层状双氢氧化物 (LDH) 是一类重要的层状纳米无机材料,以其独特的负离子容量、负离子交换能力[1]、结构记忆效应和阻隔性等特点,在腐蚀防护领域得到了广泛的发展[2-4]。近年来,已有不少研究LDH薄膜用于Mg、Al合金的表面防护[5,6]。制备LDH的方法有多种,如共沉积法[7]、水热法[1,8,9]、电沉积法[4]、离子交换法[10,11]和超声辅助法。比较之下,在密闭容器中,以溶液作为反应介质的水热合成是一种环境友好、成本低廉、操作简单的合成方法。然而,通过水热法制备的LDH薄膜在基底上通常是随机生长的,具有巢状形态的多孔膜,可能为腐蚀介质提供扩散路径,降低了LDH涂层的保护能力[12]。因此,LDH涂层亟需修饰改性提高其耐腐蚀性能。
可利用无机盐[13-15]、杂环化合物[16,17]、碳纳米材料[18-20]、金属氧化物[21-23]和聚合物[24-26]等多种材料对LDH薄膜修饰改性。鉴于LDH表面含有大量的羟基,选择含多种官能团的高分子聚合物来改性LDH,LDH和聚合物之间会有较强的相互作用,有利于显著提升LDH复合涂层的综合性能。例如,Liu等[25]报道了单层LDH纳米片与聚乙烯醇缩丁醛 (PVB) 通过氢键相互作用,使LDH纳米片在基底表面形成“砖壁结构”,提高了碳钢在3.5% (质量分数) NaCl溶液中的耐蚀性能。Zhou等[27]将LDH作为增强剂加入到聚乙烯醇 (PVA) 基体中,LDH中的羟基与PVA链中的羟基发生作用,形成较强的界面相互作用而增强复合材料的物理化学性能。另一方面,采用高分子聚合物改性LDH,也是LDH表面功能化和多元化研究的内容之一,有助于推进LDH复合涂层的广泛应用。
聚苯胺 (PANI) 被认为是一类绿色,成本低廉的导电聚合物,在金属防腐蚀领域受到特别的关注[28, 29]。PANI分子结构中含有大量的胺和亚胺官能团,对无机或有机分子具有很强的亲和力,因此是一种理想的LDH改性材料。Gao等[30]采用化学氧化法,将PANI组装到MgFe-LDH纳米片中,提高了电容器的长期稳定性。Hu等[31]研究了十钒酸盐插层和γ-氨基丙基三乙氧基硅烷接枝的ZnAl-LDH,与化学氧化制备的PANI纳米材料复合,得到的ZnAl-LDH复合涂层的耐腐蚀和阻燃性能显著提升。采用化学氧化法合成的PANI通常是粉末状,后续需要额外的成膜技术或方法在基底表面构建涂层,耗能又耗时。本文拟采用原位化学氧化法直接在得到的LDH涂层表面构建PANI膜。所谓原位聚合PANI,是将涂覆LDH的基底材料置于含苯胺的酸性溶液中,向溶液内添加氧化剂,聚合反应开始,生成的PANI颗粒逐步增大,随后PANI沉积在LDH涂层表面而成膜。这样直接成膜的方式不仅操作简便,且对环境无任何附加的污染。此外,采用这样原位聚合的方式,从理论上分析有利于增强LDH和PANI间的相互作用,从而提升LDH复合材料的综合性能。
在酸性介质中,由于电位差限制,不锈钢易发生化学或电化学腐蚀,还不能满足实际应用的需求。本文选择304不锈钢为基底材料,运用水热法在其表面制备LDH涂层,再利用原位化学氧化法构建PANI涂层改性LDH,得到LDH/PANI复合涂层。在1 mol/L H2SO4溶液中,对比研究了合成的4种类型LDH涂层对304不锈钢的保护性能,并对复合LDH涂层进行了表征。结合浸泡实验,深入讨论了LDH复合涂层的防护机理。
1 实验方法
选用304不锈钢,规格为15 mm×50 mm×2 mm。依次经过600#、1000#、1200#、1500#、2000#的碳化硅砂纸打磨,之后在丙酮中用超声波清洗5 min,再依次用无水乙醇、去离子水超声清洗5 min,以除去表面的油污和油脂,放入干燥箱中干燥备用。
304不锈钢表面NiFe-LDH涂层的水热合成过程为:将一定量的Fe(NO3)3·9H2O、Ni(NO3)2·6H2O和尿素 (利用其分解提供碱性环境) 溶解在于离子水中,3种组分的摩尔比为1∶3∶100。将混合溶液转移到聚四氟乙烯衬里的高压釜中。将304不锈钢置于反应釜中,在120 ℃的烘箱中反应2 h。得到的样品用去离子水洗涤,在60 ℃烘箱中干燥。考虑到304不锈钢基底材料可提供Fe,制备了一组未添加Fe(NO3)3·9H2O原料的来比较,标记为NiFe(s)-LDH涂层。
将干燥后的涂覆LDH涂层的304不锈钢置于100 mL苯胺和H3PO4混合溶液 (二者摩尔比为1∶2) 中。在0~5 ℃下,向混合溶液中缓慢滴加20 mL,浓度为 0.5 mol/L的过硫酸铵 (APS)。继续搅拌1 h,取出清洗干燥,在304不锈钢表面得到PANI改性的复合涂层。此涂层的厚度约为5 µm。
