现服役的油套管大部分还是碳钢,比如N80 (S) 油套管、P110 (S) 油套管等。针对油套管在含H2S-CO2体系中,浓度、温度、腐蚀产物等对其腐蚀行为影响较多,Li等[9]采用高温高压釜研究了P110S钢管在高H2S和CO2含量环境下的腐蚀行为,认为腐蚀产物的形貌与腐蚀速率有关。Yin等[10]探究了SM 80ss油套管在含H2S和CO2溶液中的腐蚀行为,研究表明,H2S在腐蚀中占主导地位,样品表面生成的硫铁膜对腐蚀有抑制作用,这种作用与H2S浓度有关。此外,针对不同钢级的油套管在含硫环境下的使用也有大量研究成果,Elgaddafi等[11]探究了API碳钢 (T95,C110和Q125) 在高压酸性环境下的性能及其腐蚀行为。刘飞等[12]探究了在不同温度含饱和H2S/CO2腐蚀溶液中P110钢的腐蚀速率。针对油套管的选材,不仅要考虑油套管的耐H2S腐蚀性能,还要考虑其强度,一旦油套管被腐蚀,套管壁变薄,强度下降,很容易发生断裂进而发生安全事故[13]。与低钢级油套管相比,P110钢级的油套管具有更高的屈服强度,更适合于高压环境中使用。油套管的服役环境中往往含有H2S,因此要求油套管具有一定的抗硫性能。P110S油套管作为抗硫钢,不仅具有高屈服强度,而且具有一定的抗H2S断裂性能,在低H2S油田中得到使用。然而在服役过程中,难免可能会发生点蚀穿孔或者断裂等,因此有必要研究其在低含硫环境中的腐蚀行为。本文通过浸泡实验、电化学实验等表征了P110S油套管在Na2S溶液中的腐蚀行为。随后对腐蚀产物的形貌、截面形貌以及腐蚀产物的成分进行了表征,探讨P110S在低含硫环境中的腐蚀行为。
1 实验方法
本文材料为P110S钢级油套管,合金成分 (质量分数,%) 为:C 0.26、Cr 0.5、Mo 0.68、Mn 0.65、Si 0.2、S 0.003、Nb 0.02。首先将油套管加工成10 mm×10 mm×3 mm的小块样品,之后依次用240#、400#、800#、1200#、1500#砂纸打磨,最后用2.5 μm金刚石抛光膏机械抛光至镜面,用4% (体积分数) 硝酸酒精溶液侵蚀。该油套管的金相组织为回火索氏体。
实验溶液采用3种不同浓度的Na2S溶液来模拟不同浓度的含硫环境,溶液分别为:4%NaCl+0.01 mol/L Na2S、4%NaCl+0.05 mol/L Na2S、4%NaCl+0.1 mol/L Na2S,利用稀盐酸调节pH至4左右。
浸泡实验每组采用4个平行样,试样尺寸为25 mm×15 mm×3 mm。试样背面打钢印做好标记,然后依次用240#、400#、800#、1200#、1500#砂纸打磨,抛光至镜面。所有样品经过称重后放入3种溶液中浸泡,浸泡周期为7 d,温度为室温 (25 ℃)。浸泡实验结束后,取出样品立即用去离子水和无水乙醇冲洗干燥,计算腐蚀速率、观察样品表面腐蚀产物和腐蚀形貌。
用500 mL HCl+500 mL H2O+3.5 g六次甲基四胺的除锈液去除了样品表明的腐蚀产物。去除腐蚀产物后再称重并计算其平均值,用失重法计算了在3种不同浓度的Na2S溶液中样品的腐蚀速率,腐蚀速率的计算如
式中,CR为腐蚀速率 (mm/a),
首先用Bruker D8 Focus X射线衍射仪 (XRD) 以及Renishaw inVia型拉曼光谱分析了表面腐蚀产物的成分。对于溶液中脱落的腐蚀产物,则用漏斗过滤出滤渣,滤渣分别用去离子水以及无水乙醇冲洗并烘干,同样用XRD分析了其成分,XRD测试范围为10°~90°,扫描速率为5°/min,拉曼光谱激光波长为532 nm,光谱分辨率为2 cm-1。
