表面活性剂分子结构中含有亲水基和亲油基,作为缓蚀剂时,亲水基团吸附在金属表面,而亲油基则背对钢表面形成一层疏水屏障[4]。阳离子表面活性剂对钢在酸中的腐蚀有良好的缓蚀效果,如溴化十六烷基吡啶[5]、十六烷基三甲基溴化铵[6]、十六烷基苄基二甲基氯化铵[7]、木质素胺[8]等。季铵盐型阳离子表面活性剂具有杀菌作用,季铵亲水基团可通过物理和化学吸附在金属/溶液界面,而疏水烷基链则背对金属表面形成疏水屏障,因而会表现出缓蚀性能。十二烷基二甲基苄基氯化铵(1227)对钢在HCl中具有优良的缓蚀性能,最大缓蚀率可高达99%[9]。然而,1227在金属表面的吸附与腐蚀介质密切相关,其在有机酸介质中冷轧钢表面的吸附及缓蚀性能研究报道目前较为少见。
1 实验方法
冷轧钢(攀枝花钢铁厂生产,厚度为0.60 mm)试样的各元素含量(质量分数,%)为:C 0.06、Si 0.02、Mn 0.21、P 0.012、S 0.01、余量为Fe。丙酮(CH3COCH3)、三氯乙酸(Cl3CCOOH)为分析纯;十二烷基二甲基苄基氯化铵(1227)为化学纯(纯度98%)。
由于三氯乙酸酸性较强,对金属有较强腐蚀作用,结合本课题组前期研究结果[11,12],本缓蚀体系中选取0.10 mol/L Cl3CCOOH。将尺寸为25.0 mm ×20.0 mm × 0.50 mm的钢片试样依次用60目、500目、1000目、1500目砂纸打磨,经蒸馏水洗净后用丙酮脱脂,冷风烘干,保存在干燥真空容器中。准确称量后,在20、30、40和50℃恒温条件下,将其浸没悬挂于含有0~100 mg/L 1227的250 mL的0.10 mol/L Cl3CCOOH溶液中6 h。实验取出后蒸馏水洗净、干燥并称重,计算出钢片腐蚀反应前后质量差,并通过其计算得出相对的腐蚀速率(v)和缓蚀率(ηw )[13]。
通过PARSTAT 2273电化学工作站进行电化学测试,测试软件为Power suite。采用三电极体系,以饱和甘汞电极(SCE)为参比电极、辅助电极(铂电极),工作电极为环氧树脂灌封的冷轧钢片(工作面积1 cm2)。测试前用系列砂纸打磨工作电极裸露面,用蒸馏水洗净后,全浸于测试溶液内30 min,开路电位稳定后测试。动电位极化曲线测量相关参数:扫描速率0.50 mV/s,扫描区间-250~+250 mV。EIS测试频率为105~10-2 Hz,交流激励幅值为10 mV,数据采集点为30个。
采用Sigma 300型扫描电子显微镜(SEM)、Bruker Dimension ICON原子力显微镜(AFM)和JC2000C1接触角测量仪对冷轧钢片试样进行SEM、AFM和接触角表面分析测试。
2 结果与讨论
2.1 失重法测试缓蚀作用
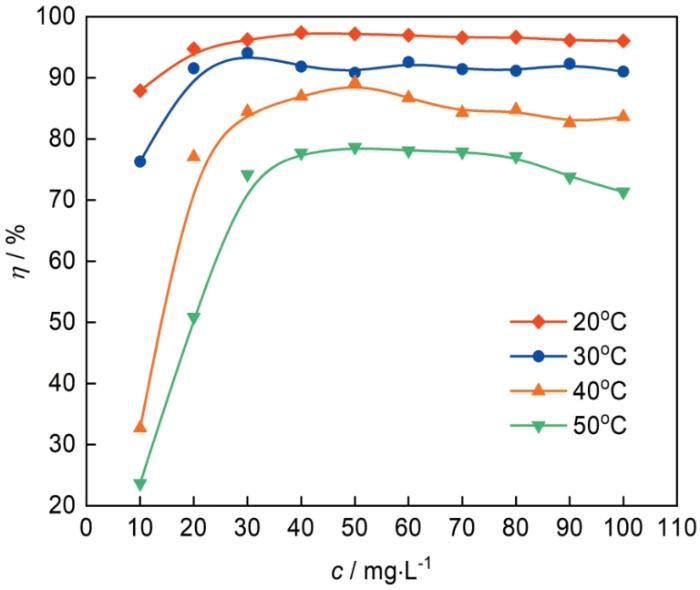