采用CHI660电化学工作站进行电化学测试,采用三电极体系,304不锈钢为工作电极,涂层的工作面积为1 cm2,铂片为辅助电极,测试介质为1 mol/L H2SO4溶液。考虑到H2SO4溶液的浓度较大,选择Hg/Hg2SO4为参比电极。测试涂层的动电位极化曲线和电化学阻抗谱。动电位极化曲线的扫描速率为1 mV/s,测试范围为自腐蚀电位±250 mV;阻抗测量频率范围为105~10-2 Hz,交流信号振幅为5 mV,采用Zview软件对实验结果进行拟合分析。
采用JSM-7800F场发射扫描电子显微镜 (SEM) 观察涂层表面形貌。采用EMAX能谱仪 (EDS) 分析样品的元素分布和组成。采用BX53M型偏光显微镜对涂层的金属基底的表面形貌进行观测。采用 D8 ADVANCE型X射线衍射仪 (XRD) 检测样品的物相组分及材料晶型。采用FTIR-8400S傅里叶变换红外光谱仪对复合涂层进行分析。采用Thermo k-alpha X射线光电子能谱仪 (XPS) 对样品的组成和价态进行分析。采用ASTM D3359-17标准,将不同涂层交叉切成1 mm×1 mm的方格,胶带在涂层上按压2 min,然后将胶带从基材上剥离。通过光学图片观察涂层与基底的附着力[32,33]。
2 结果与讨论
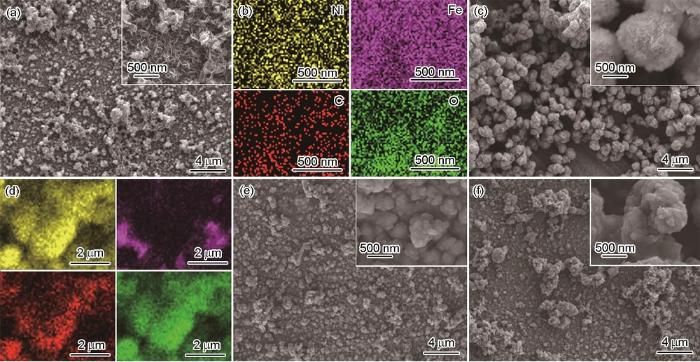
图1显示了304不锈钢表面4种复合涂层表面SEM图和EDS元素面分析结果。比较图1a和c可见,两种LDH涂层的表面结构存在显著差异。对于添加Fe(NO3)3得到的NiFe-LDH涂层,样品表面由500 nm左右的纳米片组成,呈现出预期的层状结构 (图1a)。由304不锈钢基底提供Fe的NiFe(s)-LDH涂层表面为球状颗粒,平均颗粒直径约为1 µm (图1c)。图1b和d所示为NiFe-LDH和NiFe(s)-LDH涂层中不同元素的空间分布情况。对比可见,NiFe-LDH的整个涂层中Ni、Fe、C和O分布非常均匀,而NiFe(s)-LDH涂层中主要是由Ni、C和O组成,存在部分区域堆积,且Fe含量明显较低。表明基底304不锈钢的反应活性较弱,提供的Fe有限。采用原位化学氧化法在两种LDH涂层表面构建将PANI涂层,如图1e和f所示。比较之下,经PANI改性后NiFe-LDH涂层的表面变得更致密,且排列更紧凑。
图1
图1
304不锈钢上所制备涂层表面SEM图和相应EDS元素面扫描图
Fig.1
SEM surface images (a, c, e, f) of NiFe-LDH (a), NiFe(s)-LDH (c), NiFe-LDH/PANI (e) and NiFe(s)-LDH/PANI (f) coatings on 304 stainless steel, and EDS element mappings of NiFe-LDH (b) and NiFe(s)-LDH (d) coatings
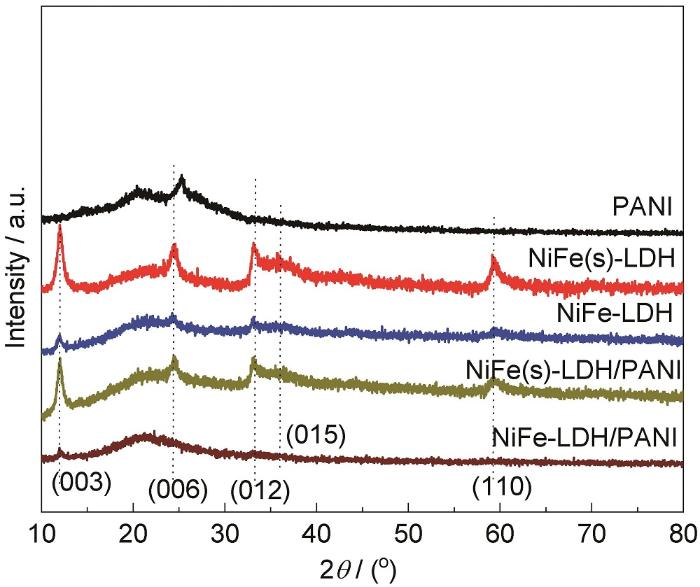
图2为304不锈钢表面不同涂层的XRD图。在2θ=12.0°、36.1°和59.4°出现的衍射峰,分别对应NiFe-LDH的 (003)、(015) 和 (110) 晶面 (JCPDS 49-0188)[34-37]。然而,与卡片中 (006) 和 (012) 晶面所对应的2θ=24.4°和33.2°衍射峰位置相比发生偏移,这可能由于溶液中NO3-的插入。加入Fe(NO3)3后,LDH涂层对应的XRD衍射强度明显降低,表明NiFe-LDH涂层中颗粒的分散性增强,粒度变小。这与SEM分析的结果一致。采用相同的化学氧化法,制备了一组纯PANI。其XRD如图2中的曲线1所示。在2θ=20.3°和25.3°处有两个宽衍射峰,归属于PANI的Bragg峰[38,39]。经PANI改性后的LDH复合涂层,在2θ=20.3°也出现了较宽的衍射峰,表明LDH复合涂层中PANI的存在。比较发现,加入PANI后,LDH复合涂层的特征XRD衍射峰强度也有所降低,且出现宽化现象,意味着复合涂层中不同组分间均匀分散,颗粒结合紧密。
图2
图2
304不锈钢表面所制备涂层的XRD谱图
Fig.2
XRD patterns of the PANI, NiFe(s)-LDH, NiFe-LDH, NiFe(s)-LDH/PANI and NiFe-LDH/PANI coatings on 304 strainless steel
图3为不同涂层的FT-IR谱图。在3420和1617 cm-1附近的宽吸收峰,分别与O-H的拉伸振动和H2O分子的弯曲振动有关[40],表明在304不锈钢表面已形成LDH层。在637 cm-1处出现的特征峰归属为LDH层中的M-O晶格振动。在1390 cm-1处的特征峰与CO32-的反对称伸缩有关,2216 cm-1处出现的特征峰与C=O伸缩振动[41]有关,CO32-可能是水热过程中尿素分解生成的。对于纯PANI,在1580、1488和1149 cm-1处的特征峰,分别对应PANI分子的醌环、苯环的C=C伸缩振动和C-N拉伸振动[42-44]。NiFe-LDH/PANI和NiFe(s)-LDH/PANI的IR谱图中也出现了PANI的特征峰,进一步证实采用原位化学氧化法成功得到PANI改性的LDH复合涂层。比较可见,NiFe-LDH/PANI和NiFe(s)-LDH/PANI在1488 cm-1处特征峰均发生红移,分别移至1493和1502 cm-1。PANI与LDH的复合可能存在化学键的结合,从而影响与之结合的原子周围的电子云密度,导致红移。PANI分子结构中的亚胺N原子是最活跃的作用点,具体的成键方式在后面的XPS部分详细讨论。
