,形成浅大凹坑。暴露在飞溅区和潮差区的腐蚀坑比暴露在大气区的腐蚀坑更大、更深。据报道,附着在钢材表面的污垢生物促进钢材的局部腐蚀[20~22]。因此,由于生物污损或沉积物等附着,造成了钢表面不同区带环境下的腐蚀形态发生变化,这可能是造成S420钢在实海全浸区域出现点蚀的原因之一。另一方面,由于Cl-的富集(图4)造成腐蚀产物层局部环境酸化,为点蚀的萌生和生长提供了条件。
图5
图5
S420钢在大气区、飞溅区、潮差区和全浸区暴露1 a后去除腐蚀产物后的表面形貌和3D轮廓
Fig.5
Surface morphologies (a-d, a1-d1) and 3D topographies (a2-d2) of S420 steel after 1 a exposure to atmospheric (a, a1, a2), splash (b, b1, b2), tidal (c, c1, c2) and immersion (d, d1, d2) zone. The corrosion products has been chemically removed
图6
图6
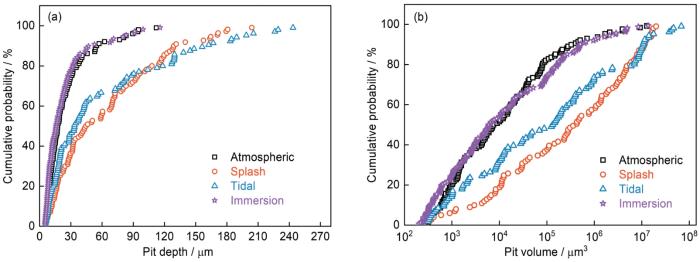
S420钢在不同环境下腐蚀1 a后蚀坑深度和体积的累计概率分布
Fig.6
Cumulative probability distribution of pit depth (a) and volume (b) of S420 steel after corrosion in different zones for 1 a
2.4 腐蚀产物成分分析
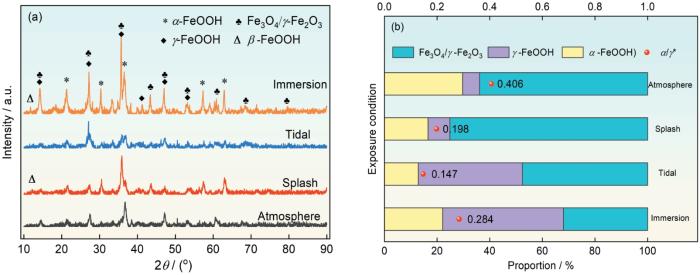
为了进一步认识S420钢在不同环境下的腐蚀演变过程,对腐蚀产物进行了XRD测试。因Fe3O4与γ-Fe2O3难以区分,并可以相互转化,因此把两者合并成Fe3O4/γ-Fe2O3。由图7的XRD结果可以看出,腐蚀产物主要由γ-FeOOH、α-FeOOH、Fe3O4/γ-Fe2O3和少量的β-FeOOH组成(在半定量分析结果中因为β-FeOOH量太少而被忽略不计)。飞溅环境中形成的锈层主要是Fe3O4/γ-Fe2O3,潮差环境下腐蚀产物以γ-FeOOH和Fe3O4/γ-Fe2O3为主。α-FeOOH的结构较为致密,Fe3O4则是疏松多孔的结构,γ-FeOOH是不稳定的相,可以向α-FeOOH转化:
图7
图7
S420钢在不同环境下腐蚀1 a后腐蚀产物的XRD谱以及不同组成相和α/γ*的比值
Fig.7
XRD patterns of the corrosion products formed on S420 steel after corrosion in different zones for 1 a (a) and the proportion of different constituting phases and α/γ* (b)
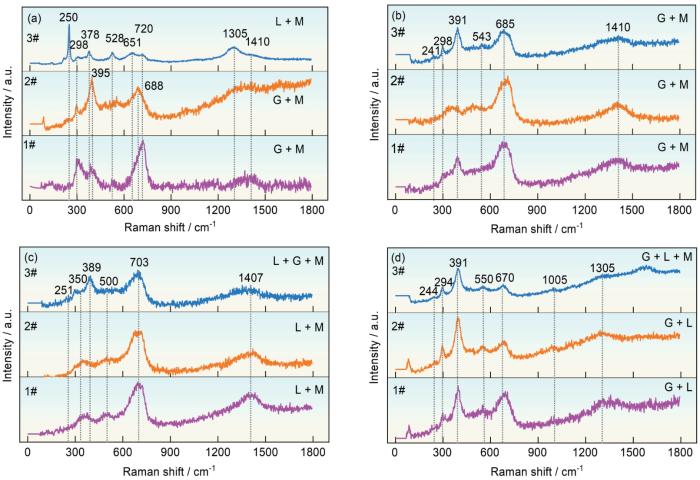
为了确定S420钢在4种区带环境下暴露1 a腐蚀产物的物相分布,对锈层截面框出的标记位置(图4)进行了Raman测试。Raman光谱的峰值位置根据文献[18, 24~26]列于表1。根据图8的结果表明,在大气区暴露的锈层内层由致密稳定的α-FeOOH和Fe3O4/γ-Fe2O3组成,外层主要为γ-FeOOH和Fe3O4/γ-Fe2O3。飞溅区内层由大量的α-FeOOH和少量的Fe3O4/γ-Fe2O3组成,外层以Fe3O4/γ-Fe2O3为主。潮差区的Raman光谱内层由γ-FeOOH和Fe3O4/γ-Fe2O3组成,外层以Fe3O4/γ-Fe2O3为主。这可能是由于潮差区锈层裂纹较多,为继续腐蚀提供了侵蚀性介质和氧,导致了γ-FeOOH和Fe3O4/γ-Fe2O3的连续产生,这也可能是潮差区比飞溅区腐蚀速率略高的原因。全浸区内层以α-FeOOH和γ-FeOOH为主,外层由于O2充分,导致了Fe3O4/γ-Fe2O3在外层占据了一定的位置。