图1为各温度下冷轧钢在0.10 mol/L Cl3CCOOH溶液中的腐蚀速率(v)与1227浓度(c)的关系曲线图。空白的0.10 mol/L Cl3CCOOH中v分别为15.97 g·m-2·h-1 (20℃)、24.48 g·m-2·h-1 (30℃)、36.20 g·m-2·h-1 (40℃)和40.93 g·m-2·h-1 (50℃)。随着1227浓度的增大,v显著下降,当1227含量为50 mg·L-1时,v已大幅度下降至0.45 g·m-2·h-1 (20℃)、2.25 g·m-2·h-1 (30℃)、3.90 g·m-2·h-1 (40℃)和8.73 g·m-2·h-1 (50℃)。当缓蚀剂浓度相同时,温度越高,v变得越大,说明随着温度升高,钢表面析氢腐蚀加快;而当1227浓度超过一定值(30 mg/L)后,各温度下的v不再随1227浓度的增加而发生显著变化,这说明随着1227浓度的增加,其在钢表面吸附趋于饱和。
图1
图1
0.10 mol/L Cl3CCOOH溶液中腐蚀速率(v)与1227浓度(c)的关系曲线
Fig.1
Relation curves between corrosion rate (v) and 1227 concentration (c) in 0.10 mol/L Cl3CCOOH solution
图2
图2
0.10 mol/L Cl3CCOOH溶液中缓蚀率(ηw )与1227浓度(c)的关系曲线
Fig.2
Relation curves between inhibition efficiency (ηw ) and 1227 concentration (c) in 0.10 mol/L Cl3CCOOH solution
不同温度下,最大缓蚀率分别为97.4% (20℃)、94.1% (30℃)、89.2% (40℃)和78.7% (50℃)。这说明在0.1 mol/L Cl3CCOOH中1227对钢具有优异的缓蚀作用。缓蚀率总体上随温度升高而下降,这是由于温度升高后,Cl3CCOOH对冷轧刚的腐蚀作用加强,1227在钢表面的吸附强度减弱,或已在钢表面吸附的12227发生脱附致使缓蚀率下降[14]。
2.2 吸附等温式
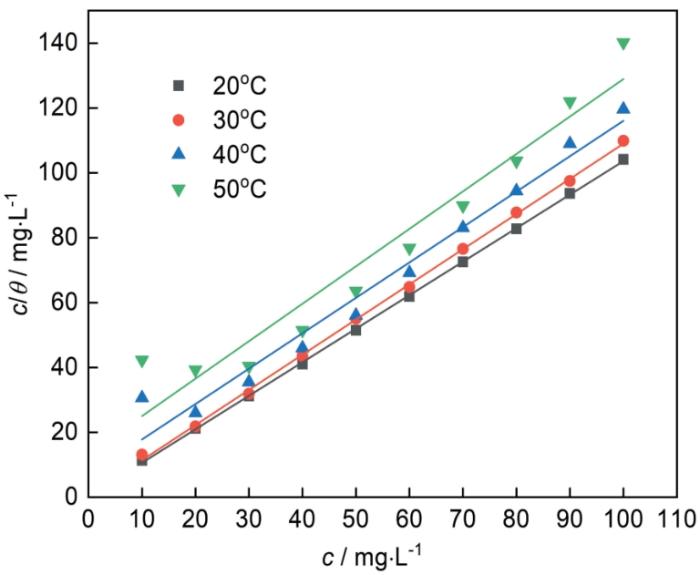
阳离子表面活性剂1227的缓蚀性能与其在钢表面的吸附密切相关。采用Langmuir吸附方程式对实验数据进行拟合[15]:
图3
图3
1227在钢表面的Langmuir吸附等温式
Fig.3
Langmuir adsorption isotherm of 1227 on steel surface
表1 c/θ-c直线回拟合参数
Table 1
| Temperature / oC | r2 | slope | K / L·mg-1 |
|---|---|---|---|
| 20 | 0.9998 | 1.03 | 3.40 |
| 30 | 0.9993 | 1.08 | 1.74 |
| 40 | 0.9727 | 1.09 | 0.15 |
| 50 | 0.9404 | 1.15 | 0.07 |
2.3 吸附热力学参数
在金属/溶液界面上的缓蚀剂吸附过程主要通过取代已吸附在金属表面的水分子,可通过(2)式计算标准吸附Gibbs自由能(ΔG0)[17]:
式中,R为气体常数,T为热力学温度,ρsolvent为该溶液中溶剂水的质量浓度(此处取其近似值106 mg/L)[17]。
吸附平衡常数(K)和温度(T)之间的关系满足Van't Hoff方程[18]:
式中,ΔH0为标准吸附焓(kJ·mol-1),B为不定积分常数。
lnK和1/T的拟合直线(r2 = 0.9673)如图4所示,故可根据拟合直线的斜率求出ΔH0。最后,标准吸附熵(ΔS0)可由
图4
上述计算所得热力学参数均列于表2中。
表2 1227在0.10 mol/L Cl3CCOOH溶液中钢表面的吸附热力学参数
Table 2
Temperature oC | ΔG0 kJ·mol-1 | ΔH0 kJ·mol-1 | ΔS0 J·mol-1·K-1 |
|---|---|---|---|
| 20 | -36.66 | -109.87 | -249.74 |
| 30 | -36.22 | -109.87 | -242.95 |
| 40 | -30.94 | -109.87 | -252.05 |
| 50 | -30.13 | -109.87 | -246.76 |
2.4 动电位极化曲线
图5
图5
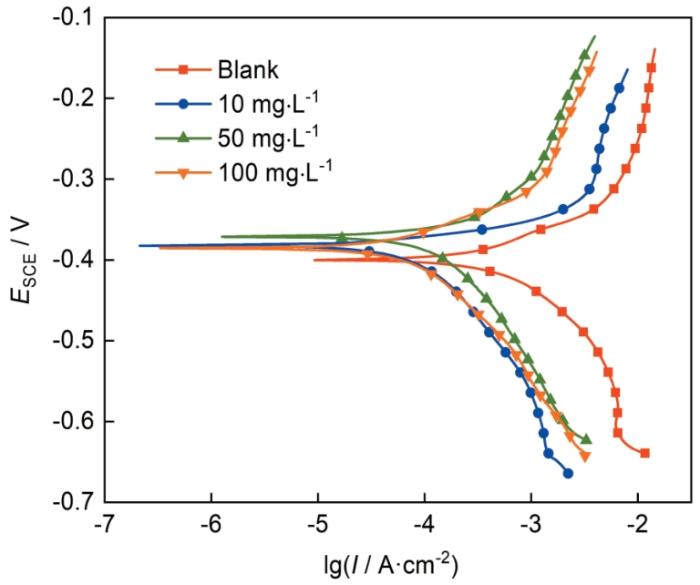
20℃时冷轧钢在0.10 mol/L Cl3CCOOH中不含和含不同1227浓度的动电位极化曲线
Fig.5
Potentiodynamic polarization curves of cold rolled steel without and with different concentrations of 1227 in 0.10 mol/L Cl3CCOOH solution at 20oC
用Tafel法拟合极化曲线后所得相关数据列于表3,通过(5)式可得到极化曲线法的缓蚀率(ηp):
其中,Icorr(0)和Icorr(inh)分别为钢电极在不含和含1227的0.10 mol/L Cl3CCOOH溶液中腐蚀电流密度。
表3 动电位极化曲线拟合参数
Table 3
| c / mg·L-1 | Ecorr / mV | bc / mV·dec-1 | ba / mV·dec-1 | Icorr / μA·cm-2 | ηp / % |
|---|---|---|---|---|---|
| 0 | -399 | -204 | 124 | 1125 | - |
| 10 | -382 | -240 | 40 | 189 | 83.2 |
| 50 | -371 | -206 | 101 | 180 | 84.0 |
| 100 | -385 | -187 | 85 | 148 | 86.8 |
