ADC12铝合金是一种压铸铝合金,主要应用于汽车零部件。它可由废弃铝材再生,一定程度上缓解了铝材的供不应求。特殊环境下,铝合金在冶金过程中形成的金属化合物粒子(IMP)会加剧腐蚀,因此在实际工业应用中会采取保护措施[1,2]。传统的保护措施是六价铬钝化膜技术,但由于Cr6+的剧毒性和致癌性,这种技术已被国际组织明令禁止[3,4]。近年来出现了越来越多的新兴无铬替代技术,如稀有金属转化膜[5]、溶胶-凝胶转化膜[6]、有机树脂转化膜[7]、锆钛基转化膜[8]、微弧氧化膜[9,10]和阳极氧化膜[11,12]等。其中,锆钛基转化膜技术因为成本低、易于过程控制、降低能耗和避免广泛的废水处理方面具有优势最早实现工业化[13~15]。Peng等[16]研究表明,金属间化合物和基底之间的伏特电位差形成的微电化学电池是形成锆钛基转化膜的驱动力,驱动力随着转化膜的增长逐渐减弱。先进的锆钛基转化膜通常以锆钛的成膜特性为基础,加入各种功能添加剂以确保不同金属 (如钢,镀锌钢和铝) 上所需的薄膜结构,涂层厚度通常不超过20~100 nm,主要成分是TiO2/ZrO2[17]。Zhang等[18]以K2ZrF6和K2TiF6为基料,KMnO4为氧化剂,NaF为催化剂,氟硅烷 (FAS-17) 为表面改性添加剂,制备出了耐蚀性良好的转化膜。Zhan等[19]将样品在含有钛/锆基离子、偏磷酸钠和硝酸铈六水合物的处理溶液中浸渍,制备了一种新型化学转化涂层,转化涂层下铝合金的腐蚀电位为-0.577 V,腐蚀电流密度为0.1148 μA/cm2。Liao等[20]以含有氟钛酸、氟锆酸、偏钒酸铵和单宁酸成分的转化液在低温条件下制备的锆钛转化膜极大地提高了铝合金的耐蚀性。
转化液的诸多成分客观上增加了工业应用的成本及难度。胺基双硅烷水溶液 (ABSE) 的硅醇密度高,可提供优异的交联密度与附着力。本文使用ABSE与氟锆酸简单复配,通过它们的协同作用在铝合金表面获得了均匀致密的转化膜。通过场发射扫描电镜 (FESEM)、Fourier变换红外光谱分析仪 (FT-IR) 与X射线光电子谱 (XPS) 研究了转化膜的形貌、结构和成分,通过电化学测试和盐雾实验等研究了转化膜下铝合金的耐蚀性能。
1 实验方法
本实验使用48 mm×75 mm×1.8 mm ADC12铝合金板进行硫酸铜点滴测试、电化学测试实验和盐雾实验;用10 mm×10 mm×1.8 mm ADC12铝合金片进行FESEM分析;用转化膜粉末进行FT-IR与XPS测试。预处理过程:依次用240目、600目和1000目SiC砂纸打磨铝板至表面光滑均亮,然后将铝板放在2% (质量分数) 的40 ℃碱洗液 (Ridoline 336) 中超声1.5 min,再将铝合金放在3% (质量分数) 的40 ℃表调液 (Aluetch) 中处理1.5 min,最后用去离子水将铝板冲洗干净。
2.5 g 45% (质量分数) H2F6Zr溶液分别与37.5, 27.5, 17.5和7.5 g水混合后,将10, 20, 30和40 g 30% (质量分数) ABSE匀速滴入搅拌的H2F6Zr溶液中,搅拌30 min后得到不同ABSE含量的复合转化原液。将预处理好的样品放在转化液(转化原液使用量:5%,温度:35 ℃)中化学反应6 min,然后用去离子水冲洗表面1~2 min,最后放在110 ℃的烘箱内固化30 min后得到转化膜。锆转化膜使用的转化液不含ABSE,制备方式与硅锆复合转化膜相同。
采用NANO SEM430型FESEM观察转化膜表面微观形貌。采用Nicolet IS10型FT-IR研究ABSE与转化膜的结构。采用K-Alpha型XPS研究了转化膜的成分。
