近年来,随着国家经济长期向好、基础设施建设事业目标更加明确,对耐候钢的需求量也与日俱增。为了大力提升发展质量和效率,取得更大的经济和社会效益,同时降低维护成本和节约资源,更好地满足国家、人民和社会日益增长的新需求,新型钢铁材料也面临着新一轮高标准的考验,如高强度、高韧性和易焊接性,甚至对于耐候性能也提出了严格要求[1]。目前,提高钢铁材料耐大气腐蚀性能的常用方法之一是向钢中添加Cu、Cr和Ni等一定量的合金元素。随着腐蚀过程的逐步进行,钢材与环境相互接触的表面会产生结构紧密、保护性优异的锈层,此外会发生合金元素的富集,形成非晶态层,阻碍侵蚀性离子的进一步侵蚀,进而提高钢材在大气环境中的的耐蚀能力[1]。研究表明,不同的合金元素在锈层中起着不同的作用,如Cu的加入有利于内锈层中α-FeOOH的形成,增强阳离子选择性[2,3];Cr可以促进Fe3O4和α-FeOOH的形成,且有助于生成Fe(O,OH)6纳米网络[4,5];而Ni可以促进锈层中形成纳米结构的NiFe2O4,提供大量形核位点,促进γ-FeOOH向纳米相α-FeOOH的转变,同时增强锈层的阳离子选择性[6,7]。但海洋大气环境是一种十分严苛的服役环境,具有高温、高湿和高盐雾等特点,能够显著破坏锈层,加速钢铁材料腐蚀,因此,在海洋大气环境中普通耐候钢的应用效果并不尽如人意[8]。
为了攻克耐候钢在海洋大气环境中的服役这一棘手问题,有学者提出采用合金元素协同作用来进一步提高普通耐候钢的耐蚀性能,如Al和Si[9]、Sn和Sb[10]、Ni和Cu[11]等,均表现出优异的耐腐蚀性能。而Cu作为一种价格相对低廉的合金元素,在考虑经济成本来优化合金元素组成和含量进而增强合金元素之间的相互协同作用方面一直都得到较为广泛的应用。如Hao等[2]研究了低成本MnCuP耐候钢的耐大气腐蚀性能,表明锈层中的Cu和Mn分别以Cu (I) 和Mn (II) 的形式存在,提高了锈层的阳离子选择性,从而大大增强其致密性和保护性。张天翼等[12]研究表明,Cu和Ni二者的协同作用可提高低合金钢的耐蚀性,主要是由于随着Cu和Ni含量的增加,锈层致密性增加且逐渐变薄,加速了保护性锈层的形成所致。除此之外,合金元素三元复合协同作用提高钢的耐蚀性也开始引起研究者关注。Wu等[13]在3%Ni钢中加入不同含量的Cu和Sb进行三元复合,研究认为0.5%Cu和0.2%Sb之间存在显著的协同作用,锈层中会形成富含 (Cu,Sb,Ni) 的内锈层,其中电负性尖晶石氧化物如CuFeO2以及耐酸氧化物如Sb2O3的存在使得锈层结构致密,从而显著提高耐腐蚀性。Sun等[14]也认为Sn、Cr和Mo的协同作用可以促进α-FeOOH、SnO2、SnO、Cr(OH)3和钼酸盐的形成,从而提高锈层的保护性和稳定性,同时这种协同效应还赋予内锈层以阳离子选择性,防止Cl-进一步渗透并抑制钢的局部腐蚀。本课题组前期也曾研究了Cu、Ni和Si对SPA-H钢在海洋大气环境耐蚀性的影响,表明三者之间存在交互作用,其中,Cu为高度显著影响因子,Ni和Si为非显著影响因子[15]。另外,也有研究[16~18]表明,Mo的标准电极电位 (vs SHE) 为-0.22 V,高于Fe (-0.44 V)、Cr (-0.74 V) 和Cu (-0.337 V),这意味着相比Fe、Cr和Cu来说,Mo是更加惰性的元素。Mo的添加有助于提高钢的耐热性和耐蚀性,其所形成的氧化物可以在锈层缺陷处偏聚,使锈层更完整,从而具有较好的稳定性和保护性,进而提高钢的耐蚀性能[19]。Sun等[19]对于在含Cr耐候钢中添加Mo是否能取得优异的效果以及两种元素之间的协同机制进行探索,提出Mo可以促进Cr转化为更稳定的Cr2O3和Fe2CrO4。Xu等[20]认为在1Ni钢中添加Mo (Ni含量为1%) 后钢表面可以形成较厚的保护锈层,以防止NiFe2O4的溶解,有助于提高钢的长期耐腐蚀性能。然而,关于海洋大气环境中Cu与Mo协同作用的研究较少见到报道。