由表3可知,空白Cl3CCOOH溶液中腐蚀电流密度Icorr为1225 μA·cm-2,而在添加1227后Icorr急剧降低,并且随着1227浓度的增大而减小;ηp的值与1227浓度呈正相关,1227浓度为100 mg/L时,ηp为86.8%,说明1227有良好的缓蚀性能,对钢在Cl3CCOOH中的电化学腐蚀有良好抑制效果。加入1227后腐蚀电位(Ecorr)变化幅度较小,表明1227在Cl3CCOOH溶液介质中为通过“几何覆盖效应”起作用的混合抑制型缓蚀剂。在1227浓度为50和100 mg/L时,阴、阳极的Tafel斜率(bc、ba)变化不大,说明浓度较高时,添加缓蚀剂后,体系中阴阳极的极化变化规律受到影响较小。
2.5 EIS
图6为20℃时钢在0.1 mol/L Cl3CCOOH溶液中不含和含有不同浓度1227的EIS图。由图6a可知,未添加缓蚀剂1227的Nyquist图由高频区的容抗弧和低频区的感抗弧组成;高频区的容抗弧表明钢在三氯乙酸介质中的腐蚀主要由电荷转移控制[22],而低频区的感抗弧可能与酸根离子(Cl3CCOO-)在电极表面的吸-脱附造成的不稳定状态有关[23]。值得注意的是,加入1227后整个Nyquist图谱则主要由容抗弧构成,低频区有小段的不完整的容抗弧,这主要与缓蚀剂在电极形成膜层有关[24]。容抗弧随着加入的1227浓度的增加而增大,说明1227有效抑制了钢在Cl3CCOOH溶液中受到的腐蚀。容抗弧不是完整的半圆,主要是由于钢腐蚀过程中电极/溶液界面的异质性、粗糙度等引起的弥散效应[25]。
图6
图6
20℃时钢在不同浓度1227的0.10 mol/L Cl3CCOOH溶液中的Nyquist和Bode图
Fig.6
Nyquist spectra (a), Bode modulus graphs (b) and Bode phase angle graphs (c) of steel in 0.10 mol/L Cl3CCOOH solution with different concentrations of 1227 at 20oC
式中,fmax为最大特征频率(Hz),a为弥散系数。
图7
表4 20℃时冷轧钢在不同1227的0.10 mol/L Cl3CCOOH溶液中的EIS参数
Table 4
| c | Rs | Rt | RL | Q | a | Cdl | χ2 | ηR |
|---|---|---|---|---|---|---|---|---|
| mg·L-1 | Ω·cm2 | Ω·cm2 | Ω·cm2 | μΩ-1·S a ·cm-2 | μF·cm-2 | % | ||
| 0 | 9.7 | 8.0 | 11.32 | 323 | 0.9397 | 223 | 3.5 × 10-3 | - |
| 10 | 11.3 | 137.1 | - | 146 | 0.7871 | 50 | 2.5 × 10-3 | 94.1 |
| 50 | 12.1 | 365.9 | - | 147 | 0.7018 | 46 | 2.4 × 10-3 | 97.8 |
| 100 | 11.7 | 409.8 | - | 179 | 0.7079 | 57 | 3.6 × 10-3 | 98.0 |
式中,Rt(inh)和Rt(0)分别为缓蚀溶液和空白溶液中的电荷转移电阻。各浓度的ηR均超过94%,故1227表现出良好的缓蚀性能。