参照GB6807-86进行硫酸铜点滴测试,点滴液组分:CuSO4·5H2O:41 g/L,NaCl:35 g/L,0.1 mol/L的HCl:13 mL/L。采用CHI660E型电化学工作站测试稳态极化曲线与电化学阻抗,采用三电极体系,其中参比电极是饱和甘汞电极,辅助电极为铂丝电极,3.5% (质量分数) NaCl溶液作为测试溶液。用环氧树脂密封铝合金表面,留出1 cm2作为测试工作面。极化曲线参数:扫描速率 0.01 V·s-1,灵敏度10-3A/V。电化学阻抗谱 (EIS) 参数:频率范围10-2~105 Hz,扫描振幅0.005 V。测试完成后用Zview软件对电化学阻抗进行拟合,用CHI660E分析软件对极化曲线进行拟合得到相关参数。采用YWX/Q型盐雾箱进行中性盐雾实验,温度 (35±2) ℃,NaCl浓度 (50±5) g/L,pH 6.5~7.2之间,定期观察样品在不同时间盐雾实验后的外观变化,拍照记录。
2 结果与讨论
2.1 ABSE含量的优化
2.1.1 硫酸铜点滴测试
为了方便叙述,本论文以转化原液为20%,40%,60%和80% (质量分数) ABSE的转化液制备的转化膜分别用20Si-ZrCC、40Si-ZrCC、60Si-ZrCC和80Si-ZrCC代替。硫酸铜点滴测试显示空白样的耐点滴时间只有6 s,对点滴液的防护能力很弱,耐蚀性能很差。相比于空白样,不加ABSE的锆转化膜耐点滴时间增加至23 s,说明锆转化膜对铝合金的耐蚀性能有一定的提高,这是因为锆转化膜中的主要物质是锆氧化物,锆氧化物的耐蚀性远强于铝合金表面自身的铝氧化物。随着ABSE含量的增加,硅锆复合转化膜的耐点滴时间先增加后减小。20Si-ZrCC和40Si-ZrCC耐点滴时间分别为47和63 s,60Si-ZrCC为79 s耐点滴时间最优,分别是空白样和单一锆转化膜耐点滴时间的13倍和3倍以上。
2.1.2 极化曲线测试
空白样与不同转化膜的极化曲线见图1,极化曲线拟合数据列于表1。自腐蚀电位 (Ecorr)、自腐蚀电流密度 (Icorr) 可以通过CHI660B自带的极化曲线外推法得到,小孔腐蚀电位 (Epit) 可直接在CHI660B软件中读出,钝化区 (ΔE) 可通过公式ΔE=Epit-Ecorr得到。极化曲线中的Ecorr可以反映出转化膜被腐蚀的难易程度,Ecorr越正代表转化膜越难被腐蚀;Icorr可以反映出转化膜的腐蚀速率,Icorr越小代表着转化膜的腐蚀速率越慢;ΔE可以反映出转化膜发生点腐蚀的难易程度,ΔE越宽转化膜越难发生点腐蚀[21~24]。从图1与表1可以看出,随着ABSE含量的增加,转化膜的Ecorr先增大后减小;Icorr先减小最后增大。其中,60%含量的ABSE硅锆复合转化膜耐蚀性能最优,相比于空白样,Ecorr正移了0.297 V,比单一锆转化膜正移了0.211 V;Icorr比空白样减小了约95.4%,比单一锆转化膜减小了约90.9%。空白样与单一锆转化膜不存在明显的钝化区,而60Si-ZrCC出现了明显的钝化区,宽为0.176 V。通过以上分析可以看出,ABSE的引入可以明显改善膜层的耐蚀性,硅锆复合转化膜的耐蚀性明显要强于单一的锆转化膜。另外,当转化原液中的ABSE含量为60%时与H2F6Zr的协同效果最好,耐蚀性能最优。
图1
表1 不同样品的极化曲线数据
Table 1
| Sample | Ecorr / V | Epit / V | ΔE / V | Icorr / (A·cm-2) |