本文以新型Cu-Mo试验耐候钢为研究对象,采用含Cr耐候钢和Q355C碳钢作为对比,同时通过干、湿交替周期浸润实验探究其在海洋大气环境中的腐蚀动力学规律。利用场发射扫描电镜 (FE-SEM)、场发射电子探针 (FE-EPMA)、X射线衍射仪 (XRD)、显微Raman技术以及电化学测试技术观察和分析新型Cu-Mo试验钢锈层微观形貌、锈层截面形貌及元素分布、锈层物相分析以及锈层电化学性能,揭示了新型Cu-Mo试验钢在模拟海洋大气环境中耐蚀规律及机理,为新型Cu-Mo试验钢的技术开发及其在海洋大气环境中服役安全及防护举措的制定提供理论根据和数据支撑。
1 实验方法
实验所用新型Cu-Mo试验钢、含Cr耐候钢和Q355C碳钢主要化学成分见表1。其中新型Cu-Mo试验钢是在含Cr耐候钢成分基础上,增加Cu含量并用Mo替代Cr。
表1 3种钢的化学成分
Table 1
| Sample | C | Si | Mn | P | S | Ni | Cu | Cr | Mo | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| New Cu-Mo test steel | <0.21 | 0.28 | 1.00 | 0.013 | <0.010 | 0.16 | 0.53 | - | 0.52 | Bal. |
| Cr-bearing weathering steel | <0.21 | 0.30 | 1.04 | 0.014 | <0.010 | 0.15 | 0.32 | 0.50 | <0.1 | Bal. |
| Q355C | <0.21 | 0.27 | 1.46 | 0.017 | 0.010 | - | - | - | - | Bal. |
采用Nova400 NanoSEM型FE-SEM对3种钢试样的显微组织及表面锈层的微观形貌进行观察,同时使用EPMA-8050G型EPMA观察试样锈层截面及合金元素分布。研磨刮取各试样锈层后取等量粉末,采用Philips X' Pert Pro型XRD检测锈层的物相组成,Cu靶,电压为40 kV,电流40 mA,2θ范围为10°~90°。同时使用Renishaw inVia型Raman光谱仪分析试样锈层的截面物相分布。
利用Auto Lab PGSTAT302F型电化学工作站进行电化学阻抗谱 (EIS) 的测量。以工作面积为1 cm2的带锈试样为工作电极,铂 (Pt) 片为对电极,饱和甘汞电极 (SCE) 为参比电极,近中性3.5%NaCl溶液为腐蚀介质组装为三电极体系后进行电化学测试。电化学阻抗谱扫描频率范围为105~10-2 Hz,正弦波幅值为10 mV,实验溶液温度为 (25 ±1) °C。
2 结果与讨论
2.1 显微组织
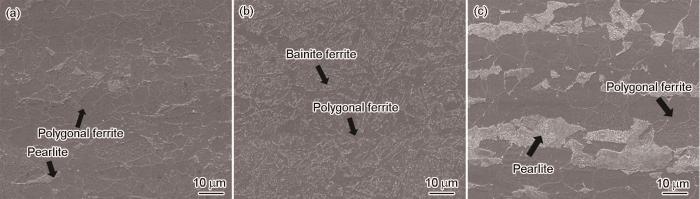
图1给出了3种钢试样的显微组织照片。可以看到,新型Cu-Mo试验钢显微组织以多边形铁素体 (PF) 为主,同时也含有少量片层状珠光体 (P);含Cr耐候钢以均匀的贝氏体-铁素体 (BF) 为主,同时含有少量PF;Q355C碳钢则主要由PF和P组成。