2.6 SEM形貌
图8
图8
钢片表面的SEM像
Fig.8
SEM microscopic image of steel sheet surface: (a) polished surface, (b) after corrosion in 0.10 mol/L Cl3CCOOH solution for 6 h at 20oC, (c) after corrosion in 100 mg/L 1227 + 0.10 mol/L Cl3CCOOH solution for 6 h at 20oC
2.7 3D-AFM形貌
图9
图9
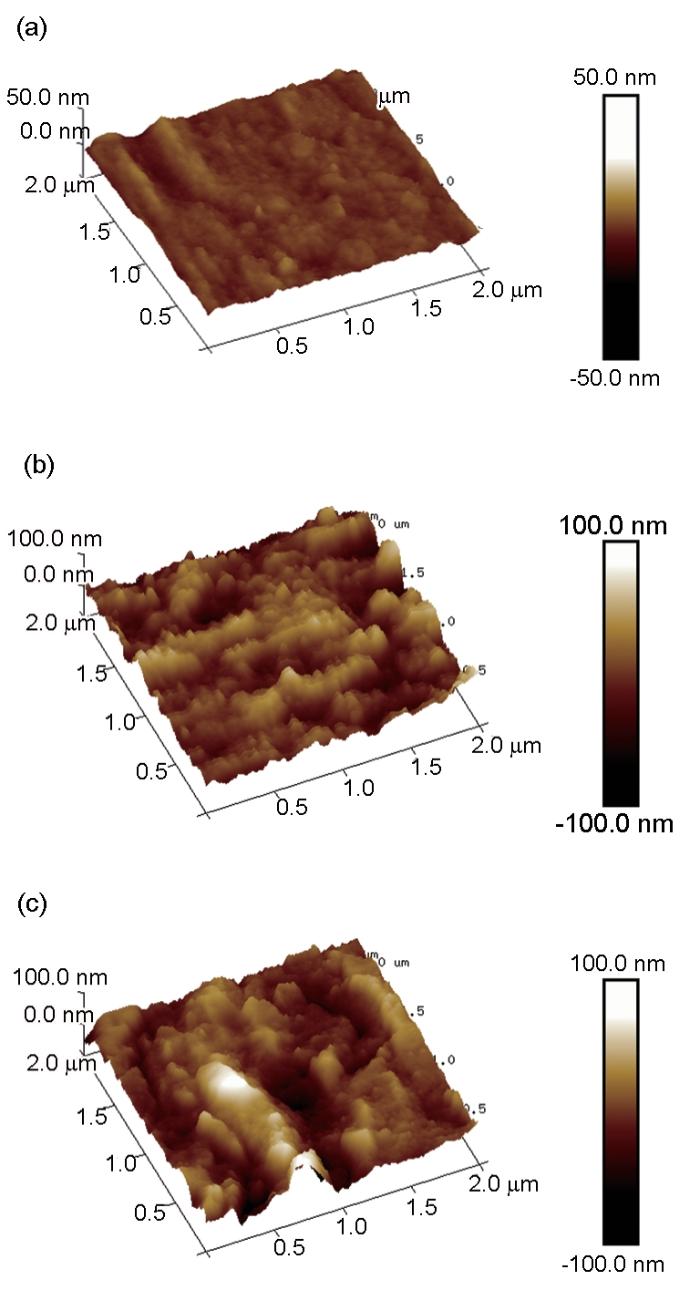
钢片表面的3D-AFM微观图像
Fig.9
3D-AFM microscopic images of steel sheet surfaces: (a) polished surface, (b) after corrosion in 0.10 mol/L Cl3CCOOH solution for 6 h at 20oC, (c) after corrosion in 100 mg/L 1227 + 0.10 mol/L Cl3CCOOH solution for 6 h at 20oC
AFM测试不仅能给出高分辨率的三维微观形貌,而且能给出表面粗糙度的定量化数值[27]。钢在0.10 mol/L Cl3CCOOH溶液浸泡前后的AFM微观形貌表面粗糙度参数见表5。其中Ra、Rq和Rmax分别为平均表面粗糙度、均方根表面粗糙度以及最大起伏度,从表中可以看出浸泡前的钢片3个参数均为最小,说明其表面最为平整。钢片浸没于0.10 mol/L Cl3CCOOH溶液中6 h后,相关数值均显著增大,说明此时钢片表面覆盖大量腐蚀产物,导致钢片表面粗糙。而在溶液中加入1227后,相关数值较未加缓蚀剂仍在一定程度上增加,这可能是由于缓蚀剂分子吸附在钢片产生的缓蚀层不均匀所引起的,或者部分区域钢片腐蚀较为严重导致表面粗糙度增大。
表5 钢表面的3D-AFM表面粗糙度参数
Table 5
| Steel | Ra / nm | Rq / nm | Rmax / nm |
|---|---|---|---|
| Before immersion | 1.81 | 2.36 | 19.2 |
| Cl3CCOOH | 15.3 | 19.1 | 134 |
| Cl3CCOOH+1227 | 16.9 | 22.1 | 172 |
2.8 接触角分析
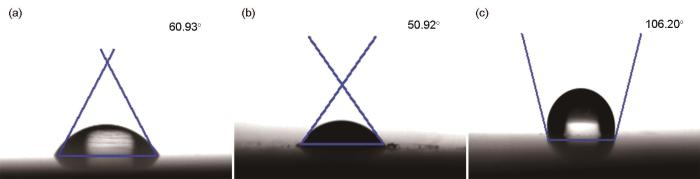