采用KYKY-6200扫描电镜 (SEM) 观察浸泡之后样品表面带锈和除锈后的腐蚀形貌和截面形貌,并用激光共聚焦观察腐蚀产物的厚度和致密程度。
电化学样品尺寸为10 mm×10 mm×3 mm,样品背面用Cu导线焊接,除工作面以外其余各面均用环氧树脂密封。密封之后,工作面依次用240#、400#、800#、1200#、1500#砂纸打磨,之后用去离子水以及无水乙醇冲洗并干燥。电化学测试采用三电极体系,参比电极为饱和甘汞电极 (SCE) ,对电极为铂电极 (CE)。利用CHI660E电化学工作站对其电化学阻抗谱和动电位极化曲线进行测试。电化学阻抗谱的测试参数为:测试频率为105~10-2 Hz,施加的振幅为0.01 V。动电位极化曲线测试参数为:电位范围为-0.5~+0.5V (vs. OCP),扫描速率为0.0005 V/s。
2 结果分析
2.1 腐蚀形貌和产物分析
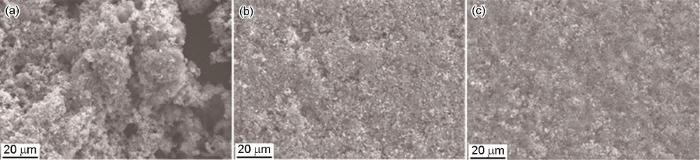
图1
图1
P110S钢在不同浓度Na2S溶液中浸泡7 d后的SEM像
Fig.1
SEM surface images of P110S steel after 7 d immersion in different solutions: (a) 4%NaCl+0.01 mol/L Na2S, (b) 4%NaCl+0.05 mol/L Na2S, (c) 4%NaCl+0.1 mol/L Na2S
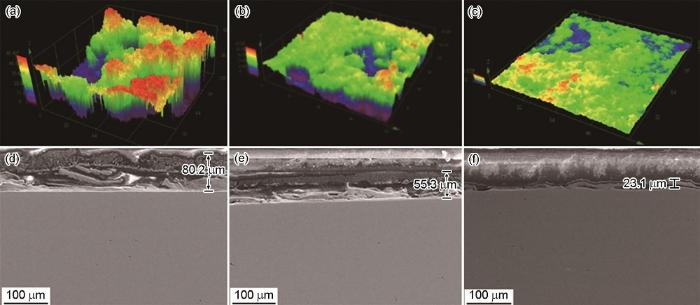
图2
图2
P110S钢在不同浓度Na2S溶液中浸泡7 d后的腐蚀形貌CLSM图以及截面SEM像
Fig.2
CLSM surface (a-c) and SEM cross-sectional (d-f) images of P110S steel after 7 d immersion in different solutions: (a, d) 4%NaCl+0.01 mol/L Na2S, (b, e) 4%NaCl+0.05 mol/L Na2S, (c, f) 4%NaCl+0.1 mol/L Na2S
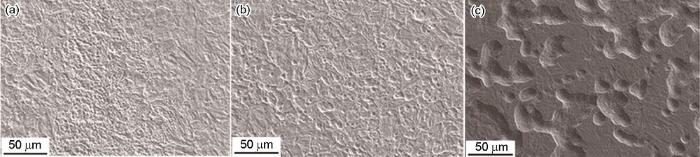
图3
图3
P110S钢在不同浓度Na2S溶液中浸泡7 d后去除腐蚀产物的SEM像
Fig.3
SEM images of P110S steel after 7 d immersion in 4%NaCl+0.01 mol/L Na2S (a), 4%NaCl+0.05 mol/L Na2S (b) and 4%NaCl+0.1 mol/L Na2S (c) solutions and then removing corrosion products
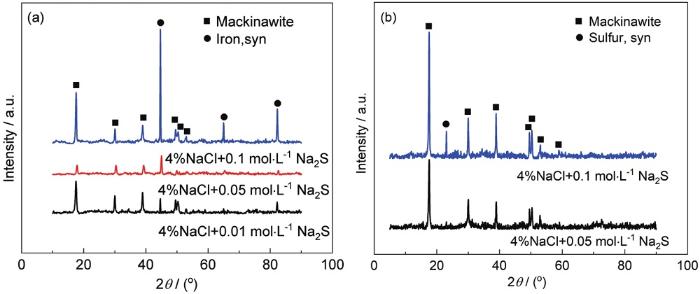
图4
图4
基体及溶液中腐蚀产物的XRD谱
Fig.4
XRD patterns of corrosion products on the steel substrate (a) and in the solution (b)
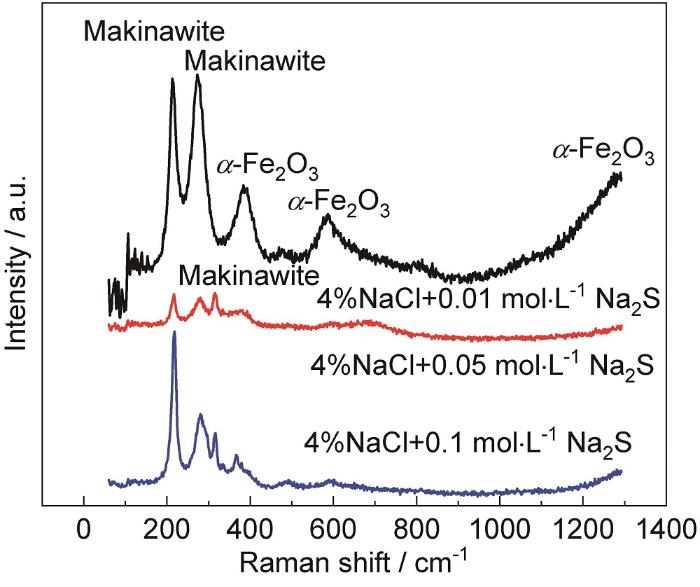
为了进一步分析腐蚀产物的成分,对油套管表面上的腐蚀产物进行了拉曼光谱的测试,其结果如图5所示。
图5