|---|---|---|---|---|
| Blank | -1.201 | - | 0 | 31.63×10-7 |
| ZrCC | -1.046 | - | 0 | 15.86×10-7 |
| 20Si-ZrCC | -1.043 | - | 0 | 5.902×10-7 |
| 40Si-ZrCC | -0.921 | -0.766 | 0.155 | 1.950×10-7 |
| 60Si-ZrCC | -0.904 | -0.727 | 0.176 | 1.44×10-7 |
| 80Si-ZrCC | -1.046 | - | 0 | 5.421×10-7 |
通过硫酸铜点滴测试与极化曲线测试确定了转化原液中ABSE的含量,使用60%时钝化效果最优。
2.2 转化膜的结构、形貌及成分分析
2.2.1 FT-IR分析
锆转化膜与硅锆复合转化膜粉末的红外光谱见图2。图中2906 cm-1是由C-H的伸缩振动引起的,3425 cm-1是由Si-OH的伸缩振动引起的。这两个峰的强度明显增强,说明ABSE成功地与基体相结合,并参与了成膜过程。另外,与锆转化膜的红外光谱相比,硅锆复合转化膜出现了3个新峰,推测它们分别是670 cm-1处由Si-O-Al的伸缩振动引起的红外峰,1085 cm-1处由Si-O-Zr的伸缩振动引起的红外峰及1150 cm-1处由Si-O-Si的伸缩振动引起的红外峰。说明ABSE上的Si-OH不仅发生了彼此的交联反应,还可能与基体表面的Al-OH、溶液中的活性基团Zr-(OH)4发生缩合反应生成了Si-O-Al结构与Si-O-Zr结构。
图2
2.2.2 FESEM分析
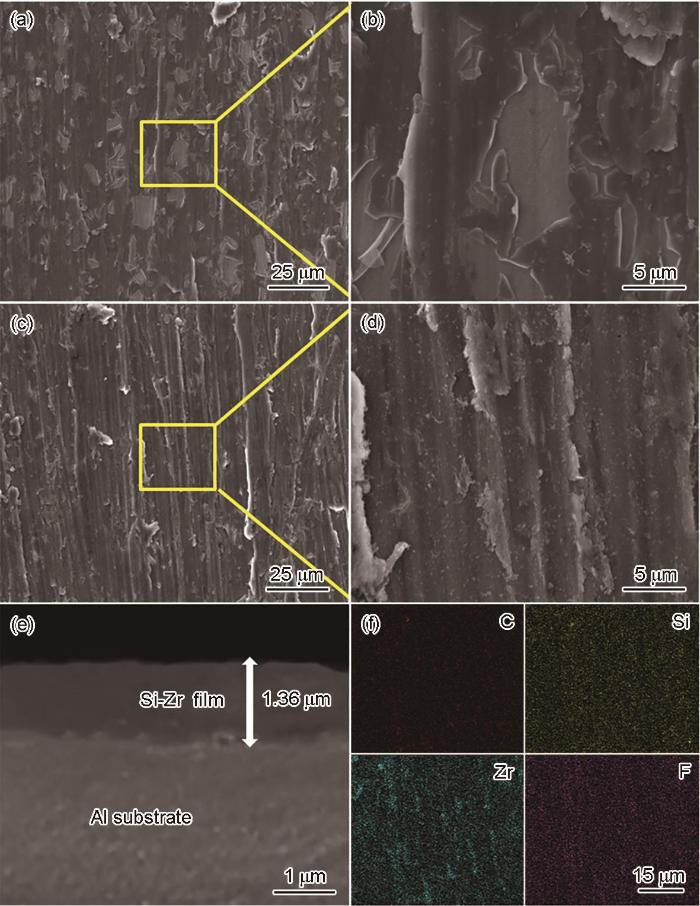
图3是锆转化膜与硅锆复合转化膜在不同放大倍数下的微观形貌图。从图3a和c可以看出,锆转化膜表面膜层有很多明显的裂纹,部分区域没有被转化膜覆盖。单一的锆转化膜属于脆性材料,抗断强度低、延展性低,导致膜层在固化过程中因内应力出现脱落与裂开的现象。图3c和d可以看出,硅锆复合转化膜在高放大倍数下观察不到微裂纹,且从图3e可以看出,硅锆复合转化膜均匀完整地覆盖在基体上,厚度约为1.36 μm。从图3f可以看出,来自ABSE的C、Si呈均匀分布。这些现象说明ABSE有效参与了成膜并改善了膜层的脆性,可协同氟锆酸在基体表面生成较厚的致密转化膜。另外,从FT-IR分析结果推测致密转化膜形成的原因可能是:ABSE是有机大分子软链,硅醇Si-OH密度高可发生彼此间的高强度交联,通过Si-OH与Zr-(OH)4的缩合可与锆氧化物等无机物质杂糅在一起,甚至将它们包裹起来;ABSE两头的硅醇基Si-OH可与基体表面Al-OH发生缩合反应形成稳定的Si-O-Al结构,通过这些反应,转化膜中的各个物质彼此紧密交联,固化时不易脱落与裂开。致密的转化膜可以有效阻止氧气与腐蚀介质的侵入,对铝合金有非常好的保护效果。
图3
图3
锆转化膜与硅锆复合转化膜的FESEM和硅锆复合转化膜的EDS谱
Fig.3
FESEM of zirconium conversion film (a, b) and silicon-zirconium composite conversion film (c-e) and EDS mappings of silicon-zirconium composite conversion film (f)
2.2.3 XPS分析
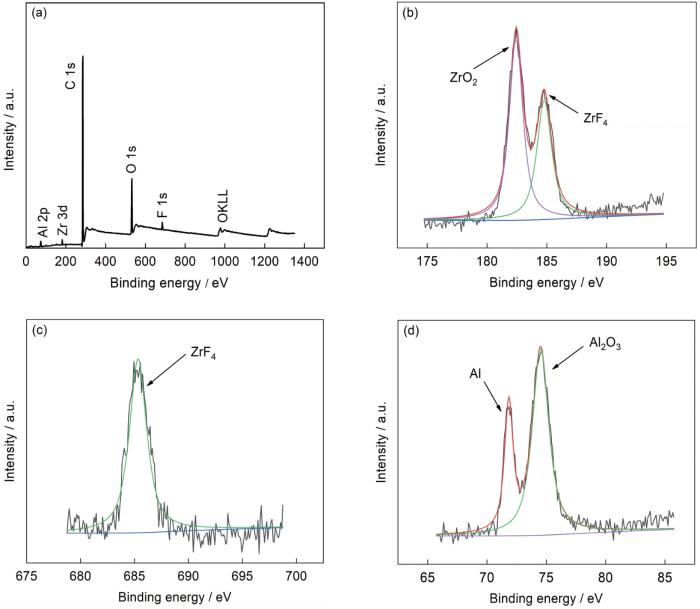