图1
图1
3种钢的显微组织照片
Fig.1
Microstructural images of new Cu-Mo test steel (a), Cr-bearing weathering steel (b) and Q355C steel (c)
2.2 干、湿交替周期浸润腐蚀动力学曲线
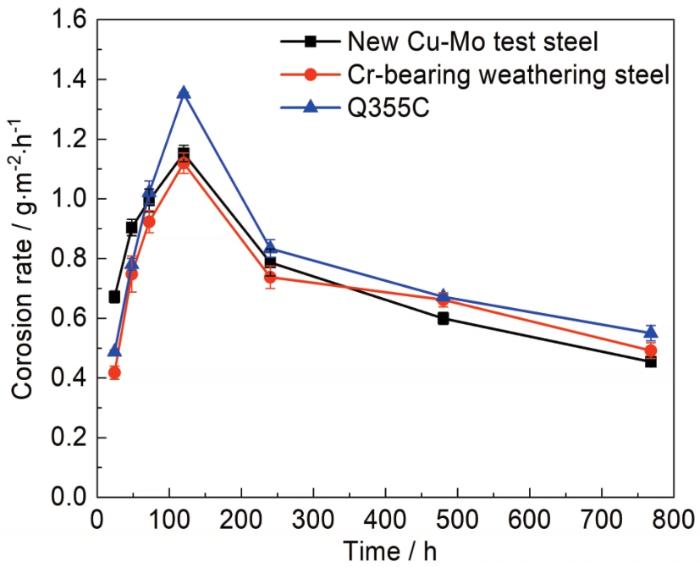
3种钢试样干、湿交替周期浸润腐蚀平均速率随时间的动力学变化曲线见图2。可以看出,3种钢试样的曲线变化规律大体一致,均表现为初始阶段腐蚀速率显著上升,在120 h时达到最大值,后均呈下降趋势,并随着加速腐蚀时间的延长,下降渐趋平稳缓慢。进一步观察表明,3种钢试样腐蚀速率的相对大小也会随腐蚀时间的延长而变化。腐蚀初期 (24 h),新型Cu-Mo试验钢腐蚀速率最大,而含Cr耐候钢的最小;随腐蚀时间延长 (48至120 h),新型Cu-Mo试验钢腐蚀速率增长变得缓慢,而Q355C碳钢的增长加速,72 h后,新型Cu-Mo试验钢腐蚀速率位于含Cr耐候钢和Q355C碳钢之间,直至腐蚀120 h后,3种钢试样的腐蚀速率保持上述规律并开始逐渐下降,当腐蚀时间进一步延长至480 h后,新型Cu-Mo试验钢腐蚀速率开始快速减小,逐渐表现出比含Cr耐候钢更优的耐蚀性。
图2
图2
3种钢试样平均腐蚀速率随时间变化曲线
Fig.2
Average corrosion rate of three steels changes with time
2.3 锈层表征
2.3.1 锈层表面形貌
图3
图3
腐蚀768 h后3种钢试样锈层表面微观形貌
Fig.3
Micromorphologies of rust layer surface of new Cu-Mo test steel (a1, b1), Cr-bearing weathering steel (a2, b2) and Q355C steel (a3, b3) after corrosion for 768 h (b1, b2 and b3 are local enlargements of a1, a2 and a3, respectively)
2.3.2 锈层截面形貌及元素分布
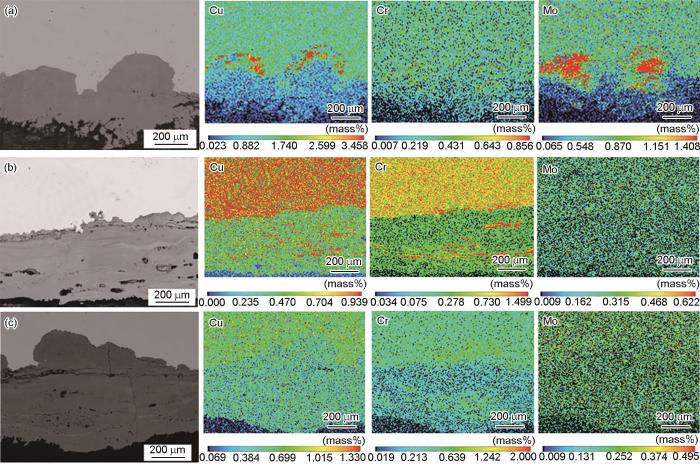
图4给出了3种钢试样周浸腐蚀768 h后锈层截面形貌及元素分布图。可以看出,新型Cu-Mo试验钢锈层最为致密,且与钢基体靠近的内部锈层中呈现出显著的Mo和Cu富集;而含Cr耐候钢锈层中出现Cr的富集和少量Cu的聚集;Q355C碳钢锈层则相较疏松,完全没有合金元素富集。
图4
图4
腐蚀768 h后3种钢试样锈层截面形貌及元素分布图
Fig.4
Cross-sectional morphologies and element distribution of rust layers of new Cu-Mo test steel (a), Cr-bearing weathering steel (b) and Q355C steel (c) after immersion for 768 h
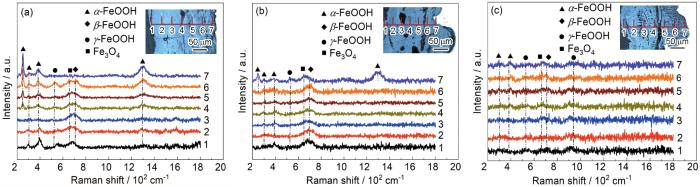
2.3.3 腐蚀产物物相演变及分布
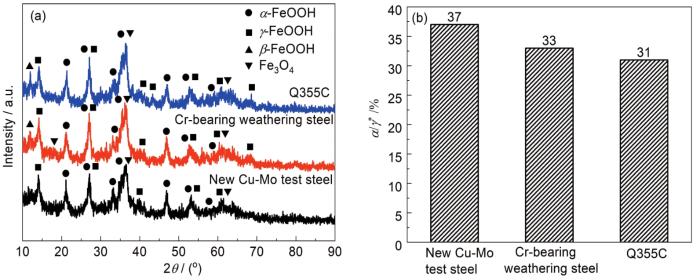