Table 1
| Phase | Raman shift / cm-1 |
|---|---|
| Lepidocrocite (γ-FeOOH) | (248-252)*, (378-380), (528-530), (478-530), (650-655), (1300-1310) |
| Goethite (α-FeOOH) | (241-250), (298-301), (385-395)*, (478-483), (549-552), (680-685), (1000-1120) |
| Magnetite (Fe3O4) | (298-302), (540-550), (663-670)* |
| Maghemite (γ-Fe2O3) | 350, (500-506)*, (700-720)*, (1400-1440)* |
图8
图8
S420钢在大气区、飞溅区、潮差区和全浸区环境下腐蚀1 a后腐蚀产物的物相分布
Fig.8
Phase distributions of the corrosion products formed on S420 steel after 1 a exposure to atmospheric (a), splash (b), tidal (c), and immersion (d) zone (G, L, and M represent goethite (α-FeOOH), lepidocrocite (γ-FeOOH), and magnetite/maghemite (Fe3O4/γ-Fe2O3), respectively)
3 分析讨论
3.1 电解液存在形式对腐蚀速率的影响
在全浸环境下氧扩散受到限制,但这并不足以抑制阴极反应的发生。全浸环境下也存在溶解氧,并且充足的水分促进了阴极反应的发生,加速OH-和Fe2+的扩散以及Fe(OH)2的形成。大气环境下,尤其是白天日照和风力充足的环境,因水分不足电解质薄液膜极其薄,可以使得阴极和阳极反应受到抑制,最终导致了大气环境下腐蚀速率最低。在全浸和大气环境下产生的腐蚀产物都比较致密(图3a, g和图4a, d)。与此相比,潮差环境的腐蚀速率最高。潮差环境和全浸环境之间腐蚀速率的差异表明了干燥期对腐蚀的影响,因为两种环境相同点是存在湿润期。在干燥期,电解液的蒸发和变薄使得Cl-浓度增加,电导率增加,加速了阳极反应。此外,电解液厚度的减小有利于氧扩散到腐蚀前沿,加速阴极去极化反应[33]。对比飞溅和潮差环境,浪花飞溅对S420钢有冲刷作用。而冲刷作用一方面可以剥离疏松的锈层,为O2扩散提供更有效的通道,另一方面,锈层厚度的减小降低了锈层的持水能力,从而降低了腐蚀速率。
3.2 电解液存在形式对腐蚀产物的影响
在海洋环境中,钢的腐蚀开始于铁的溶解和O2的阴极反应,形成Fe(OH)2:
Fe(OH)2被氧化为亚稳态的γ-FeOOH [34]:
而后γ-FeOOH通过固态相变进一步转变为热力学稳定的α-FeOOH。Stratmann等[35]报道了FeOOH与Fe2+在露水凝结水中的反应生成Fe3O4:
上述反应生成的腐蚀产物包括α-FeOOH、γ-FeOOH和Fe3O4。锈层不同相的相对含量表明锈蚀阶段不同。在大气条件下,腐蚀最轻微,α-FeOOH的相对含量最高,α/γ*值最高(图7b),此环境下的腐蚀产物对基体具有较高的保护作用。随着环境腐蚀性的增强,全浸区环境为阴极反应提供了充足的去极化剂,使得γ-FeOOH含量升高。飞溅区环境相比于全浸区环境具有充足的氧,容易生成Fe3O4。Nishimura等[8]也证实了干湿交替频繁,O2含量充足,阴极反应充分,易生成Fe3O4和Fe2O3。在潮差区,γ-FeOOH在锈层中的比例比飞溅区高,和全浸区相差不大,这可能和潮差区腐蚀产物层更易保持水介质有关。一方面,和全浸区相比,干湿交替增加了氧含量,促进了阴阳极反应,加速了Fe(OH)2被氧化为γ-FeOOH的过程和Fe3O4的产生,使得潮差区的Fe3O4的比例比全浸区略高。另一方面,相对于飞溅区,潮差区的干湿交替频率稍低,从而降低了氧化速率,使得潮差区的Fe3O4的比例比飞溅区的低。