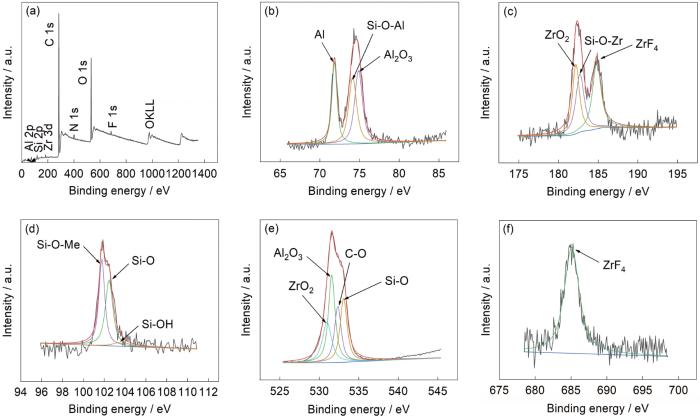
使用XPS测试转化膜的元素原子含量见表2。可以看出,相较锆转化膜,硅锆复合转化膜C的原子百分比有了明显增长,且出现了N(0.75%)与Si(1.95%)。N与Si的原子比值接近于ABSE中N与Si的原子比值1∶2,这进一步说明ABSE有效参与了铝合金表面的成膜过程。锆转化膜和硅锆复合转化膜全谱扫描结果及各元素的窄扫描谱图分别见图4和5。从Al2p的拟合结果可以看出,结合能72 eV的峰对应着Al,结合能74.7 eV的峰对应着Al2O3,硅锆复合转化膜多出Si-O-Al的74.1 eV峰,推测ABSE的硅醇Si-OH在成膜过程中与铝合金基体表面发生了反应生成了铝络合物,与红外测试结果一致。从Zr3d的拟合结果可以看出,结合能182.2 eV的峰对应着ZrO2,结合能184.9 eV的峰对应着ZrF4,硅锆复合转化膜多出Si-O-Zr的182.8 eV峰,推测ABSE的硅醇Si-OH在成膜过程中与溶液中的活性基团Zr-(OH)4发生交联形成Si-O-Zr结构,生成了锆络合物,与红外测试结果一致。从Si2p的拟合结果可以看出,结合能101.52 eV的峰对应着Si-O-Me,结合能102.46 eV的峰对应着Si-O,结合能103.38 eV的峰对应着Si-OH。从O1s的拟合结果可以看出,结合能530.9 eV的峰对应着ZrO2,结合能531.3 eV的峰对应着Al2O3,结合能532.5 eV的峰对应着C-O,结合能532.9 eV的峰对应着Si-O。综上所述,可见转化膜的主要成分是由锆络合物、铝络合物、ZrF4、Al2O3与ZrO2组成。
表2 不同转化膜的各个元素的原子百分含量 (atomic fraction / %)
Table 2
| Sample | Al | C | F | N | O | Si | Zr |
|---|---|---|---|---|---|---|---|
| Zr film | 6.02 | 57.67 | 8.13 | - | 26.39 | - | 1.79 |
| Si-Zr film | 3.64 | 73.08 | 0.98 | 0.75 | 19.36 | 1.95 | 0.23 |
图4
图4
锆转化膜的XPS谱
Fig.4
XPS spectra of Zr conversion film: (a) survey, (b) Zr 3d, (c), F 1s, (d) Al 2p
图5
图5
硅锆复合转化膜的XPS谱
Fig.5
XPS spectra of Si-Zr composite conversion film: (a) survey, (b) Al 2p, (c) Zr 3d, (d) Si 2p, (e) O 1s, (f) F 1s
2.3 成膜机理推测
根据FT-IR与XPS的分析结果可推测硅锆复合转化膜的成膜机理如下:
主体金属Al在水分子作用下形成Al3+,微阳极区反应如下:
溶液存在大量的H+,在微阴极区反应如下:
溶液中还存在下列反应:
随着反应不断进行,微阴极区的pH不断增加,溶液中的Al3+与Zr4+与OH-发生反应生成沉淀物:
Zr4+还会与F-生成沉淀:
同时溶液中还存在ABSE,双硅烷中间的胺基可以吸附在铝合金表面,两端的硅醇Si-OH可以与活性基团Zr-(OH)4发生交联形成Si-O-Zr结构,还可以与铝合金表面Al-OH形成Si-O-Al结构。固化过程中,多余的硅醇Si-OH又可以彼此交联形成Si-O-Si结构,Al(OH)3和Zr(OH)4会脱水形成Al2O3与ZrO2。在协同作用下,各种结构高强度黏连成了交联程度很高的三维膜层。