图3
图3
304不锈钢表面所制备涂层的FT-IR图
Fig.3
FT-IR spectroscopies of the as-prepared coatings on 304 strainless steel
采用ASTM D3359-17标准评估不同涂层与基底材料间的附着力等级,并利用偏光显微镜观察涂层变化。图4为不同涂层附着力试验后的光学照片。由图可见,NiFe(s)-LDH涂层大部分都从基底表面脱落,与基底的结合力较弱,而NiFe-LDH涂层仅有部分涂层脱落。对于NiFe(s)-LDH/PANI和NiFe-LDH/PANI复合涂层,其横切边缘均完整光滑,无脱落,与基底表现出良好的粘附强度。这表明添加PANI提升了LDH涂层与基底304不锈钢的结合力。
图4
图4
304不锈钢表面不同涂层附着力测试后的光学形貌
Fig.4
Microscope images of NiFe(s)-LDH (a), NiFe-LDH (b), NiFe(s)-LDH/PANI (c) and NiFe-LDH/PANI (d) coatings on 304 strainless steel after adhesion test
通过极化曲线测试涂层在1 mol/L H2SO4溶液中的耐蚀性能。图5为不同涂层的动电位极化曲线。相比于空白304不锈钢,所有涂层的腐蚀电位 (Ecorr) 均大幅度正移。通常较高的Ecorr值意味着涂层具有较好的屏蔽保护作用。比较可见,相比于由基底304不锈钢提供Fe的涂层样品,添加Fe(NO3)3得到的LDH涂层,其Ecorr值发生了一定程度的左移 (约200 mV)。可能是由于NiFe-LDH的层状结构,具有更强的阴离子交换能力,导致Ecorr负移。从图中还可看到,PANI改性LDH涂层后,对应样品的腐蚀电流密度Icorr有所上升。这并不意味着添加PANI降低了NiFe-LDH涂层的耐蚀性能。有研究表明,在1 mol/L H2SO4溶液中,聚乙烯吡咯烷酮 (PVP)-PANI复合涂层涂覆的304不锈钢的Icorr增加了1个数量级,但高的Icorr不是源于基底材料的腐蚀,而是PANI的氧化或溶液与复合物之间的离子交换所致[38]。因此,PANI改性后LDH涂层的Icorr增加归因于PANI的阳极氧化作用。
图5
图5
304不锈钢表面不同涂层在1 mol/L H2SO4溶液中的动电位极化曲线
Fig.5
Potential polarization curves of the 304 strainless steel samples with different coatings in 1 mol/L H2SO4 solution
图6a为304不锈钢表面四种不同涂层的Nyquist图。可以看出,NiFe(s)-LDH和NiFe-LDH涂层都表现出一个容抗弧,采用图6b的等效电路来拟合。当PANI改性LDH涂层后,其Nyquist图发生显著变化,如图6a中的插图所示。在高频区出现一个较小的容抗弧,而低频区出现一条倾斜角接近90°的直线。考虑到图中的直线几乎垂直于实轴,在拟合电路中选择ZT来取代常用的Zd,表明PANI改性后的LDH涂层可有效阻挡溶液中离子的扩散,拟合电路如图6c所示。在两个等效电路图中,Rs表示溶液电阻,Rct和CPEdl分别对应于电荷转移电阻和与之关联的双电层电容,Rct值越大表明涂层的物理屏蔽作用越强,耐蚀性能越好。CPEf和Rf分别表示涂层的电容和电阻。拟合得到的结果列于表1。
图6
图6
304不锈钢表面不同涂层在1 mol/L H2SO4溶液中的Nyquist图及等效电路
Fig.6
Nyquist plots (a) of the 304 strainless steel samples with different coatings in 1 mol/L H2SO4 solution, and equivalent circuits for NiFe(s)-LDH and NiFe-LDH coatings (b) and NiFe(s)-LDH/PANI and NiFe-LDH/PANI coatings (c)
表1 304不锈钢表面不同涂层在1 mol/L H2SO4溶液中浸泡不同时间后的电化学阻抗谱拟合参数
Table 1