金属表面的疏水性强弱与缓蚀剂的吸附及其缓蚀性能密切相关[28],钢片在不同条件下接触角测试图如图10所示。图10a为浸泡前处理好的钢片表面,接触角为60.93°,说明钢是亲水性的,表明钢表面在Cl3CCOOH的水溶液中易被湿润发生腐蚀。图 10b显示了在0.10 mol/L Cl3CCOOH溶液中将钢片浸泡6 h后所测得的接触角降为50.92°,说明钢片的亲水性进一步增强,表明钢会在0.10 mol/L Cl3CCOOH溶液中不断被腐蚀。图11c是在添加100 mg/L 1227的 0.10 mol/L Cl3CCOOH溶液中将钢片浸泡6 h后所测得的接触角为106.20°,将打磨好的钢片与未加入1227的钢片相比,表明添加1227后,钢表面疏水性增强,且由原来的亲水性变为了疏水性。
图10
图10
钢片表面接触角分析
Fig.10
Analysis of contact angles of steel sheet surface: (a) polished surface, (b) after corrosion in 0.10 mol/L Cl3CCOOH solution for 6 h at 20oC, (c) after corrosion in 100 mg/L 1227 + 0.10 mol/L Cl3CCOOH solution for 6 h at 20oC
图11
图11
钢表面1227吸附作用示意图
Fig.11
Schematic diagram of 1227 adsorption on steel surface
2.9 缓蚀作用机理
冷轧钢在0.10 mol/L Cl3CCOOH溶液中发生析氢腐蚀(Fe + 2H+ → Fe2+ + H2),腐蚀速率与温度呈正相关。当向体系中加入1227后,腐蚀速率下降显著,说明1227缓蚀效果优良。1227在水中发生电离生成正离子的1227和Cl-。钢在酸溶液中发生析氢腐蚀时表面带正电荷[29],因此,溶液中的Cl-通过静电作用力特性吸附在钢表面[30],从而使钢表面带负电荷,以便使带正电荷的1227通过静电作用力而物理吸附在钢表面。在Cl3CCOOH溶液中,带负电荷Cl3CCOO-在钢表面吸附,从而使阳离子在钢表面更多吸附。然后由于静电的吸引,带正电荷的N原子也可以吸附在钢的表面。1227中N原子与Fe原子中的空3d轨道通过孤对电子形成配位键,从而以化学吸附的形式停留在钢表面。而1227中苯环的共轭
3 结论
(1) 1227对冷轧钢在0.10 mol/L Cl3CCOOH溶液中的腐蚀有较好的抑制效果,且缓蚀率与1227浓度呈正相关性,但当缓蚀剂1227浓度超过50 mg/L后,缓蚀率的变化程度很小,且随温度升高而降低;40 mg/L 1227的20℃时缓蚀率为97.39%。钢表面上1227吸附符合Langmuir吸附等温方程式,吸附过程是一个以化学吸附为主的放热过程。
(2) 1227为能同时有效抑制钢在三氯乙酸中阴极和阳极电化学腐蚀的混合抑制型缓蚀剂,作用机理为“几何覆盖效应”。Nyquist图的容抗弧随1227浓度增大而变大,电荷转移电阻增大;而Bode相位角的峰值增大。SEM和AFM测试结果说明1227能有效抑制钢表面的腐蚀,其缓蚀钢表面的接触角为钝角,即疏水性大幅度增强。
参考文献
Corrosion cost and preventive strategies in China
[J].