2.4 耐蚀性分析
2.4.1 电化学测试
图6
图6
空白样、锆转化膜与硅锆复合转化膜的EIS谱
Fig.6
Nyquist (a) and Bode (b, c) plots of blank, Zr film and Si-Zr film
图7
图7
转化膜电化学阻抗谱的拟合电路图
Fig.7
Equivalent circuits for fitting impedance diagrams of blank (a), Zr and Si-Zr conversion films (b)
表3 电化学阻抗谱拟合结果
Table 3
| Sample | Rc Ω·cm2 | CPEc F·cm-2 | nc | CPEdl F·cm-2 | Rct Ω·cm2 |
|---|---|---|---|---|---|
| Blank | - | - | - | 2.099×10-5 | 13.69 |
| Zr film | 49466 | 1.094×10-5 | 0.829 | 1.695×10-5 | 54226 |
| Si-Zr film | 419080 | 8.816×10-6 | 0.908 | 9.500×10-6 | 167750 |
2.4.2 中性盐雾实验结果
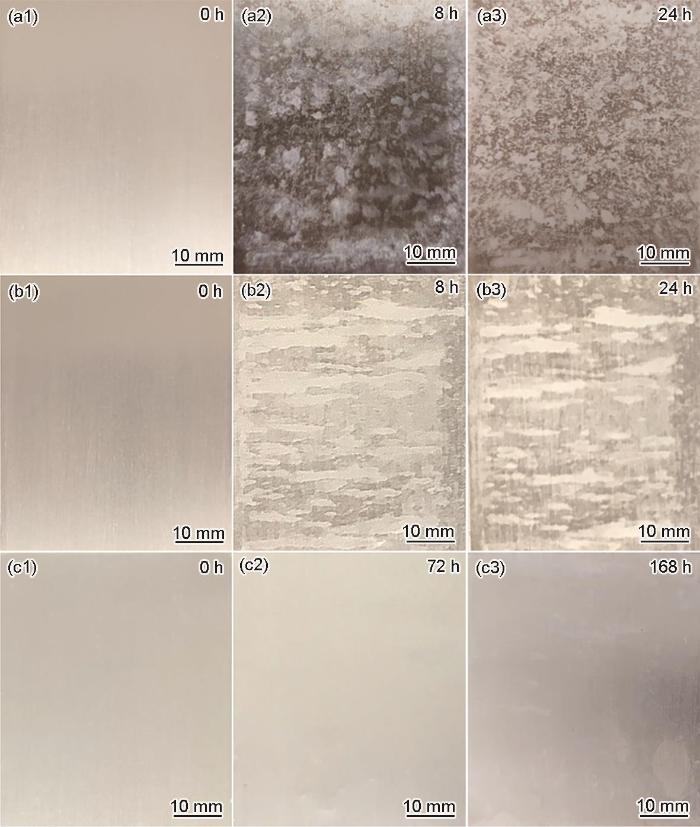
图8为铝合金基体、锆转化膜和硅锆复合转化膜盐雾实验后的形貌。可以看出,裸板经过8 h盐雾后,表面出现大量蚀孔,颜色发黑。24 h盐雾后,颜色发红,表面堆积了大量腐蚀产物。锆转化膜在经过8 h盐雾后,表面被部分腐蚀,腐蚀面积约50%,被腐蚀部分呈灰色。经过24 h后,表面基本全部被腐蚀,且腐蚀部分变黑。硅锆复合转化膜在经过72 h盐雾后表面没有出现明显的腐蚀产物。在经过168 h的盐雾后,有小部分区域发生了腐蚀,腐蚀面积约10%。从盐雾结果可以看出,锆转化膜防护能力差,转化膜不够致密,O2及Cl-等腐蚀介质易通过表面的裂纹进入铝合金内部发生腐蚀反应。硅锆复合转化膜防护能力强,耐盐雾时间比锆转化膜提升了20倍以上,耐蚀水平已达到工业应用的水平。
图8
图8
铝合金基体、锆转化膜和硅锆复合转化膜盐雾实验后的形貌
Fig.8
Photos of aluminum alloy substrate (a1-a3), Zr conversion film (b1-b3) and Si-Zr composite conversion film (c1-c3) after salt spray experiment
3 结论
(1) 当转化原液中的ABSE含量为60%时,钝化效果最好。
(2) 通过SEM观察可见,ABSE改善了锆转化膜不致密的缺陷,在表面生成了一层完整、连续和致密的转化膜。转化膜的主要成分是由锆络合物、铝络合物、ZrF4、Al2O3与ZrO2组成。
(3) 与锆转化膜相比,硅锆复合转化膜容抗弧半径增加了3倍以上;低频阻抗值增加了3倍以上;耐盐雾时间提升了20倍以上。ABSE的加入提高了锆转化膜的耐蚀性,能更好地保护膜层。
参考文献
Improved corrosion and wear resistance of micro-arc oxidation coatings on the 2024 aluminum alloy by incorporation of quasi-two-dimensional sericite microplates
[J].
