发展超 (超) 临界机组是实现“碳中和、碳达峰”战略目标的重要措施之一[1]。近些年,600 ℃超 (超) 临界技术逐步成熟,为了进一步提高发电效率、降低污染物排放,欧洲、美国、日本和中国先后启动了蒸气温度达到700 ℃以上的先进超 (超) 临界发电技术研究计划[2]。然而,高温蒸气环境中合金管材发生氧化反应,形成包含孔隙的氧化膜[3,4],剥落的氧化膜引起超温、爆管,严重危害电站的安全运行[5-7]。随着蒸气参数的升高,对高温管材抗氧化腐蚀性能的要求也逐步提高[8]。因此,研究高温蒸气环境中合金管材的氧化特性,对减缓氧化膜生长、预防氧化膜剥落、提高超临界机组运行可靠性具有重要科学意义和实际应用价值。
前期研究基于ReaxFF-MD方法,揭示了反应初期合金表面蒸气分子的吸附和分解机制[23]。本文在此基础上,揭示合金表面氧化膜形成机理,阐述氧化膜内原子迁移机制和形貌特性,进一步探讨了环境温度对合金氧化膜生长的影响。研究成果为超 (超) 临界机组过热器/再热器管道选材、减缓合金氧化速率提供理论基础。
1 ReaxFF力场
式中,系统的总能量 (Esystem) 包括:键能 (Ebond)、过协调能量 (Eover)、欠协调能量 (Eunder)、孤对电子项 (Elp)、键角能量 (Eval、Epen)、共轭能量 (Econj)、扭转能量 (Etors)、范德华相互作用力 (EvdWaals)、库仑力相互作用力 (ECoulomb)。本文采用Shin等[30]开发的Fe/Cr/H/O反应力场研究FeCr合金氧化机理。该力场可预测合金在超临界蒸气环境下的氧化动力学,并与实验结果吻合[31,32]。在前期研究中,采用密度泛函理论研究了H2O在FeCr合金表面的吸附和解离过程[33]研究结果表明,H2O解离成OH-和H+,然后它们分别转移到桥位和空位;紧接着,OH化学键发生断裂,解离的O和H原子移动到两个相邻的空位。该密度泛函理论研究结果与本文计算结果具有较高一致性,进而验证了ReaxFF反应力场参数的准确性。
2 模拟过程
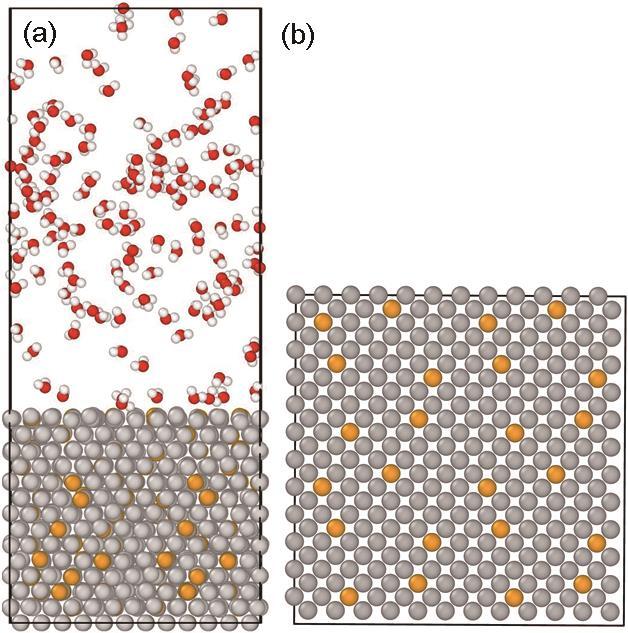
Das等[34]通过将Fe基体中Fe原子替换成Cr原子,建立FeCr合金的分子动力学模型,研究FeCr合金-氧化膜交界面原子迁移机理。Wang等[35]通过将Fe基体中Fe原子替换成Cr、Ni原子,建立FeCrNi合金的分子动力学模型,研究FeCrNi合金-水蒸气界面原子反应行为。国内外学者通过原子替换法建立氧化模型,本文借鉴该方法,建立2.99 nm×2.99 nm×2.74 nm Fe基体模型,模型中包含2016个Fe原子;参考T91合金 (具有优异的综合性能,已在世界各国超 (超) 临界发电机组高温承压部件上广泛应用) 中Cr原子浓度,并将模型中9%的Fe原子替换成Cr原子,如图1所示。在FeCr合金表面添加均匀分布的蒸气分子 (密度为88.15 kg/m3),然后将FeCr合金底层固定,使用NVT正则系综弛豫50 ps以达到平衡态,从而获得稳定构型。每隔一定时间步向FeCr合金表面添加蒸气分子,以保持蒸气密度。模型XY方向设置为周期性边界条件,Z方向设置为非周期性边界条件,并在上边界设置了反射壁。采用NVT正则系综进行氧化模拟,利用Velocity-verlet算法对时间步长为0.2 fs的运动方程进行积分。每隔1个时间步,使用电荷平衡方法 (QEq) 计算系统的电荷。
图1
图1
合金高温蒸气氧化分子动力学模型
Fig.1
Molecular dynamics model of alloy in high temperature steam (The white, red, gray and yellow atoms are H, O, Fe and Cr, respectively): (a) main view, (b) top view
3 模拟结果与分析
图2
图2
氧化过程中顶层Fe、Cr原子和底层O原子的Z方向坐标演变曲线
Fig.2