由结果可知,在低浓度Na2S溶液中浸泡的样品表面腐蚀产物在212和282 cm-1处出现拉曼峰,其他两种溶液中的样品腐蚀产物在315 cm-1处也出现了拉曼峰。212,282及315 cm-1处出现的峰为马基诺矿的特征吸收峰[14]。此外,在低浓度的Na2S溶液中的样品腐蚀产物出现389和600 cm-1附近的拉曼峰,该处峰为α-Fe2O3的特征吸收峰,1300 cm-1附近的拉曼峰对应于声子二次散射振动峰[15]。因而可知,Na2S溶液中样品的腐蚀产物为马基诺矿和α-Fe2O3。在低温以及低H2S分压下,腐蚀产物主要为马基诺矿[16-18]。马基诺矿疏松多孔,对基体的保护作用较差[19,20]。因而低浓度的Na2S溶液,腐蚀速率主要取决于Na2S浓度,低浓度Na2S限制了阴极反应致使腐蚀速率低。
2.2 电化学结果分析
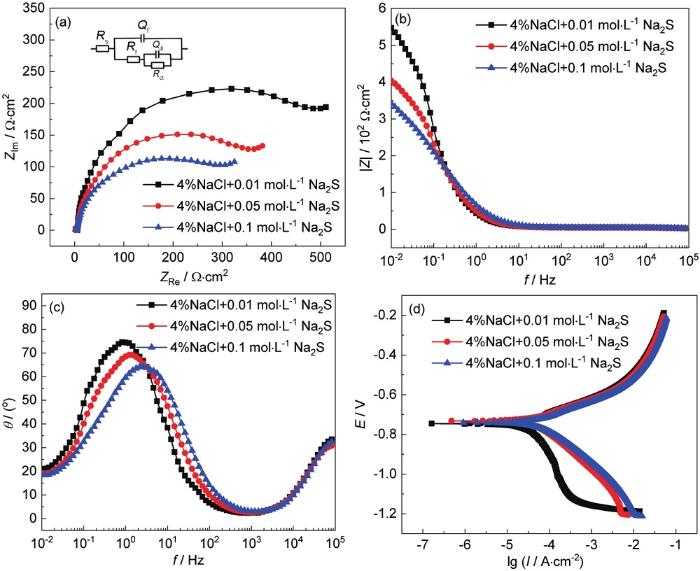
图6为P110S钢在3种浓度Na2S溶液中的电化学测试结果。图6a~c为电化学阻抗谱的Nyquist以及Bode图。在3种浓度溶液中,样品均出现明显的容抗弧。其中4%NaCl+0.01 mol/L Na2S溶液中样品的容抗弧直径最大,4%NaCl+0.05 mol/L Na2S溶液中样品的容抗弧稍小,4%NaCl+0.1 mol/L Na2S溶液中样品的容抗弧直径最小。为了进一步分析数据,使用ZSimpWin软件对阻抗谱进行拟合,拟合结果如表1所示。其中Rs为溶液电阻,Qf为腐蚀产物膜与溶液间的电容,Rf为腐蚀产物膜的电阻,Qct为基体与腐蚀产物膜之间的电容,Rct为电荷转移电阻。从电化学阻抗谱的拟合结果可知,Rct随Na2S浓度的升高而减小,表明随溶液中Na2S浓度的升高,样品的腐蚀倾向逐渐增大。
图6
图6
P110S钢的电化学测试结果
Fig.6
Nyquist plots (a), Bode plots (b, c) and polarization curves (d) of P110S steel in the solutions containing different concentrations of Na2S
表1 图6中电化学阻抗谱的拟合结果及极化曲线的拟合结果
Table 1
| Solution / mol·L-1 | Rs / Ω·cm2 | Qf / F·cm2 | Rf / Ω·cm2 | Qct / F·cm2 | Rct / Ω·cm2 | Icorr / μA·cm-2 | Ecorr / V |
|---|---|---|---|---|---|---|---|
| 0.01 | 1.233 | 3.61×10-6 | 4.343 | 4.45×10-3 | 553.3 | 33.58 | -0.7365 |
| 0.05 | 1.612 | 1.66×10-6 | 3.004 | 3.09×10-3 | 352.2 | 54.81 | -0.7353 |
| 0.1 | 1.297 | 1.39×10-6 | 3.007 | 3.56×10-3 | 328.9 | 61.78 | -0.7419 |
3 结论
(1) 随着Na2S浓度的增大,P110S油套管腐蚀速率依次增大,腐蚀形貌由点蚀转变为均匀腐蚀。
(2) P110S钢级油套管在Na2S溶液中的腐蚀产物主要为马基诺矿和α-Fe2O3。随着溶液中Na2S浓度的升高,基体上附着的腐蚀产物均较疏松且由厚变薄,溶液中脱落的腐蚀产物逐渐增多。
(3) 随着溶液中Na2S浓度的升高,样品的容抗弧直径减小,腐蚀电流密度增加,体系由阴极控制转变为混合控制。
参考文献
The influence of hydrogen sulfide on internal pressure strength of carbon steel production casing in the gas well
[J].