Effects of phytce acid additives on the characteristics of micro-arc oxidation coatings on 6061 aluminum alloy
[J].
植酸添加剂对6061铝合金微弧氧化膜层性能的影响
[J].
Vanadium and tannic acid-based composite conversion coating for 6063 aluminum alloy
[J].In this study, a vanadium (V) and tannic acid-based composite conversion coating (VTACC) was prepared on 6063 aluminum alloy (AA6063) to increase its corrosion resistance. The surface morphology and compositions of the VTACCs were characterized using scanning electron microscopy (SEM), energy dispersive spectrometry (EDS), and X-ray photoelectron spectroscopy (XPS). The corrosion resistance of the coatings was investigated by linear polarization and electrochemical impedance spectra (EIS). The self-healing ability of the coating was detected by SEM, EDS, and scanning vibrating electrode technique (SVET) measurements. The coating mainly consisted of metal oxides, including Al2O3, VO2, V2O3, and V2O5, and metal organic complexes (Al and V-complexes). The electrochemical measurement results indicated that the best corrosion resistance of VTACC was acquired when the treatment time was 12 min. Furthermore, because a new coating with vanadium rich oxide was developed on the scratch area, artificial scratch VTACC surfaces were repaired after several days of immersion in 3.5-wt% NaCl solution.
Alternative corrosion protection pretreatments for aluminum alloys
[J].
Formation and corrosion resistance of a novel Co-Ti-Mo composite chromium-free chemical conversion coating on LY12 aluminum alloy
[J].
Corrosion protection of 6061 aluminum alloys by sol-gel coating modified with ZnLaAl-LDHs
[J].In this work, ZnLaAl layered double hydroxides (LDHs) were prepared by the co-precipitation method, and the ZnLaAl-LDHs nanosheets were embedded in sol-gel coating for the corrosion protection of 6061 aluminum alloys. The structure, morphology, and long-term anti-corrosion performance of sol-gel coating modified with ZnLaAl-LDHs were investigated. The structure and morphology analysis showed that nanosheets of ZnLaAl-LDHs are finer than those of ZnAl-LDHs, with the results suggesting that the La can refine the size of LDHs’ nanosheets and improve their nucleation rate. The results of long-term corrosion tests showed that the sol-gel coating with ZnLaAl-LDHs exhibits higher corrosion resistance and better stability compared with the sol-gel coating with ZnAl-LDHs, which indicates that the addition of La enhances the anti-corrosion performance of the LDHs and improves the stability of sol-gel coating with LDHs. Finally, the formation mechanism of ZnLaAl-LDHs and the corrosion mechanism of sol-gel coating with ZnLaAl-LDHs on 6061 aluminum alloys are both discussed in detail.
Study on passivation of ADC12 aluminum alloy by self-crosslinking cationic acrylic emulsion
[J].
自交联阳离子丙烯酸乳液对ADC12铝合金的钝化研究
[J].
Design and performance of Zr- and/or Ti-based chemical conversion coatings for light alloys
[J].
轻合金锆/钛基转化膜的设计及性能研究
[J].通过对传统锆/钛基转化膜成膜机理的分析,提出了提升锆/钛基转化膜性能的设计理念,并分别在5083铝合金和AZ91D镁合金上进行了实践,显著提升了合金基体的耐腐蚀性能及其与有机涂层的附着性能。通过解析影响锆/钛基转化膜性能的关键因素,指出了当前锆/钛基转化膜研究的不足及其未来的发展方向。
Improvement in corrosion resistance of micro-arc oxidation coating on PVD Ti-coated aluminum alloy 7075
[J].
Preparation and properties of micro-arc oxide film with single dense layer on surface of 5083 aluminum alloy
[J].
5083铝合金表面单致密微弧氧化膜的制备及其性能研究
[J].采用响应曲面法对微弧氧化电解液体系进行了优化,并在5083铝合金表面进行了单致密微弧氧化涂层的制备。通过扫描电镜、X射线衍射仪、显微硬度计和极化曲线对铝合金微弧氧化膜的微观组织、显微硬度和耐蚀性进行了研究。结果表明:随着微弧氧化膜层厚度从30 μm增加到90 μm,烧结颗粒的尺寸从约为10 μm增加到约为50 μm,致密层由最初的20 μm增加到70 μm,陶瓷膜层中α-Al<sub>2</sub>O<sub>3</sub>的比例明显增加,涂层的硬度由900 HV提高到1500 HV。依据极化曲线和盐雾实验结果发现:随着微弧氧化时间的增加,微弧氧化膜的自腐蚀电流密度逐渐降低,钝化能力逐渐增强,膜层的耐腐蚀性能大幅度提高。
Facile fabrication of novel superhydrophobic Al2O3/polysiloxane hybrids coatings for aluminum alloy corrosion protection
[J].