Average positions of top Fe, Cr atoms and bottom O atoms along the Z direction during the oxidation process at 900 K (a), 1000 K (b) and 1100 K (c)
图3
图3
1100 K氧化过程中合金表面蒸气分子消耗量演变曲线
Fig.3
Consumption of steam molecules during the 1100 K oxidation process
3.1 氧化膜形成阶段表面反应和原子迁移特征
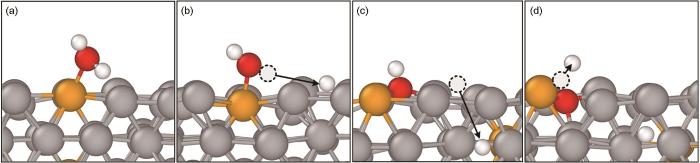
在图2c和图3中氧化初始阶段 (0~200 ps),合金表面反应剧烈,Fe、Cr、O原子迁移速度较快。图4给出了该阶段FeCr合金表面单个蒸气分子的分解和反应路径:首先蒸气分子迅速吸附到合金表面的Cr原子附近,形成金属水合物 (图4a);然后,图4b中O-H化学键发生断裂,分离出来的H原子电荷降低了0.29 e-,并重新吸附到合金表面的Fe原子附近,余下的O-H离子与附近Fe和Cr原子成键,形成金属氢氧化物 (
图4
图4
合金表面蒸气吸附和分解
Fig.4
Steam chemisorption and decomposition on the alloy surface: (a) 1 ps, (b) 12 ps, (c) 14.3 ps, (d) 15 ps (The white, red, gray and yellow atoms are H, O, Fe and Cr, respectively)
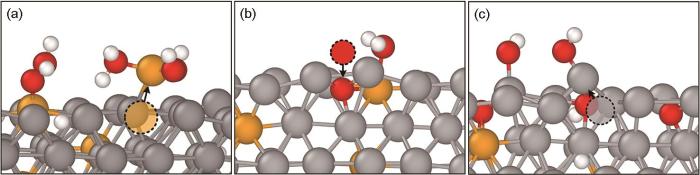
在图2c中氧化膜形成阶段,合金表面Fe、Cr、O原子都发生迁移。图5给出了合金表面的原子迁移路径:首先,O原子吸附到FeCr合金表面后,Cr原子从合金表面析出,析出的Cr原子以氢氧化物的形式游离于合金表面 (图5a),这与文献[13]中观察到的Cr蒸发现象一致;反应到15 ps,发现蒸气分子中O原子吸附到合金表面,并与表层Fe、Cr原子成键,形成金属氧化物 (图5b);接着,O原子向内迁移、氧化合金内部金属原子,导致金属原子在氧化物生长内应力作用下向外迁移 (图5c)。氧化反应放热为表面Fe、Cr原子提供动能,动能增加引起晶格膨胀从而产生内应力,在内应力与动能的共同作用下使得表面金属原子向外迁移。
图5
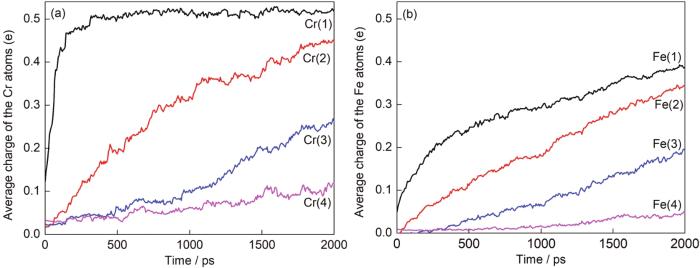
图6
图6
合金表面四层金属原子平均电荷变化
Fig.6
Average charge change of the top four layers of FeCr alloy: (a) Cr, (b) Fe (The numbers in brackets represent the number of top three layers)
3.2 氧化膜生长阶段形貌和原子迁移特征
图7
图7
不同时刻氧化膜元素分布
Fig.7
Distribution of elements in oxide film at 300 ps (a) and 2000 ps (b)
其中,ri (0) 为原子i的初始位置;ri (t) 是原子i在t时刻的位置;N为原子总数;t为时间。图8给出了1500~2000 ps氧化膜内Fe、Cr、O原子沿Z方向的MSD,表明Fe原子向外迁移速率明显快于Cr原子。Cr原子半径较大,形成的空位可参与Fe、Cr原子迁移,而Fe原子形成的空位不利于Cr原子迁移。在FeCr合金中Cr原子数量较少,难以形成适合Cr原子迁移的空位,迁移速率较慢。相反,Fe原子数量较多半径较小,可以通过Fe、Cr原子形成的空位进行迁移,因此Fe原子迁移速率较快。由于Fe原子和Cr原子迁移速率的差异,导致合金表面氧化膜元素分布不均,进而出现实验观察到的双层氧化膜现象。
图8
图8
氧化膜内Fe、Cr原子的均方位移
Fig.8
Mean square displacement of Fe, Cr atoms in the oxide layer
3.3 环境温度对合金蒸气氧化的影响