Cracking behavior of cold-welding layer on A350 LF2 steel in H2S environment
[J].
冷焊修复层在H2S环境下的开裂行为研究
[J].
Electrochemical impedance spectroscopy of iron corrosion in H2S solutions
[J].
Corrosion analysis and anti-corrosion measures of oil casing of sulfur content gas wells: A case study of Daniudi gas field in the Ordos Basin
[J].
H2S corrosion of mild steel: A quantitative analysis of the mechanism of the cathodic reaction
[J].In the context of H2S corrosion of mild steel, the direct electrochemical reduction of H2S is currently believed to be the main contribution of this species to cathodic currents. That is perhaps due to the distinct behavior of the cathodic polarization curves observed in the presence of H2S, as compared to those obtained in strong acids solutions or in the presence of other weak acids such as carboxylic acids and carbonic acid. In the presence of aqueous H2S, the cathodic polarization curves show a "double wave" shape, that is widely considered to be the result of the direct reduction of H2S. In the present study, the mechanism of H2S corrosion of mild steel is theoretically investigated with the focus on the buffering ability of H2S. It is shown that all characteristic behaviors of cathodic currents that were previously associated with the direct reduction of H2S, including the "double wave", can be fully explained in terms of the H2S dissociation reaction and its buffering effect. In order to further evaluate this mechanistic argument, a comprehensive mathematical model for the H2S system was developed and the calculated cathodic polarization curves were compared with the existing experimental data in the open literature. The results showed that the model, built with H+ reduction as the sole cathodic reaction, is able to reasonably capture all characteristic behavior of cathodic currents, further supporting this mechanistic argument. (C) 2018 Elsevier Ltd.
Mechanism of (Mg, Al, Ca)-oxide inclusion-induced pitting corrosion in 316L stainless steel exposed to sulphur environments containing chloride ion
[J].
Influence of H2S on the pitting corrosion of 316L stainless steel in oilfield brine
[J].
Corrosion rules of commonly used oil well pipes in hydrogen sulfide environment
[J].
硫化氢环境下常用油井管材质腐蚀规律研究
[J].
Corrosion behavior of 110S Tube steel in environments of high H2S and CO2 content
[J].
Corrosion behavior of SM 80SS tube steel in stimulant solution containing H2S and CO2
[J].
Modeling and experimental studies on CO2-H2S corrosion of API carbon steels under high-pressure
[J].
Corrosion behavior of P110 S steel in corrosion solution with saturated H2S and CO2 at different temperatures
[J].
P110钢在不同温度含饱和H2S/CO2腐蚀溶液中的腐蚀行为
[J].
Analysis of corrosion failure and materials selection for CO2-H2S gas well
[J].
Characterisation of mackinawite by Raman spectroscopy: Effects of crystallisation, drying and oxidation
[J].
Effect of Cr/Mo carbides on corrosion behaviour of Fe_Mn_C twinning induced plasticity steel
[J].
Effect of crystal structure and ion selectivity of corrosion products on corrosion behavior of P110S low alloy steel in H2S/CO2 environment
[J].
腐蚀产物晶体结构及离子选择性对P110S低合金钢在H2S/CO2环境中腐蚀行为的影响
[J].
An in-situ Raman study on the oxidation of mackinawite as a corrosion product layer formed on mild steel in marginally sour environments
[J].
Corrosion behavior of X52 anti-H2S pipeline steel exposed to high H2S concentration solutions at 90°C
[J].
Initiation and developmental stages of steel corrosion in wet H2S environments
[J].