图5给出了3种钢试样周期浸润腐蚀768 h后锈层粉末XRD谱及物相半定量分析结果。可以看出,3种钢试样锈层均由α-FeOOH、γ-FeOOH、β-FeOOH和Fe3O4组成。从特征峰的强度可以得出,新型Cu-Mo试验钢中β-FeOOH的最强峰最低 (11.56°),且γ-FeOOH的特征峰减少 (43°和68°),说明在腐蚀后期,部分γ-FeOOH发生演化生成α-FeOOH,且反应的γ-FeOOH的含量相比两种对比钢更多[23]。根据文献[24]计算锈层保护因子α/γ*,结果见图5。可知,新型Cu-Mo试验钢中α/γ*值比含Cr耐候钢及Q355C碳钢大,说明新型Cu-Mo试验钢锈层中致密相α-FeOOH相对含量最高,锈层对基体的保护作用最好。
图5
图5
腐蚀768 h后3种钢试样锈层的XRD谱及半定量分析
Fig.5
XRD patterns (a) and semi-quantiative analysis of XRD patterns of rust layers (b) of three steels after 768 h corrosion
图6
图6
腐蚀768 h后3种钢试样锈层截面物相分布图
Fig.6
Phase distribution of rust layer cross section of new Cu-Mo test steel (a), Cr-bearing weathering steel (b) and Q355C steel (c) after 768 h corrosion
2.4 电化学阻抗谱
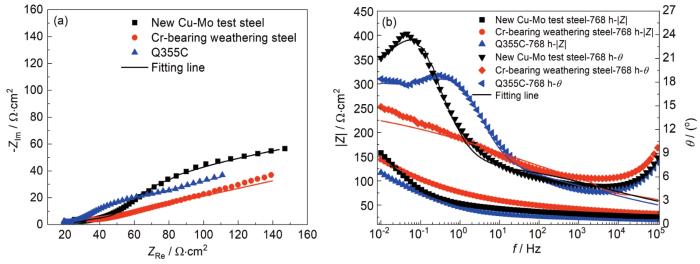
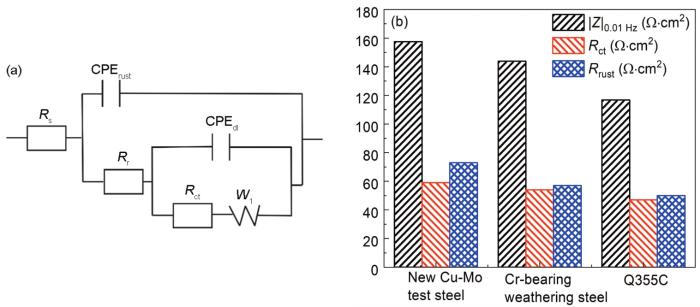
图7给出了3种钢试样周期浸润腐蚀768 h后电化学阻抗谱的Nyquist图和Bode图。3种钢试样的Nyquist曲线都由一条压缩的弧线构成,并且出现了扩散尾。另外,由Bode-相位角和Bode-阻抗模值图可知,3种钢试样中都存在两个时间常数,分别位于高频区域 (>102 Hz) 和低频区域 (<0.5 Hz),且新型Cu-Mo试验钢中|Z|0.01 Hz值大于两种对比钢[26]。图8为该实验条件下模拟的等效电路[27]及拟合结果,图中Rs代表溶液电阻,Rr代表锈层电阻,Rct为电荷转移电阻,W1为Warberg阻抗,CPErust为锈层电容,CPEdl为双电层电容。在相同腐蚀时间下,新型Cu-Mo试验钢的Rr和Rct值均大于另外两种钢,表明前者锈层对基体的保护作用更强。
图7
图7
腐蚀768 h后3种钢试样的电化学阻抗谱
Fig.7
Nyquist (a) and Bode (b) spectrums of three steels after 768 h corrosion
图8
图8
腐蚀768 h后3种钢试样EIS拟合结果
Fig.8
Equivalent circuit for fitting EIS spectra (a) and EIS fitting results (b) of three steels after 768 h corrosion
2.5 腐蚀动力学行为
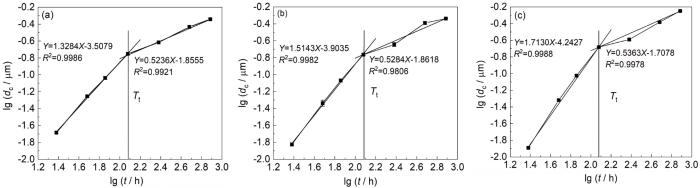
研究[28]表明,耐候钢大气腐蚀实验失重规律应满足动力学经验公式:
式中,dc值代表试验钢腐蚀深度 (µm);t代表试验钢腐蚀时间 (h);A和n值为与环境和材料有关的常数。当n>1时,腐蚀进程加快,当n<1时,则腐蚀进程减缓。从图9中可以看出,3种钢试样在模拟海洋大气中的腐蚀过程可以分为两个阶段:n>1的加速腐蚀阶段和n<1的减速腐蚀阶段,且均在腐蚀120 h左右时出现转折点。第一阶段 (24~120 h),n值均大于1,n值越大,腐蚀速率上升越快[22],此时3种钢n值大小为:新型Cu-Mo试验钢 (1.3284)<含Cr耐候钢 (1.5143)<Q355C碳钢 (1.7130),可见新型Cu-Mo试验钢腐蚀速率上升最慢。在第二阶段 (120~768 h),n值均小于1,3种钢表面均形成了致密的保护性锈层 (图4),可以在一定程度上减缓侵蚀性离子对钢基体的腐蚀。
图9
图9
3种钢试样腐蚀深度-时间双对数曲线
Fig.9
Corrosion depth-time double logarithmic curves of new Cu-Mo test steel (a), Cr-bearing weathering steel (b) and Q355C steel (c)
2.6 新型Cu-Mo试验钢在海洋大气环境中耐腐蚀机理探讨
本研究采用3.5%中性NaCl溶液来模拟海洋大气环境,由于模拟环境为近中性,故钢发生腐蚀时,阳极为Fe的溶解,阴极为O2的还原,反应式如下[29]:
随着反应的进行,Fe2+和OH-反应生成的中间产物Fe(OH)2会进一步氧化生成β-FeOOH (在后续的腐蚀过程中,β-FeOOH也会部分转化为γ-FeOOH) 和γ-FeOOH,腐蚀反应式如下[30]:
随着腐蚀时间延长,部分反应产物β/γ-FeOOH参与阴极反应,如
而在本研究中可见新型Cu-Mo试验钢的腐蚀速率变化可以分为腐蚀速率增加的加速阶段和腐蚀速率减小的减速阶段 (图2和9),且加速程度最小、减速程度最大,这与锈层的形成过程有关。在腐蚀前期,稳定性锈层尚未形成,显微组织对耐蚀性能的影响较大,当钢含有多种显微组织时,二者之间可能会发生微电偶腐蚀,从而影响其腐蚀速率。F和P的电化学活性差异比较大,而F和B的差异很小,F和P之间可以形成原电池,分别作为阴极和阳极,从而促进腐蚀[32]。本研究中,新型Cu-Mo耐候钢和Q355C碳钢显微组织主要以P和F为主,电化学活性差异较大,在腐蚀初期会形成微电偶腐蚀,腐蚀速率较之以BF组织为主的含Cr耐候钢更大。此外,相比于Q355C碳钢,新型Cu-Mo试验钢中P的含量较低,“大阴极小阳极”效应会使其初期腐蚀加剧 (图1和2)[27,33]。
腐蚀后期,Cu和Mo复合添加促进钢中γ-FeOOH向热力学稳定的α-FeOOH的转化,其促进效果明显高于两种对比钢 (图5和6)。此外,新型Cu-Mo试验钢在锈层与基体的交界处出现Cu和Mo明显富集,且二者富集区具有一定的重合,呈现元素团聚现象。而含Cr耐候钢锈层中Cr富集在锈层中间偏外侧位置,同时有少量Cu的聚集。锈层中合金元素富集和致密相α-FeOOH含量的增加 (图4~6),使得新型Cu-Mo试验钢的锈层更为致密,锈层的裂纹和气孔较少,锈层相应的电化学阻抗值更大 (图4和5),增强了锈层对基体的保护作用,从而表现出较好的耐候性 (图2)。综上所述,在传统耐候钢成分基础上,增加Cu含量并用Mo替代Cr有助于α-FeOOH的形成以及合金元素的富集,大大提高了锈层的保护性和稳定性。因此,有理由推测Cu和Mo在模拟海洋大气环境下具有优异的协同作用。
3 结论
(1) 新型Cu-Mo试验钢以多边形铁素体为主,同时含有少量片层状珠光体;含Cr耐候钢以均匀的贝氏体-铁素体为主,同时含有少量多边形铁素体;Q355C碳钢的组织主要由多边形铁素体和珠光体组成。