| Sample | Time / h | CPEf / Ω-1·cm-2·S-n | Rf / Ω·cm2 | CPEdl / Ω-1·cm-2·S-n | Rct / Ω·cm2 | T / Ω-1·cm-2·S-0.5 | t / S-0.5 |
|---|---|---|---|---|---|---|---|
| NiFe(s)-LDH | 2 | 4.89×10-5 | 1.566 | 6.23×10-5 | 25.90 | --- | --- |
| NiFe-LDH | 0 | --- | --- | 4.08×10-5 | 4.36×105 | --- | --- |
| 2 | --- | --- | 4.32×10-5 | 3.39×106 | --- | --- | |
| 8 | --- | --- | 4.42×10-5 | 1.98×106 | --- | --- | |
| 24 | 4.27×10-5 | 422.5 | 3.31×10-5 | 1.90×105 | --- | --- | |
| 36 | 5.63×10-5 | 400.0 | 5.78×10-5 | 9.31×104 | --- | --- | |
| 72 | 4.85×10-5 | 413.6 | 2.00×10-4 | 4.55×104 | --- | --- | |
| 168 | 2.60×10-5 | 438.4 | 1.82×10-4 | 7.59×104 | --- | --- | |
| NiFe(s)-LDH/PANI | 0 | 4.59×10-5 | 162.3 | --- | --- | 0.906 | 1.873 |
| 2 | 6.08×10-5 | 183.1 | --- | --- | 0.814 | 1.574 | |
| 8 | 1.35×10-4 | 175.3 | --- | --- | 0.108 | 1.295 | |
| 24 | 2.14×10-4 | 224.4 | --- | --- | 0.109 | 1.081 | |
| 36 | 3.46×10-4 | 190.4 | --- | --- | 0.128 | 0.827 | |
| NiFe-LDH/PANI | 0 | 3.01×10-5 | 43.2 | --- | --- | 0.477 | 2.764 |
| 2 | 3.95×10-5 | 32.6 | --- | --- | 0.386 | 2.690 | |
| 8 | 3.95×10-5 | 32.6 | --- | --- | 0.254 | 1.533 | |
| 24 | 4.33×10-5 | 29.9 | --- | --- | 0.209 | 1.308 | |
| 36 | 4.62×10-5 | 34.7 | --- | --- | 0.221 | 1.213 | |
| 72 | 5.15×10-5 | 39.4 | --- | --- | 0.195 | 1.101 | |
| 168 | 5.23×10-5 | 39.1 | --- | --- | 0.186 | 0.935 |
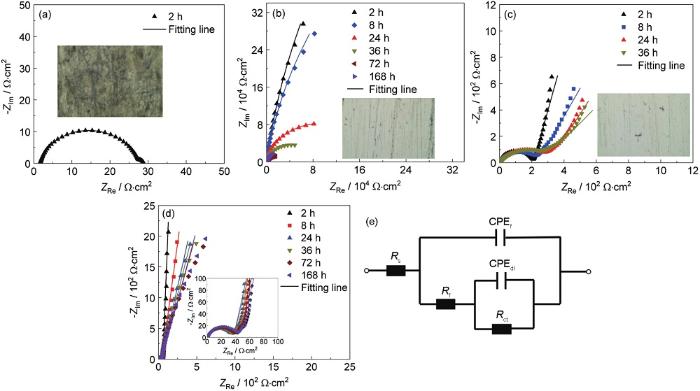
图7为4种涂层在1 mol/L H2SO4中浸泡不同时间的Nyquist图。从图7a可以看出,NiFe(s)-LDH涂层浸泡2 h后,其Nyquist图变成一个非常小的半圆弧。对于NiFe-LDH涂层,其Nyquist图中的圆弧半径随浸泡时间的延长逐渐变小,浸泡24 h后圆弧半径明显下降,如图7b所示。因此,采用图7e中的等效电路来拟合NiFe(s)-LDH涂层浸泡2 h和NiFe-LDH浸泡24 h及之后的EIS谱,拟合结果见表1。可以看出,浸泡2 h后,NiFe(s)-LDH涂层样品的Rct值从5.55×105 Ω·cm2迅速下降至25.90 Ω·cm2,表明其防腐蚀性能已基本丧失。在实验中观察到,此LDH涂层在1 mol/L H2SO4中浸泡2 h后表面开裂,从裂缝处将其剥离304不锈钢基底,采用偏光显微镜观察304不锈钢。插入的图片显示,304不锈钢表面已腐蚀。而对于NiFe-LDH涂层,浸泡168 h后,Rct值从最初的4.36×105 Ω·cm2降低为7.59×104 Ω·cm2。插入的图片则显示,304不锈钢表面出现轻微点蚀。以上结果进一步证实,添加Fe(NO3)3增强了LDH涂层的物理屏蔽作用。
图7
图7
不同涂层在1 mol/L H2SO4溶液中浸泡不同时长的Nyquist图及等效电路图
Fig.7
Nyquist diagrams of different coatings immersed in 1 mol/L H2SO4 solution for different durations (a-d) and equivalent circuit diagram (e)