我国腐蚀成本及其防控策略
[J].
The cost of corrosion in China
[J].Corrosion is a ubiquitous and costly problem for a variety of industries. Understanding and reducing the cost of corrosion remain primary interests for corrosion professionals and relevant asset owners. The present study summarises the findings that arose from the landmark “Study of Corrosion Status and Control Strategies in China”, a key consulting project of the Chinese Academy of Engineering in 2015, which sought to determine the national cost of corrosion and costs associated with representative industries in China. The study estimated that the cost of corrosion in China was approximately 2127.8 billion RMB (~ 310 billion USD), representing about 3.34% of the gross domestic product. The transportation and electronics industries were the two that generated the highest costs among all those surveyed. Based on the survey results, corrosion is a major and significant issue, with several key general strategies to reduce the cost of corrosion also outlined.
Application of corrosion inhibitors for steels in acidic media for the oil and gas industry: a review
[J].
A review of surfactants as corrosion inhibitors and associated modeling
[J].
Adsorption and inhibitive action of hexadecylpyridinium bromide on steel in phosphoric acid produced by dihydrate wet method process
[J].
Corrosion inhibition and adsorption behavior of some cationic surfactants on carbon steel in hydrochloric acid solution
[J].
The influence of N-hexadecyl benzyl dimethyl ammonium chloride on the corrosion of mild steel in acids
[J].
Investigation of the adsorption behavior of dodecylamine on carbon steel
[J].
十二胺在碳钢表面的吸附行为
[J].
Corrosion inhibition of cold rolled steel in hydrochloric acid medium 1227
[A].
盐酸介质中1227对冷轧钢的缓蚀作用
[A].
Corrosion inhibition of 3s aluminium in trichloroacetic acid by methyl pyridines
[J].
Corrosion inhibition of anionic surfactant on steel in trichloroacetic acid medium
[J].
三氯乙酸介质中阴离子表面活性剂对钢的缓蚀性
[J].
Inhibition action of calcium lignosulphonate on cold rolled steel in trichloroacetic acid media
[J].
三氯乙酸介质中木质素磺酸钙对冷轧钢的缓蚀性能
[J].
Application of surfactants as corrosion inhibitor for different metals and alloys: a review
[J].
Experimental and computational studies on propanone derivatives of quinoxalin-6-yl-4,5-dihydropyrazole as inhibitors of mild steel corrosion in hydrochloric acid
[J].
A effect of sodium dodecylsulfate on the corrosion of copper in sulphuric acid media
[J].
Mechanism of temperature influence on adsorption of Schiff Base
[J].
温度影响席夫碱缓蚀剂吸附的机理研究
[J].研究了所合成的两种含有苯基基团的席夫碱缓蚀剂 (BB-S缓蚀剂和B-S缓蚀剂) 在不同温度下对N80钢在0.5%盐酸溶液中的缓蚀作用,探讨了温度影响席夫碱缓蚀剂的吸附机理。结果表明,BB-S缓蚀剂和B-S缓蚀剂的缓蚀效率随着温度的升高而降低,且B-S缓蚀剂的缓蚀效率在不同温度下始终大于BB-S缓蚀剂的缓蚀效率。分子动力学和量子化学计算方法表明,两种席夫碱缓蚀剂的缓蚀效率随温度的升高而降低,该现象与席夫碱缓蚀剂中苯环较大的空间位阻、分子热运动、分子吸附构型以及前线轨道能级密切相关。
Comprehensive investigation of steel corrosion inhibition at macro/micro level by ecofriendly green corrosion inhibitor in 15% HCl medium
[J].
The adsorption and corrosion inhibition of anion surfactants on aluminium surface in hydrochloric acid
[J].