Active corrosion protection by epoxy coating on Li2CO3-pretreated anodized aluminum alloy 2024-T3
[J].The fast leaching and robust barrier property of inhibitors are the basic fundamentals for the formation of active protective coatings to protect aluminum alloys. Herein, an active protective surface was developed based on an epoxy coating and an underlying lithium carbonate (Li2CO3)-treated anodized aluminum alloy 2024-T3. The morphology of the Li-LDH layer was studied to know its formation mechanism. The electrochemical studies revealed that the fast and adequate leaching of lithium led to a substantial increment of corrosion resistance of the scratched coating in 3.5 wt% NaCl from 1 to 8 days. Time of flight secondary ion mass spectroscopy (ToF-SIMS) results indicated that Li was distributed in the lateral direction and covered the scratched area. The 3D images indicated that different lithium compounds were formed and 90% of the scratched area was covered with the lithium protective layer over immersion time. A combined approach of morphology observations, electrochemical measurements, and ToF-SIMS showed the lithium protective layer offered good corrosion resistance. On the contrary, lithium provided fast and adequate leaching from the coating, demonstrating good active protection for aluminum and its alloys.
Review-conversion coatings based on zirconium and/or titanium
[J].
Review of Cr-free coatings for the corrosion protection of aluminum aerospace alloys
[J].Aluminum alloys are known to have many advantages (e.g., light weight and low cost) but they are not immune to corrosion. So, it is important to assess their corrosion behavior, in particular under atmospheric conditions. To protect aluminum alloys against corrosion, paints are generally applied onto the materials. Corrosion protection in the aerospace industry consists of a conversion or anodized coating, an inhibited primer, and a top-coat. Chromate conversion coating (CCC) and primers containing chromate pigments have been widely used in the aerospace industry over the last decades. However, new environmental regulations have led to major changes for aluminum corrosion protection. By limiting or prohibiting some chemicals, for instance Cr(VI), the European regulation REACH (Regulation on Registration Evaluation, Authorization and Restriction of Chemicals) has induced major changes to some of the finishing processes of aluminum alloys (e.g., chromate conversion, chromic acid anodizing, and chromate sealing). Interesting results have been obtained while seeking replacements for Cr(VI), for example, with the incorporation of cerium, lithium salt, or nanocontainers loaded with corrosion inhibitors in organic coatings. For several years, hybrid sol–gel coatings able to replace the pre-treatment and primer steps have been under development, showing interesting results. New prospects for the future involve the use of photopolymerization to reduce the energy-intensive heat treatment needed in sol–gel technology. It will also be necessary to test these new technologies in service conditions or in accelerated corrosion tests before being able to conclude on the real effectiveness of these coatings. This review summarizes the recent developments in Cr-free coatings for aluminum alloys. Their advantages and drawbacks are also discussed.
Chromate-free chemical conversion coatings for aluminum alloys
[J].Corrosion of metallic components represents a major issue in the aeronautical sector, giving rise to safety concerns and significant financial damages. Conversion coatings (CC) based on hexavalent chromium provide exceptional corrosion protection at relatively low cost. However, environmental issues and health concerns raised a growing interest in the development of alternative technologies. These must not only be cost effective and environmentally friendly but also provide corrosion resistance and adhesion performance comparable to Cr6+-based CCs. Simultaneously fulfilling all of these criteria is a difficult challenge, and an industrial application has so far only been achieved by a small number of systems. This review critically summarizes the recent scientific literature and patents for chromate-free CCs on aluminum alloys and tries to assess their potential regarding the highly demanding aerospace requirements. The bath composition and coating characteristics of the trivalent chromium process, rare earth chemical conversion coatings, transition metal oxyanion additives, Zr/Ti-based chemical conversion coatings, sol-gel coatings, and smart coatings providing stimulus-related inhibitor release are discussed. The advantages and disadvantages of the alternative technologies with regard to their practical implementation are debated, as the aeronautics industry is confronted with the necessity to move away from chromates in the near term.
The formation and corrosion behavior of a zirconium-based conversion coating on the aluminum alloy AA6061
[J].
The effect of surface pre-conditioning treatments on the local composition of Zr-based conversion coatings formed on aluminium alloys
[J].
Preparation of Ti-Zr-based conversion coating on 5052 aluminum alloy, and its corrosion resistance and antifouling performance
[J].
Preparation and corrosion resistance of titanium–zirconium–cerium based conversion coating on 6061 aluminum alloy
[J].This paper aims to develop a chromium-free chemical conversion coating with good corrosion resistance. A novel chemical conversion coating was prepared on 6061 aluminum alloy by dipping in the treatment solution containing titanium/zirconium based-ions and sodium metaphosphate and cerium nitrate hexahydrate as additives. The morphology and composition of the conversion coatings were observed by scanning electron microscopy and energy dispersive X-ray spectroscopy. The microarea structure of conversion coatings at different formation stages was analyzed by electron probe microanalyzer. The electrochemical polarization curve revealed that the corrosion potential of the conversion coating was -0.577 V and the corrosion current density was 0.1148 mu A/cm(2). The equivalent circuit fitted by AC impedance showed that the film resistance reaches 68,140 omega. The formation of coating preferentially grows on the Al (Fe) Si intermetallic to form oxides of Ti and Zr; then TiO2 formed by a higher concentration of Ti4+ gradually covered ZrO2. Ce3+ could adsorb on the intermetallic compound, the hydrolysis of which causes the local pH of the solution to decrease and promotes the aluminum alloy dissolved.