3.3 电解液存在形式对局部腐蚀行为的影响
高强度低合金钢在海水中的局部腐蚀与腐蚀产物层微环境有着紧密的联系。Jeffrey和Melchers[10]指出锈层下的微环境促进了金属基材的腐蚀,点蚀慢慢长大并且相连构成了较大的阳极区,小蚀坑明显合并为较大的局部蚀坑,锈层下的微环境最终表现为促进了碳钢点蚀直到最终转变为均匀腐蚀。
电解液层厚度的不同,直接决定了电解液层氧浓度、盐浓度和电导率的差异。一方面,电解液层的厚度影响氧向金属表面的扩散速率,在干湿循环过程中的干燥期,由于水分的蒸发,盐浓度增大,为保持电中性,金属离子发生水解,生成氢离子,这为点蚀的萌生提供了酸性高盐的环境条件。另一方面,由于钢表面存在夹杂物和缺陷,钢表面不同位置的活化能和表面状态有所不同。在这种情况下,钢表面形成微电池,导致点蚀萌生。在干湿循环的干燥期,钢表面不同位置的电解液层的厚度是不均匀的,不同位置之间容易形成浓度差,从而进一步加剧了钢的点蚀。因此,可以认为在含Cl-腐蚀介质和周期性干湿循环的双重作用下,微电池和盐浓度差的形成触发了钢的点蚀。
4 结论
(1) 不同的海洋区带通过电解液存在形式的不同影响S420钢的腐蚀速率、腐蚀产物和局部腐蚀行为。
(2) S420钢在潮差环境下腐蚀速率最高,大气区环境下腐蚀速率最低。在潮差环境的干燥期,腐蚀产物中较为充足的水含量和充足的氧供应是促进阴阳极反应的关键。
(3) S420钢的腐蚀产物由α-FeOOH、γ-FeOOH和Fe3O4组成。电解液层的持续存在有利于γ-FeOOH的形成,使其在潮差和全浸环境中含量较高。充足的O2和干湿循环促进了Fe3O4的生成,导致Fe3O4相对含量在飞溅区较高。
(4) S420钢在潮差和飞溅环境下表现出最大点蚀深度和高点蚀体积的数量较多。含Cl-和干湿循环的环境是点蚀萌生和长大的关键。
参考文献
Corrosion and pitting of 6060 series aluminium after 2 years exposure in seawater splash, tidal and immersion zones
[J].
Comparative study of the stress corrosion behavior of a multiuse bainite steel in the simulated tropical marine atmosphere and seawater environments
[J].
Comparative study of the SCC behavior of E690 steel and simulated HAZ microstructures in a SO2-polluted marine atmosphere
[J].
Passivation behavior and surface chemistry of 2507 super duplex stainless steel in acidified artificial seawater containing thiosulfate
[J].
Corrosion behavior of steel in Chengdao offshore oil exploitation area
[J].
Influence of outer rust layers on corrosion of carbon steel and weathering steel during wet–dry cycles
[J].
Corrosion behavior of X65 pipeline steel: comparison of wet-dry cycle and full immersion
[J].