图9给出了不同环境温度下,氧化过程中由快速形成转变为缓慢生长的临界时间tm。在900 K环境温度下,临界时间tm为385 ps,蒸气分子消耗速率为0.145 ps-1。当环境温度提高到1000 K,临界时间tm降低到325 ps,蒸气分子消耗速率增加到0.178 ps-1;当环境温度进一步提高到1100 K,临界时间tm缩短到200 ps,蒸气分子消耗速率上升为0.275 ps-1。这些结果表明,升高温度有助于蒸气分子的解离,从而加速氧化膜的形成,使得氧化膜由形成阶段向生长阶段的转变提前。在900、1000和1100 K条件下,合金表面呈现抛物线氧化规律,氧化膜降低了表面的金属活度,后期氧化膜内原子迁移成为反应速率控制方式,使得反应速率随着氧化时间的延长而降低。
图9
图9
不同温度下合金表面消耗的蒸气分子数
Fig.9
Variation trend of the number of steam molecules consumed at different temperatures
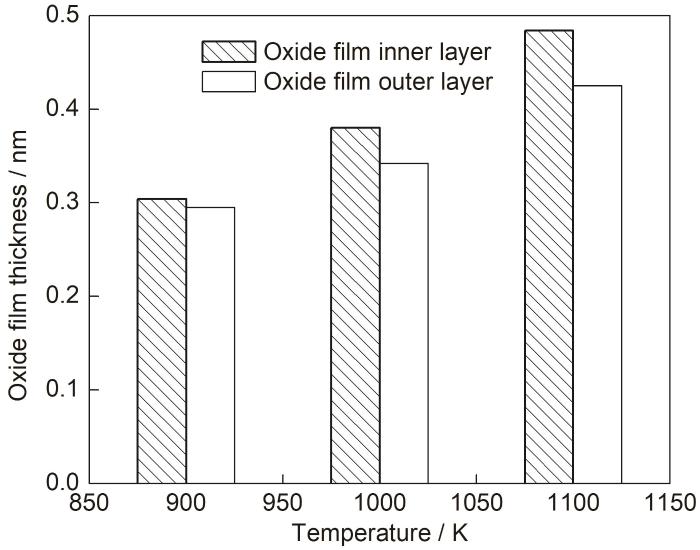
图10给出了模拟2000 ps后不同环境温度中氧化膜的厚度,可以看出温度越高形成的氧化膜越厚。Zhu等[8]通过研究铁素体-马氏体钢在不同实验温度下的氧化行为,也发现氧化速率随温度的提高而升高,这与本文模拟结果一致。如图10所示,环境温度由900 K升高到1000 K时,外氧化膜厚度增加了0.047 nm,内氧化膜厚度变化了0.076 nm;当温度进一步提高到1100 K后,外氧化膜厚度增长了0.083 nm,而内氧化膜厚度变化了0.104 nm。可以看出升高温度使得内、外氧化膜厚度同时增加,并且温度对内层氧化膜厚度影响更大。这表明提高环境温度对氧化膜内原子的迁移行为有促进作用,并且对O原子向内迁移的作用更显著。
图10
图10
模拟2000 ps后不同反应温度的氧化膜厚度
Fig.10
Thickness of the oxide layer at different reaction temperatures was simulated after 2000 ps
4 结论
本文基于Reax-FF反应分子动力学,建立高温蒸气环境中T91 FeCr合金氧化模型,从原子尺度揭示氧化初期合金表面蒸气化学吸附、分解和迁移机制,阐述FeCr合金表面双层氧化膜形成机理,进一步探讨了环境温度合金氧化特性的影响。主要研究结论包含:
(1) 在合金氧化初期,蒸气分子迅速吸附到合金表面的Cr原子附近,并逐步分解成O和H原子。迁移到FeCr合金内部的O原子氧化金属原子,形成内层FeCr氧化物。氧化反应放热为表面Fe、Cr原子提供动能,动能增加引起晶格膨胀从而产生内应力,在内应力与动能的共同作用下使得表面金属原子向外迁移。
(2) 一旦合金表面形成完整的氧化膜,合金的蒸气氧化特征发生变化。合金表面Fe原子呈现层状分布,并且在内层氧化膜生长内应力的作用下,Fe原子逐层向外迁移,形成外层Fe氧化膜。由于氧化膜内Fe原子和Cr原子迁移速率的差异,导致氧化层元素分布不均,进而出现实验观察到的双层氧化膜现象。
(3) 环境温度对FeCr合金蒸气氧化特性有重要影响,并且随着环境温度的升高,原子在氧化膜中的扩散速率加快,氧化膜对合金的保护作用逐步降低。合金氧化包含氧化膜快速形成和缓慢生长两个阶段,提高温度可加速合金氧化转变为缓慢生长阶段。
参考文献
Industry development and frontier technology roadmap of thermal power generation
[J].
火力发电产业发展与前沿技术路线
[J].
Investigating the role of oxygenated treatment on oxide exfoliation in TP347H alloy tube based on finite element method
[J].
基于有限元的溶氧蒸汽环境TP347H管氧化膜剥落特性分析
[J].
A research on the corrosion and stress corrosion cracking susceptibility of 316L stainless steel exposed to supercritical water