Rapid formation of titanium–zirconium-based conversion coating on aluminum alloy at low temperatures
[J].
铝合金上低温钛锆转化膜快速成膜工艺
[J].
Vanadium pentoxide-enwrapped polydiphenylamine/polyurethane nanocomposite: high-performance anticorrosive coating
[J].
Poly(2-aminothiazole): An emerging functional polymer
[J].
Corrosion resistance of silane film modified by hexagonal boron nitride
[J].
六方氮化硼改性硅烷膜耐蚀性能研究
[J].采用多巴胺对氮化硼进行非共价改性,通过SEM、红外光谱和TGA对粉末进行了表征。使用浸泡法在40Cr合金钢表面制备了掺杂改性氮化硼 (mBN) 的硅烷复合膜,运用SEM、红外光谱、润湿性测试以及动电位极化曲线研究了硅烷复合膜的耐蚀性能。结果表明,聚多巴胺成功附着在氮化硼表面,掺杂mBN的硅烷膜厚度增大至1.812 μm,mBN/BTESPT硅烷复合膜的表面接触角达到91.97°,动电位腐蚀电流密度为9.187×10<sup>-8</sup> A/cm<sup>2</sup>,耐蚀性能相比单一硅烷膜提高了约30倍,在中性盐雾测试中表现出较好的耐蚀性。mBN通过对硅烷膜的物理填充与化学键结合,阻碍了腐蚀介质的扩散,显著增强了金属的耐蚀性能。
Preparation of PTFE/Al2O3–13% TiO2 composite coating and its corrosion resistance
[J].
聚四氟乙烯/Al2O3-13% TiO2复合涂层的制备及耐蚀性研究
[J].
Influence of Cr content on corrosion resistance of composite Ni/Ni-Cr/Ni-Cr-Al-Si films on Cu
[J].
Cr含量对Cu合金表面Ni/Ni-Cr/Ni-Cr-Al-Si膜层耐蚀性的影响
[J].为了解决Cu合金在海水中的腐蚀问题,促进Cu合金部件在海洋工程装备和设施中的应用,采用电镀与磁控溅射相结合的方法,首先在Cu衬底上电镀Ni层,随后在Ni层上溅射沉积Ni-Cr合金薄膜,最后在Ni-Cr层上再溅射沉积不同Cr含量的Ni-Cr-Al-Si合金薄膜,获得Ni/Ni-Cr/Ni-Cr-Al-Si梯度复合膜层,并研究了该梯度复合膜层的结构与腐蚀性能。结果表明:在海水中,Cr含量为43.05%的梯度复合膜层 (S2样品),其低频端阻抗值、容抗弧半径、最大相位角、电荷转移电阻R<sub>f</sub>和腐蚀电位最大,表明其耐腐蚀性能最好。在所研究的范围内,梯度膜层的Ni-Cr-Al-Si表层中[Al]/[Cr]原子比率越大,其耐腐蚀性越好。梯度复合膜层的腐蚀过程主要为点蚀,膜层表面团簇的界面处是点蚀的中心。适当Cr含量的Ni/Ni-Cr/Ni-Cr-Al-Si梯度复合膜层具有很好的耐海水腐蚀和导热能力,适合作为海水中使用的Cu换热器表面的防护涂层。
The long-term corrosion performance and adhesion properties of 7B04 aluminum alloy/anodic film/epoxy primer system in acidic NaCl solution
[J].
Corrosion resistance of epoxy coatings modified by bis-silane prepolymer on aluminum alloy
[J].In this communication, a bis-silane prepolymer was used to modify epoxy resin, aiming to enhance the corrosion resistance of epoxy coatings on aluminum alloy substrates. The bis-silane prepolymer was prepared by tetraethoxysilane (TEOS) and γ-glycidoxypropyl trimethoxysilane (GPTMS). The corrosion behavior of silane-epoxy coatings was studied. Compared with silane monomer-modified epoxy coatings, bis-silane-modified epoxy coatings have lower coating capacitance (Cc), higher charge transfer resistances (Rdl), and lower double layer capacitance (Cdl) during long-time immersion. It indicates that bis-silane-modified epoxy coating has stronger waterproof permeability and substrate corrosion protection ability. In addition, due to the leaching of the silane component and cross-linking reaction between different silanes during the immersion process, the bis-silane-modified epoxy coatings exhibit much stronger “self-healing” ability.