(2) 在模拟海洋大气环境下,新型Cu-Mo试验钢在腐蚀前期 (24~240 h) 耐蚀性劣于含Cr耐候钢;但在腐蚀后期 (>480 h) 逐渐表现出优异的耐蚀性能。
(3) 在腐蚀前期,显微组织对耐蚀性有较大影响,多边形铁素体和较低含量的珠光体组织使新型Cu-Mo试验钢耐蚀性能劣于含Cr耐候钢和Q355C碳钢。在腐蚀后期,耐蚀性能主要由元素分布及锈层结构决定,新型Cu-Mo试验钢锈层中Cu和Mo之间存在协同效应,这种协同作用导致富含 (Cu,Mo) 锈层的形成,该层结构致密,具有高稳定性和保护性,有助于显著提高耐蚀性。
致谢
感谢武汉科技大学分析测试中心在场发射电子探针 (EPMA) 和显微Raman光谱测试中给予的帮助。
参考文献
Corrosion behavior of 690 MPa grade high strength bainite steel in a simulated rural atmosphere
[J].
690 MPa级高强贝氏体钢在模拟乡村大气中的腐蚀行为
[J].通过湿/干循环实验 (在蒸馏水中润湿并在空气中干燥)、场发射扫描电子显微镜 (FE-SEM)、X射线衍射 (XRD)、电子探针 (EPMA) 和其他表面测试技术以及电化学阻抗谱 (EIS),研究了690 MPa高强度贝氏体钢 (简称Q690钢) 在模拟乡村大气中的长期腐蚀行为。结果表明,在整个腐蚀过程中,Q690钢的腐蚀过程可以分为两个阶段,即加速阶段和减速阶段。在腐蚀的早期阶段,以板条贝氏体 (LB) 为主的Q690钢的耐蚀性优于含有铁素体 (F) 和珠光体 (P) 组织的Corten-A钢。在腐蚀后期,Q690钢锈垢中Cr的富集和α-FeOOH的增加增强了锈层的防护性,导致Q690钢的腐蚀速率降低,因此表明Q690钢耐腐蚀性能明显优于Corten-A钢。
Atmospheric corrosion resistance of MnCuP weathering steel in simulated environments
[J].
Understanding the role of alloyed Cu and P in the initial rust composition of weathering steel formed in a simulated coastal-industrial atmosphere
[J].
The function of Cr on the rust formed on weathering steel performed in a simulated tropical marine atmosphere environment
[J].
Fundamental understanding on the effect of Cr on corrosion resistance of weathering steel in simulated tropical marine atmosphere
[J].
Benefit of the corrosion product film formed on a new weathering steel containing 3% nickel under marine atmosphere in Maldives
[J].
Corrosion behavior of nickel-containing weathering steel in simulated marine atmospheric environment
[J].