[J].
Influence of temperature on the oxide spallation of T91 alloy superheater tubes in power plant
[J].
Corrosion behavior and mechanism of heat-resistant steel T91 in supercritical carbon dioxide environment
[J].
超临界二氧化碳环境下耐热钢T91腐蚀行为机理研究
[J].
Preliminary calculations of erosion wear resulting from exfoliation of iron oxides in austenitic superheaters
[J].
Investigation of overheating of the final super-heater in a 660 MW power plant
[J].
Influence of temperature on the oxidation behaviour of a ferritic-martensitic steel in supercritical water
[J].
Influence of exposure environment on the corrosion resistance of 2-9% Cr steels
[J].
Oxidation of low-alloy steel in high temperature steam and supercritical water
[J].
Corrosion of engineering materials in a supercritical water cooled reactor: characterization of oxide scales on Alloy 800H and stainless steel 316
[J].
Understanding the surface oxide evolution of T91 ferritic-martensitic steel in supercritical water through advanced characterization
[J].
Oxidation and chromia evaporation of austenitic steel TP347HFG in supercritical water
[J].
Corrosion behavior of austenitic stainless steel with different Cr contents in 700 ℃ coal ash/high sulfur flue-gas environment
[J].
不同Cr含量的奥氏体不锈钢在700 ℃煤灰/高硫烟气环境中的腐蚀行为
[J].研究了3种不同Cr含量(质量分数)的奥氏体不锈钢在700℃下煤灰/高硫烟气环境中的腐蚀行为。结果表明,低Cr合金(19.13%)腐蚀最为严重,氧化膜由外层Fe<sub>2</sub>O<sub>3</sub>、内层Cr<sub>2</sub>O<sub>3</sub>及CrS组成,外层氧化膜剥落严重;中Cr合金(22.78%)氧化膜结构与低Cr合金类似,但腐蚀程度更轻;高Cr合金(24.00%)表面形成了稳定而致密的Cr<sub>2</sub>O<sub>3</sub>层,表现出比其他2种合金更好的抗高温腐蚀性。合金中的NbC会被氧化成Nb<sub>2</sub>O<sub>5</sub>分布在氧化膜中,Nb<sub>2</sub>O<sub>5</sub>的形成会破坏氧化膜的完整性,导致氧化膜更容易发生开裂。
Influence of water vapor on oxide scale morphology of Incoloy800HT at 850 ℃
[J].