NiFe(s)-LDH/PANI和NiFe-LDH/PANI复合涂层在1 mol/L H2SO4溶液中的稳定性如图7c和d所示。从NiFe(s)-LDH/PANI涂层的Nyquist图中发现,随着浸泡时间的延长,低频区的直线与实轴的夹角越来越小。浸泡36 h后,夹角降低为45°,这表明腐蚀性离子在涂层中的扩散显著,意味着阻挡层的作用在慢慢减弱。与此同时,NiFe(s)-LDH/PANI涂层从304不锈钢表面脱落。与两种NiFe(s)-LDH和NiFe-LDH涂层的情形不同的是,此时304不锈钢表面尚未发生明显变化,如插入的图片所示,未观察到304不锈钢的腐蚀。对于NiFe-LDH/PANI涂层,即使浸泡168 h后,低频区的直线与实轴的夹角也较大,意味着涂层仍然具有良好的保护作用。以上分析表明,PANI的改性显著提高了两种LDH涂层在1 mol/L H2SO4溶液中对基底保护作用的持久性。
Yao等[45]研究指出,腐蚀性离子自溶液向金属基底的渗透可分为两个过程。第一个过程是离子快速进入金属表面的涂层,第二个过程则是离子在涂层内缓慢地扩散,最终趋于饱和。一般认为,CPEf值与第一个过程相关,较小的CPEf值表明溶液中较少的离子进入涂层。从表1可见,在浸泡期间,NiFe-LDH/PANI涂层相比于NiFe(s)-LDH/PANI具有较小的CPEf值,这一点表明NiFe-LDH/PANI涂层拥有较好的阻挡层性能,可较好地抑制腐蚀性离子向涂层地快速扩散。此外,t值反映了离子在涂层中的扩散时间,与第二个过程相关。在浸泡初始时刻,NiFe-LDH/PANI涂层对应的t值为2.764,而NiFe(s)-LDH/PANI涂层的t值仅为1.873。在浸泡过程中,前者的t值总是大于后者的。较高的t值表明NiFe-LDH/PANI涂层的致密结构有效地阻挡了腐蚀性离子在涂层内的扩散,有利于保护金属基底免于腐蚀性离子的入侵。
另一方面,PANI改性的两种LDH涂层浸泡于H2SO4溶液中时,溶液中的腐蚀性离子可参与PANI的氧化还原反应,可能降低PANI对金属的阳极保护效应。幸运地是,LDH涂层中的阳离子,如Fe3+、Ni2+或其氢氧化物具有比PANI高的电位,可原位氧化还原态的PANI,在浸泡期间保持PANI良好的阳极保护作用。Wang等[46]研究表明,Rf数值与复合涂层中PANI组分的氧化态和还原态的比值相关。若Rf值较小,意味着PANI呈现较多的氧化态;反之,则有较多还原态PANI。由表1可见,NiFe-LDH/PANI样品对应的Rf值明显小于NiFe(s)-LDH/PANI的,这表明NiFe-LDH/PANI复合涂层中含有更多氧化态的PANI,有助于提升PANI对304不锈钢的阳极保护效应。此外,整个168 h浸泡期内,NiFe-LDH/PANI的Rf值亦变化较小,表明该复合涂层在长期浸泡中保持了较高的化学稳定性。
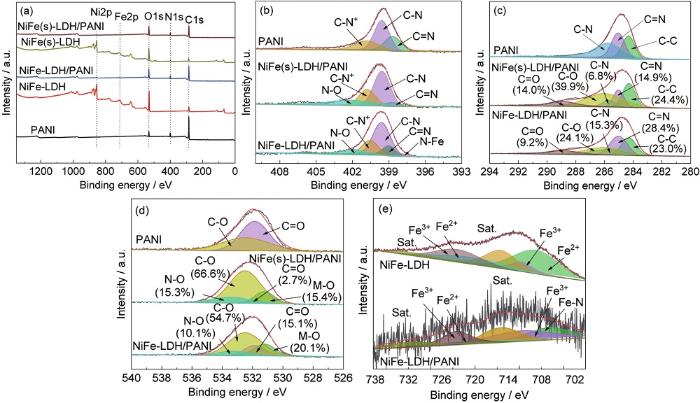
采用XPS对涂层进行表征,以分析不同LDH涂层表面化学组成及涂层的微结构,如图8所示。图8a为304不锈钢表面不同涂层的XPS全谱图。验证了NiFe-LDH和NiFe(s)-LDH涂层中Ni、Fe、C、N和O的存在,这与EDS mapping图元素映射数据一致。图8b中,N 1s高分辨谱分峰解析得到结合能为398.6、399.5和400.7 eV的3个峰,分别对应于PANI材料中的C=N键、C-N键和C-N+键。与纯PANI相比,NiFe-LDH/PANI样品的N 1s谱中还存在结合能为401.9 eV和397.8 eV两个峰,分别归属为N-O键和M-N键。N-O键可能源于水热反应中NO3-的插层。至于M-N键,推测可能是Fe-N键。另一方面,由Fe 2p的高分辨光谱图 (图8e) 可见,在NiFe-LDH/PANI复合涂层中,除了Fe2+和Fe3+特征峰外,在结合能为706.7 eV还出现一个新的特征峰,据此可确认Fe-N键的存在。这也证实,PANI与NiFe-LDH之间存在化学键的结合,即PANI结构中的亚胺N原子和Fe发生作用,一部分正电荷离域至芳环,使芳环的电子云密度降低,从而提高NiFe-LDH/PANI复合物的化学稳定性。
图8
图8
304不锈钢表面不同涂层的XPS谱图
Fig.8
XPS survey spectra of various coatings (a), and fine spectra of N 1s (b), C 1s (c) and O 1s (d) for PANI, NiFe-LDH/PANI and NiFe(s)-LDH/PANI coatings, and Fe 2p (e) for NiFe-LDH and NiFe-LDH/PANI coatings