Comparative study of rust layers on the advanced 3Ni steel, Q235 carbon steel and conventional weathering steel exposed to atmosphere environment of tropical islands
[J].
新型3Ni钢和Q235碳钢、普通耐候钢在热带岛屿大气环境中暴晒后的锈层对比分析
[J].
Electrochemical behaviour and structure of rust formed on Si- and Al-bearing steel after atmospheric exposure
[J].
Effects of Sn and Sb on the corrosion resistance of AH 32 steel in a cargo oil tank environment
[J].
Understanding the role of alloyed Ni and Cu on improving corrosion resistance of low alloy steel in the simulated Beishan groundwater
[J].The corrosion behavior of NiCu low alloy steel and Q235 low carbon steel as the candidate materials for geological disposal containers of high-level radioactive waste in the simulated Beishan groundwater was comparatively studied by weight loss test, electrochemical measurements, scanning electron microscope (SEM), electron probe microanalysis (EPMA), X-ray diffraction (XRD), Raman spectrum and X-ray photoelectron spectroscopy (XPS). The electrochemical results showed that the corrosion potential of NiCu steel and Q235 steel gradually increased with the immersion time. Simultaneously, the cathodic process transited from hydrogen evolution reaction (HER) control to the rust reduction control, while the anodic process was always dominated by the active dissolution of iron. By comparison, both the cathodic resistance and the anodic dissolution resistance of NiCu steel corrosion were apparently higher than that of Q235 steel. The results of rust layer characterization indicated that Ni and Cu elements could be enriched in the inner rust layer of NiCu steel and the rust layer was more compact. As the main corrosion products, the content of α-FeOOH in the rust layer of NiCu steel was obviously increased more than that of Q235 steel. Fe6(OH)12SO4 stably existed in the corrosion products of NiCu steel because Ni(II) or Cu(II) could substitute Fe(II) of Fe6(OH)12SO4 and increased its oxidation resistance. Moreover, Ni and Cu could also make Fe3O4 ionic selective by doping. After the long-term immersion, the corrosion mass loss of NiCu steel was significantly lower than Q235 steel, which further confirmed the benefits of Ni and Cu alloying on improving the steel corrosion resistance.
Effect of synergistic action of Cu/Ni on corrosion resistance of low alloy steel in a simulated tropical marine atmosphere
[J].
海洋大气环境Cu/Ni协同作用对低合金钢耐蚀性影响
[J].通过模拟严苛热带海洋大气环境的室内喷淋加速实验,研究了Cu/Ni协同作用对低合金钢的腐蚀速率及锈层形貌、成分的影响,测试3种合金试样在0.5% (质量分数) NaCl溶液中的电化学阻抗及线性极化曲线。结果表明,随着Cu、Ni含量的提高,钢材在喷淋实验下生成的锈层逐渐变薄且致密,同时Cu在锈层的富集作用阻碍了Cl<sup>-</sup>入侵。Cu、Ni两种元素在一定程度上提高了基体金属溶解反应的电荷转移电阻 (R<sub>t</sub>) 及极化电阻,加速了保护性锈层的形成并从阳极电化学反应抑制作用上提高了锈层的保护性。
Synergy of Cu and Sb to enhance the resistance of 3%Ni weathering steel to marine atmospheric corrosion
[J].
Distinct beneficial effect of Sn on the corrosion resistance of Cr-Mo low alloy steel
[J].In this work, the beneficial effect of Sn addition on the corrosion resistance mechanism of Cr-Mo low alloy steel was studied. Results demonstrated that Sn improves the corrosion resistance of the steel matrix mainly by influencing the microstructural transformation. Sn addition and the synergistic effect of Sn, Cr, and Mo promote the formation of α-FeOOH, SnO2, SnO, Cr(OH)3, and molybdates, lead to the improved protection and stability of the rust layer. This synergistic effect also endows the inner rust layer with cation selectivity, preventing the further penetration of Cl- and inhibiting the localized corrosion of steel.
Relative function of effects of alloy elements on corrosion resistance of weathering steels in marine atmosphere
[J].
合金元素对耐候钢在海洋大气中耐蚀性影响的交互作用
[J].
A study of the evolution of rust on Mo-Cu-bearing fire-resistant steel submitted to simulated atmospheric corrosion
[J].
Corrosion behaviors of Mo-containing low alloy steels exposed in simulated acidic marine atmosphere environment
[J].