Microstructural understanding of the oxidation of an austenitic stainless steel in high-temperature steam through advanced characterization
[J].
Point defects and cation tracer diffusion in (Cr x Fe1- x )3- δ O4 spinels
[J].
Predictions and analyses on the growth behavior of oxide scales formed on ferritic-martensitic in supercritical water
[J].
Corrosion of ferritic-martensitic steels in steam and supercritical water
[J].
Selective adsorption of CO2/N2 mixtures in carbon nanotubes: A molecular simulation study
[J].
碳纳米管吸附分离CO2/N2混合物的分子模拟研究
[J].
The oxidation of Fe/Ni alloy surface with supercritical water: a ReaxFF molecular dynamics simulation
[J].
Molecular dynamics simulations of the oxidation of aluminum nanoparticles using the ReaxFF reactive force field
[J].
Reactive molecular dynamics study of the oxidation behavior of iron‐based alloy in supercritical water
[J].
ReaxFF: a reactive force field for hydrocarbons
[J].
Catalytic effect of calcium on reaction of phenol using reactive molecular dynamics simulation
[J].
钙催化苯酚反应的分子动力学模拟
[J].
Understanding the impacts of moisture and thermal ageing on transformer's insulation by dielectric response and molecular weight measurements
[J].
Pyrolysis of triglyceride materials for the production of renewable fuels and chemicals
[J].
Revealing chemical reactions of coal pyrolysis with GPU-enabled ReaxFF molecular dynamics and cheminformatics analysis
[J].
Study on the pyrolysis mechanism of silicon insulating oil and the influence of moisture on it based on molecular simulation
[J].
基于分子模拟的硅绝缘油高温裂解及水分的影响机理研究
[J].
Development of a ReaxFF reactive force field for Fe/Cr/O/S and application to oxidation of butane over a pyrite-covered Cr2O3 catalyst
[J].
Reactive molecular dynamics simulations of thermal film growth from di-tert-butyl disulfide on an Fe(100) surface
[J].Iron sulfide films are present in many applications, including lubricated interfaces where protective films are formed through the reactions of lubricant additive molecules with steel surfaces during operation. Such films are critical to the efficiency and useful lifetime of moving components. However, the mechanisms by which films form are still poorly understood because the reactions occur between two surfaces and so cannot be directly probed experimentally. To address this, we explore the thermal contribution to film formation of di- tert-butyl disulfide-an important extreme pressure additive-on an Fe(100) surface using reactive molecular dynamics simulations, where the reactive potential parameters are validated by comparison to ab initio calculations. The reaction pathway leading to the formation of iron sulfide surfaces is characterized using the reactive simulations. Then, the film formation process is mimicked by simulations where di- tert-butyl disulfide molecules are cyclically added to the surface and subjected to temperatures comparable to those expected due to frictional heating. The use of a reactive empirical potential is a novel approach to modeling the iterative nature of thermal film growth with realistic lubricant additive molecules.
Mechanism of Al on FeCrAl steam oxidation behavior and molecular dynamics simulations
[J].
The role of Cr atom in the early steam oxidation of Fe‐based alloys: an atomistic simulation
[J].
A fundamental study of Fe-Cr binary alloy-oxide film interfaces at 288 ℃ by computational chemistry calculations
[J].
Ab initio molecular dynamics simulation on interfacial reaction behavior of Fe-Cr-Ni stainless steel in high temperature water
[J].
Molecular dynamics simulation of influence of water molecules on formation process of nascent soot particles
[J].
水分子对初始碳烟颗粒形成过程影响的分子动力学模拟
[J].
Reaction mechanism of the aluminum nanoparticle: physicochemical reaction and heat/mass transfer
[J].
Adsorption and diffusion of H and O on an Ni (1 1 1) surface containing different amounts of Cr
[J].
Diffusion of interstitial hydrogen into and through bcc Fe from first principles
[J].