图8c为C 1s的XPS谱图。NiFe-LDH/PANI样品的C 1s峰解析得到结合能为284.3、285.0、285.7、286.0和288.9 eV 5个峰,分别对应C-C键、C=N键、C-N键、C-O键和C=O键[47,48]。其中C-O键和C=O键,是源于CO32-插入到NiFe-LDH中。对比可见,在NiFe-LDH/PANI涂层中,C=N键和C-N键所占百分比明显增大,意味着此涂层中氧化态PANI的含量较高。与之前EIS分析结果一致,更多氧化态的PANI可增强NiFe-LDH/PANI复合涂层对基底材料的阳极保护效应。从图8d可见,NiFe-LDH/PANI样品的O 1s峰可解析得到结合能为531.2、531.9、532.5和533.3 eV 4个峰,分别对应晶格氧 (M-O,M为Fe或Ni)、C=O键、C-O键和N-O键[48,49] (来源于NO3-)。在纯PANI样品中,出现C=O键和C-O键可能是由于空气中带入的CO2。对于NiFe-LDH/PANI涂层,C=O键所占比例显著增大,表明更多CO32-的插入。这也反映了NiFe-LDH层状结构的特征。
3 结论
采用水热法结合原位化学氧化法在304不锈钢表面制备了4种不同LDH涂层。与基底304不锈钢提供Fe的NiFe(s)-LDH相比,添加Fe(NO3)3改变了LDH的表面结构和化学组成。电化学结果表明,添加Fe(NO3)3后,LDH样品的腐蚀电位发生一定程度的负移,是由于其特征的层状结构,拥有更强的阴离子交换能力。相比于LDH涂层,LDH/PANI复合涂层的腐蚀电流密度有所升高,这是由于PANI的阳极氧化作用所致,并不是304不锈钢的腐蚀。浸泡实验和EIS分析表明,NiFe-LDH/PANI复合涂层在强酸性溶液中对基底具有优良的保护效应和长期的稳定性。FT-IR和XPS分析表明,在NiFe-LDH/PANI复合涂层中存在Fe-N键。这样的结合方式,在LDH和PANI间产生协同效应。一方面,PANI的添加优化复合涂层的表面结构,提升涂层的物理屏蔽保护;另一方面,LDH中阳离子具有较高的电位,利于维持PANI有效的阳极保护作用。N原子和Fe原子发生作用,使得复合涂层中PANI离域程度升高,从而提升NiFe-LDH/PANI复合涂层的化学稳定性。
参考文献
A protective superhydrophobic Mg-Zn-Al LDH film on surface-alloyed magnesium
[J].
Electrodeposited Zn-Al layered double hydroxide films for corrosion protection of aluminum alloys
[J].
Research progress on anti-corrosion coatings of layered double hydroxides
[J].
层状双金属氢氧化物防腐蚀涂层材料的研究进展
[J].
Effect of Ce on corrosion resistance of films of ZnAlCe-layered double hydroxides on Mg-alloy
[J].
Ce对镁合金表面ZnAlCe-LDHs薄膜耐腐蚀性能的影响机理研究
[J].
Layered double hydroxide (LDH) for multi-functionalized corrosion protection of metals: a review
[J].Layered double hydroxide (LDH) has been widely developed in the field of corrosion and protection in recent years based on its unique characteristics including anion capacity, anion exchange ability, structure memory effect, and barrier resistance. This paper comprehensively reviews recent work on the preparations, properties of LDH in the forms of powder and film and their applications in different environments in corrosion and protection. Some novel perspectives are also proposed at the end of the review for future research in corrosion and protection field.