A new insight into corrosion inhibition mechanism of copper in aerated 3.5 wt.% NaCl solution by eco-friendly imidazopyrimidine dye: experimental and theoretical approach
[J].
Comparative study of synergistic inhibition of mild steel and pure iron by 1-hexadecylpyridinium chloride and bromide ions
[J].The comparison of the corrosion behavior of mild steel (MS) and pure iron (PI), and synergistic action of 1-Hexadecylpyridinium chloride (HDPCI) and Br- ion are studied in 0.5 M sulphuric acid solution. The study was carried out using open-circuit potential, potentiodynamic polarization, and electrochemical impedance spectroscopy measurements. In absence of inhibitor (blank), the corrosion rate of mild steel is greater than that of pure iron. In presence of HDPCl only, the inhibition efficiency for MS corrosion in H2SO4 is higher than that of PI and attributed to the different surface charges on both MS and PI in H2SO4 solution. The obtained results indicate that the inhibition efficiency (eta(i)) of HDPCI is significantly increased in the presence of Br- ions for both MS and PI. In the presence of the Br- ions, the increases significantly at relatively low concentrations of HDPCl ([HDPCl] = 8 x 10(-6) M), Le, eta(i) is 96 and 99% for MS and PI, respectively. The synergism factor (S-theta) is found to be more than unity suggesting greater eta(i) due to the addition of Br- ions to the HDPCI with a co-operative adsorption action. Chemisorption is postulated as the mode of adsorption based on extracted thermodynamic parameters.
Two novel chitosan derivatives as high efficient eco-friendly inhibitors for the corrosion of mild steel in acidic solution
[J].
The inhibition of benzimidazole derivatives on corrosion of iron in 1 M HCl solutions
[J].
The inhibition of low carbon steel corrosion in hydrochloric acid solutions by succinic acid: Part I. Weight loss, polarization, EIS, PZC, EDX and SEM studies
[J].
Preparation and corrosion inhibition of super hydrophobic adsorption film of lotus leaf extract on mild steel
[J].
Q235钢表面的超疏水吸附层形成与缓蚀研究
[J].用新鲜荷叶作为研究对象,经过简便的乙醇回流萃取取得提取物。室温条件下,荷叶提取物能够在THF/HCl水溶液的混合溶液 (体积比为1/1,1.0 mol/L HCl溶液) 中产生聚集。傅立叶变换红外光谱以及X射线光电子能谱的结果证明了荷叶提取物在Q235钢样品表面发生化学作用,能够形成超疏水的吸附层。电化学结果表明荷叶提取物对碳钢在HCl溶液中具备良好的缓蚀性能,在0.4 g/L浓度下,最大缓蚀效率达到93.14%。
Experimental and theoretical investigation of inhibition behavior of 2-((4-(dimethylamino)benzylidene)amino) benzenethiol for carbon steel in HCl solution
[J].
Effect of ethylenediamine tetraacetic acid disodium on the corrosion of cold rolled steel in the presence of benzotriazole in hydrochloric acid
[J].
Electrochemical applications of in situ scanning probe microscopy
[J].
Preparation of superhydrophobic surface on copper substrate and its corrosion resistance
[J].
铜基超疏水表面的制备及其耐蚀性研究
[J].以十二硫醇作为疏水剂,采用化学刻蚀和高温氧化在铜基体上构造超疏水表面,以提高铜基体的耐蚀性。结果表明,当化学刻蚀8 min、高温氧化6 h、十二硫醇修饰15 min,基体表面形成了具有足够粗糙度并可以捕获大量空气的网状层叠结构,此时基体表面疏水性最好,水的接触角为165.50°。动电位极化曲线表明,超疏水表面的腐蚀速率明显降低,腐蚀电流密度由7.43×10<sup>-5</sup>下降至4.31×10<sup>-6</sup> A·cm<sup>-2</sup>。电化学阻抗谱表明,超疏水表面的电荷转移电阻明显高于铜基体,说明其具耐蚀性相较于铜基体也得到了提高。与当前制备超疏水表面的方法相比,本方法具有廉价、简单、环保的特点。
Corrosion behaviour of mild steel in formic acid solutions
[J].