含Mo低合金钢在模拟酸性海洋大气环境中的腐蚀规律研究
[J].
Comparative study on the corrosion behavior of the cold rolled and hot rolled low-alloy steels containing copper and antimony in flue gas desulfurization environment
[J].
Optimization of Mo on the corrosion resistance of Cr-advanced weathering steel designed for tropical marine atmosphere
[J].
Optimizing the resistance of Ni-advanced weathering steel to marine atmospheric corrosion with the addition of Al or Mo
[J].
Five-year atmospheric corrosion of Cu, Cr and Ni weathering steels in a wide range of environments
[J].
Corrosion behavior of Q345q bridge steel in three simulated atmospheres
[J].
桥梁钢Q345q在3种模拟大气环境中的腐蚀行为研究
[J].选择3种模拟西北地区大气环境的腐蚀介质 (除冰盐介质、工业大气介质及除冰盐+工业大气介质),采用干湿交替加速腐蚀实验研究了桥梁钢Q345q的腐蚀行为,通过失重法探讨了桥梁钢Q345q在3种不同腐蚀环境下的腐蚀动力学曲线,并采用XRD、SEM和电化学工作站等分析了桥梁钢Q345q腐蚀不同时间形成的锈层物相、形貌、结构及其电化学特性。结果表明:虽然在除冰盐介质中480 h内腐蚀速率小,但表面形成含有β-FeOOH和氯化物等不稳定可溶性的腐蚀产物,导致锈层疏松,腐蚀电流增大,锈层不具保护性;在NaHSO<sub>3</sub>介质中的腐蚀速率较高,但随着腐蚀时间的延长锈层致密性增加,腐蚀速率下降较快,锈层阳极稳态腐蚀电流减小,锈层具有保护性;而在混合介质中腐蚀行为为耦合效应,由于氯化物等腐蚀产物使得锈层致密性下降,但锈层仍具有一定的保护性。
Evolution of rust layers formed on Q235 and 09CuPCrNi-A steels during initial stage of field exposure in two sites of different environment
[J].
Q235和09CuPCrNi-A钢在两种不同大气环境中腐蚀早期锈层演化研究
[J].
Insight into atmospheric corrosion evolution of mild steel in a simulated coastal atmosphere
[J].The corrosion behavior of mild steel in a simulated coastal atmosphere environment has been investigated by the indoor accelerated wet/dry cyclic corrosion acceleration test (CCT), scanning electron microscopy (SEM), Raman spectroscopy and electrochemical measurements. During the CCT test of 60 cycles, the evolution of logarithmic (corrosion rate) vs. logarithmic (CCT cycles) presents a turning point at the 5th cycle, presenting a tendency to increase first and then decrease to gradually stabilize as the CCT cycle prolonged. Before the 5th cycle, γ-FeOOH and β-FeOOH and Fe3O4 were detected, respectively. And then, α-FeOOH as a new chemical composition was detected in the subsequent corrosion cycles. It is found that, after long term corrosion, the rust separated into a relatively dense inner layer rich with α-FeOOH and a loose outer layer rich with γ-FeOOH, both of which have poor electrical conductivity. The rapid increase of corrosion rate in the early stage since reducible corrosion products are involved in the reduction process of the cathode which promotes the dissolution of the anodic metal substrate. Afterward, as the rust layer thickens, the resistance of the rust increases, and the aggressive ions diffusion is blocked, gradually suppressing the electrochemical corrosion process. At last, when the composition and distribution of the rust layer remain stable, the corrosion presents a fluctuating speed around a certain value during the cracking and self-repairing process of the rust layer.
Comparison of the rusting behaviors of S450EW weathering steel under continuous spray and wet/dry cycling
[J].
Comparative study of corrosion resistance of three weathering steels for bridges in simulated marine atmospheric environment
[J].
三种桥梁耐候钢在模拟海洋大气环境中的耐蚀性能比较
[J].
Long-term monitoring of atmospheric corrosion at weathering steel bridges by an electrochemical impedance method
[J].
Essential role of element Si in corrosion resistance of a bridge steel in chloride atmosphere
[J].
Weathering steels: from empirical development to scientific design. A review
[J].
Marine atmospheric corrosion of carbon steel in the tropical microclimate of Port Louis
[J].
Effect of micro-phase electrochemical activity on the initial corrosion dynamics of weathering steel
[J].