A self-healing corrosion protection coating with graphene oxide carrying 8-hydroxyquinoline doped in layered double hydroxide on a micro-arc oxidation coating
[J].
ZnO/ZnGaNO heterostructure with enhanced photocatalytic properties prepared from a LDH precursor using a coprecipitation method
[J].
Effect of alloy cations on corrosion resistance of LDH/MAO coating on magnesium alloy
[J].
Chemical modification of hydrotalcite coating for enhanced corrosion resistance
[J].
PPA-containing layered double hydroxide (LDH) films for corrosion protection of a magnesium alloy
[J].
A comparative study and optimization of corrosion resistance of ZnAl layered double hydroxides films intercalated with different anions on AZ31 Mg alloys
[J].
Development of a thiophene derivative modified LDH coating for Mg alloy corrosion protection
[J].
Sodium dodecyl sulfate (SDS) intercalated Mg-Al layered double hydroxides film to enhance the corrosion resistance of AZ31 magnesium alloy
[J].
A comparative study on corrosion inhibitive effect of nitrate and phosphate intercalated Zn-Al- layered double hydroxides (LDHs) nanocontainers incorporated into a hybrid silane layer and their effect on cathodic delamination of epoxy topcoat
[J].
Autogenous formation and smart behaviors of nitrite- and nitrate-intercalated layered double hydroxides (LDHs) in portland cement-metakaolin-dolomite blends
[J].
Incorporation of layered double hydroxides modified with benzotriazole into an epoxy resin for the corrosion protection of Zn-Mg coated steel
[J].
Improving the corrosion protection ability of epoxy coating using CaAl LDH intercalated with 2-mercaptobenzothiazole as a pigment on steel substrate
[J].
The growth behavior and properties of orientated LDH film composited with reduced graphene oxide
[J].
Sulfonated polyaniline assisted hierarchical assembly of graphene-LDH nanohybrid for enhanced anticorrosion performance of waterborne epoxy coatings
[J].
Facile one-step synthesis of nanocomposite based on carbon nanotubes and nickel-aluminum layered double hydroxides with high cycling stability for supercapacitors
[J].