Electrochemical behavior of rust formed on carbon steel in a wet/dry environment containing chloride ions
[J].The iron rust phase was analyzed by using the in-situ x-ray diffraction (XRD) and alternating current (AC) impedance methods after a wet/dry corrosion test using sodium chloride (NaCl) solution, which is the main composition of airborne saline particles. The corrosion content of the carbon steel depended on the concentration of Cl ions in the environment of the test chamber. As the concentration of Cl ions increased, the content of β-FeOOH increased in iron rust phases. The transition of β-FeOOH from the green rust I (GRI) was observed directly by in-situ XRD. The amount of GRI depended on the concentration of Cl ions, and β-FeOOH was transformed from GRI automatically in the dry process of the test. AC impedance showed that the resistance of the rust (Rrust) increased with the number of cycles in the corrosion test, and that the structural factor of the rust became predominant in Rrust. With the increase of the amount of rust, the resistance corresponding to the corrosion rate (Rt decreased, which was related to the reduction of β-FeOOH in the rust phase.
Effect of cathodic potentials on the SCC behavior of E690 steel in simulated seawater
[J].
The changing topography of corroding mild steel surfaces in seawater
[J].
Comparative study on corrosion behaviour of rusted X100 steel in dry/wet cycle and immersion environments
[J].
Mechanism of atmospheric rusting
[J].
Corrosion behavior of Q235 steels in atmosphere at Deyang district for one year
[J].
Q235钢在德阳大气环境中腐蚀行为研究
[J].通过失重实验、宏观形貌观察、SEM分析、腐蚀产物分析和电化学测试研究了电网设备主要金属材料碳钢在四川德阳地区暴露1 a的大气腐蚀行为。结果表明,在四川德阳3个变电站环境下碳钢的平均腐蚀速率分别为13.8、23.47和40.18 μm/a,除锈后碳钢表面存在大量点蚀坑。德阳不同地区暴露碳钢的腐蚀产物主要由α-FeOOH、γ-FeOOH和Fe<sub>3</sub>O<sub>4</sub>组成,腐蚀严重地区锈层中α-FeOOH组分比例有所增加。电化学结果表明,在重工业环境下碳钢腐蚀严重,腐蚀电流密度大,锈层电阻和电荷转移电阻增大。这一结果进一步说明碳钢表面形成的锈层在一定程度上能有效保护基体,减缓基体的进一步腐蚀。
Analysis of localized corrosion mechanism of 2024 aluminum alloy at a simulated marine splash zone
[J].
On the localized corrosion of AA5083 in a simulated dynamic seawater/air interface—Part 1: corrosion initiation mechanism
[J].
A study on corrosion of X80 steel in a simulated tidal zone
[J].
The influence of macrofouling on the corrosion behaviour of API 5L X65 carbon steel
[J].Seawater is a complex corrosive system, and biofouling is one of the factors that influences corrosion processes. The behaviour of corrosion associated with the development of macrofouling was investigated during the first 6 months of the successional process. Three treatments were compared: the 'Control' treatment (absence of macrofouling); 'Community' treatment, and 'Barnacle' treatment, where other macroorganisms were excluded. In the Community treatment, the dominant organisms were filamentous macroalgae (23.73%), barnacles (17.51%), hydroids (16.96%) and encrusting bryozoans (9.58%). In the Barnacle treatment, the cover varied between 39.38% and 62.50%. The corrosion potential ranged from -665.75 to -517.50 mV(Ag/AgC l((KCl))) and could not be associated with fouling development. The highest corrosion rate in the control suggests that macrofouling provides a protection against mass loss. The highest percentage of localised attacks was found in the Community treatment. This may indicate that not only barnacles, but also other organisms induce localised corrosion.
SEM/micro-raman characterization of the morphologies of marine atmospheric corrosion products formed on mild steel
[J].
Marine atmospheric corrosion of carbon steel: a review
[J].
A review: microbiologically influenced corrosion and the effect of cathodic polarization on typical bacteria
[J].
Electrochemical investigation of increased carbon steel corrosion via extracellular electron transfer by a sulfate reducing bacterium under carbon source starvation
[J].
Effects of anaerobe in sea bottom sediment on the corrosion of carbon steel
[J].
Composition and protective ability of rust layer formed on weathering steel exposed to various environments
[J].
Atmospheric corrosion behavior of Q 460 and Q690 low alloy steels in antarctic environment
[J/OL].
南极大气环境下Q460和Q690低合金钢的腐蚀行为
[J/OL].
Insight to atmosphere corrosion behavior of Q345NH steel in Wenchang tropical marine environment
[J].
On how the corrosion behavior and the functions of Cu, Ni and Mo of the weathering steel in environments with different NaCl concentrations
[J].