Ni-Al polyvanadate layered double hydroxide with nanoceria decoration for enhanced corrosion protection of aluminium alloy
[J].
Inhibitive effect of SiO2@ NO2 - intercalated MgAl-LDH nanocomposite on steel in Cl- contaminated saturated Ca(OH)2 solution
[J].
Selective lithium adsorption of silicon oxide coated lithium aluminum layered double hydroxide nanocrystals and their regeneration
[J].
Achieving an acid resistant surface on magnesium alloy via bio-inspired design
[J].
Direct synthesis of layered double hydroxides monolayer nanosheets for co-assembly of nanobrick wall hybrid film with excellent corrosion resistance
[J].
Extraordinary corrosion protection from polymer-clay nanobrick wall thin films
[J].
Facile synthesis of LDH nanoplates as reinforcing agents in PVA nanocomposites
[J].
Anti-corrosive performance of electropolymerized phosphomolybdic acid doped PANI coating on 304SS
[J].
Electropolymerization of camphorsulfonic acid doped conductive polypyrrole anti-corrosive coating for 304SS bipolar plates
[J].
Facile fabrication of the polyaniline/layered double hydroxide nanosheet composite for supercapacitors
[J].To overcome the structural instability of polyaniline (PANI) due to repeated volumetric swelling and shrinking during charge/discharge process, the LDH/PANI composite was designed and prepared via the assembly of organic PANI and 2D host nanosheets from the MgFe layered double hydroxide (MgFe-LDH) through incorporation of PANI between the nanosheets gap using in situ polymerization. Owing to the combined advantages of conducting PANI and 2D nanosheets, the LDH/PANI composite exhibited excellent supercapacitive performance, including low resistance, high specific capacitance, good charge/discharge stability and long-term cycling life, which greatly improved faradaic redox reaction and mass transfer. As electrode material for supercapacitors, the LDH/PANI composite showed a maximum specific capacitance of 592.5 F.g(-1) at a current density of 2 A.g(-1), and achieved remarkable capacitance retentions of 87% after 500 cycles. The LDH/PANI material may be a potential economical alternative electrode material for supercapacitors.
Preparation and enhanced properties of polyaniline/grafted intercalated ZnAl-LDH nanocomposites
[J].
Anticorrosive performance of polyaniline/waterborne epoxy/poly (methylhydrosiloxane) composite coatings
[J].
Novel anti-corrosion coatings from rubber-modified polybenzoxazine-based polyaniline composites
[J].
Crystallinity effect of NiFe LDH on the growth of Pt nanoparticles and hydrogen evolution performance
[J].
NiFe LDH nanodots anchored on 3D macro/mesoporous carbon as a high-performance ORR/OER bifunctional electrocatalyst
[J].
Facilitating active species generation by amorphous NiFe-Bi layer formation on NiFe-LDH nanoarray for efficient electrocatalytic oxygen evolution at alkaline pH
[J].
2D/2D FeNi-LDH/g-C3N4 hybrid photocatalyst for enhanced CO2 photoreduction
[J].
二维/二维FeNi-LDH/g-C3N4复合光催化剂用于促进CO2光还原反应
[J].
One-step electrochemical synthesis of poly (vinyl pyrrolidone) modified polyaniline coating on stainless steel for high corrosion protection performance
[J].
Preparation and application of polyaniline doped with different sulfonic acids for supercapacitor
[J].
2D-2D growth of NiFe LDH nanoflakes on montmorillonite for cationic and anionic dye adsorption performance
[J].
Effect of N-doping, exfoliation, defect-inducing of Ni-Fe layered double hydroxide (Ni-Fe LDH) nanosheets on catalytic hydrogen storage of N-ethylcarbazole over Ru/Ni-Fe LDH
[J].
Synthesis of Fe3O4/rGO@PANI with three-dimensional flower-like nanostructure and microwave absorption properties
[J].
An all-paper, scalable and flexible supercapacitor based on vertically aligned polyaniline (PANI) nano-dendrites@fibers
[J].
Three-dimensional needle branch-like PANI/CoNiP hybrid electrocatalysts for hydrogen evolution reaction in acid media
[J].
Corrosion protection of epoxy coatings containing 2-hydroxyphosphonocarboxylic acid doped polyaniline nanofibers
[J].
Electropolymerization and corrosion protection performance of the Nb:TiO2 nanofibers/polyaniline composite coating
[J].
Rapid and efficient removal of diclofenac sodium from aqueous solution via ternary core-shell CS@PANI@LDH composite: experimental and adsorption mechanism study
[J].
Hollow carbon spheres/graphene hybrid aerogels as high-performance adsorbents for organic pollution
[J].
Achieving high specific capacity of lithium-ion battery cathodes by modification with “N-O˙” radicals and oxygen-containing functional groups
[J].