Corrosion evolution and stress corrosion cracking behavior of a low carbon bainite steel in the marine environments: effect of the marine zones
[J].
Quantitative study of the corrosion evolution and stress corrosion cracking of high strength aluminum alloys in solution and thin electrolyte layer containing Cl-
[J].
Atmospheric corrosion characteristics and regularity of the Q235, 40Cr steels commonly-used in power grid equipment in Anhui Province
[J].
安徽省内电网设备常用Q235和40Cr钢大气腐蚀特性及其规律
[J].针对在安徽省内代表性变电站站点自然环境下曝露1和3 a后的Q235、40Cr钢试样,开展腐蚀产物、腐蚀层形貌的研究,探讨其大气腐蚀机理。采用失重法获取Q235和40Cr钢试样的腐蚀速率,结合安徽省各相关地市的主要环境因素数据,再采用灰色关联分析方法,研究主要环境因素对1和3 a期Q235、40Cr钢试样大气腐蚀的影响规律。结果表明,Q235和40Cr钢试样大气腐蚀产物为FeOOH、Fe<sub>3</sub>O<sub>4</sub>、Fe(OH)<sub>3</sub>及FeSO<sub>4</sub>;腐蚀层表面密布着棉花球状的α-FeOOH,其间分布着片状的γ-FeOOH,腐蚀层结构较致密,但发生层状开裂。安徽省内Q235和40Cr钢试样大气腐蚀等级均在C2和C3等级,两者无明显差别。影响Q235和40Cr钢试样1 a期大气腐蚀的环境因素关联度排序为:NO<sub>2</sub>>温度>SO<sub>2</sub>>相对湿度>O<sub>3</sub>;随着曝露时间延长至3 a,该关联度排序改变为:SO<sub>2</sub>、温度>NO<sub>2</sub>>相对湿度>O<sub>3</sub>。
Fundamental investigation of stress corrosion cracking of E690 steel in simulated marine thin electrolyte layer
[J].The mechanism of stress corrosion cracking (SCC) of E690 high-strength steel in a marine thin electrolyte layer (TEL) was investigated by performing in-situ mechanical-electrochemical tests, slow strain rate tensile (SSRT) tests, and characterization of corrosion morphology. It was concluded that E690 steel was highly sensitive to SCC, which was jointly determined by local anodic dissolution (AD) and hydrogen embrittlement (HE) both caused by dissolved O-2. In addition to these functions, hydrogen oxidation catalyzed by ferric ion was found. There was a critical oxygen concentration, approximately 21% by volume, between these two different roles. Below this value, the increase in the oxygen concentration promoted the synergistic effect of AD and HE, resulting in the increase in SCC susceptibility. However, above this value, worse general corrosion offset crack initiation as well as the oxidation of hydrogen catalyzed by ferric ions reduced the SCC susceptibility.
Corrosion behavior of Q690qE steel in a simulated coastal-industrial environment
[J].
Q690qE桥梁钢在模拟滨海工业环境中的腐蚀行为研究
[J].
Corrosion mechanism of materials in three typical harsh marine atmospheric environments
[J].
几种苛刻海洋大气环境下的海工材料腐蚀机制
[J].以南极低温高辐照冰雪凝-融环境、南海高温高湿高盐雾环境以及滨海氯-霾耦合环境3种典型环境为研究对象,开展了典型海工材料的腐蚀行为研究。结果表明,南极低温环境下冰层、雪层覆盖下电化学腐蚀过程依然可以发生,冰雪凝-融过程导致液膜长周期存在促进了腐蚀的进行且加速局部腐蚀。南海高温高湿高盐雾环境下有色金属材料表面存在化学氧化和电化学腐蚀协同作用机制,不同铝合金的局部腐蚀萌生扩展驱动力不同 (即扩散与电荷转移、氢致沿晶裂纹、腐蚀产物楔入效应),表面润湿时间和Cl<sup>-</sup>协同作用导致腐蚀动力学偏离幂函数规律。滨海氯-霾耦合环境下NH<sub>4</sub><sup>+</sup>加速腐蚀的关键控制因素为缓冲效应导致的持续供H<sup>+</sup>,Cl<sup>-</sup>、NH<sub>4</sub><sup>+</sup>、NO<sub>3</sub><sup>-</sup>协同作用下镁合金发生“类自催化点蚀”。
Model for corrosion of metals covered with thin electrolyte layers: pseudo-steady state diffusion of oxygen
[J].
Fundamental understanding on the effect of Cr on corrosion resistance of weathering steel in simulated tropical marine atmosphere
[J].