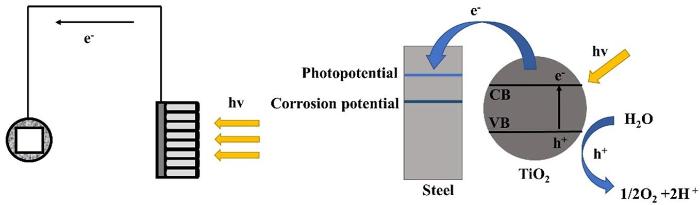
金属材料在工业、农业以及人们生活的各个领域发挥了重要的作用,给社会创造了极大的经济价值。而金属腐蚀给人类社会带来了严重的经济损失、资源浪费与环境污染。太阳能的开发和利用是人们解决资源危机和环境污染问题的重要途径[1-5]。TiO2作为一种成本低、环境友好的N型半导体材料,具有较好的物理化学稳定性、优异的光电转换性能、环境友好等特性[6-10],在太阳能利用领域受到了广泛关注和详细研究,尤其是活性相对较高的锐钛矿型TiO2备受瞩目[11-15]。光生阴极保护就是利用光照,TiO2光阳极价带上的电子跃迁到导带上,进而转移到被保护金属基体,使被保护金属电极电位负移至阴极保护状态,达到防腐蚀的目的[16]。
阳极氧化首先在Ti表面生成一层氧化膜,该氧化膜在含F-电解质中生成可溶的氟化配合物,在外部电位驱动下定向选择性溶解,最终形成TiO2纳米管阵列。为了增加TiO2纳米管阵列的有序性,一般将一次阳极氧化样品超声去除表层钛管,然后进行二次阳极氧化,这样制备的TiO2纳米管具有有序的结构和光滑的表面,以及更优越的性能[27]。
目前,TiO2纳米管主要是在单一含氟电解液中通过二次阳极氧化法制备,Ma等[28]在60 V电压下,于NH4F、H2O、乙二醇电解液中阳极氧化1 h制得TiO2纳米管,其形貌不规则,耦联304不锈钢的开路电位为-0.4V,而304不锈钢的自腐蚀电位约-0.2 V (vs. Ag/AgCl),电位降低了200 mV,而电位负移250 mV以上,才能达到良好的阴极保护效果[29],因此该文献中制备的TiO2纳米管不能对304不锈钢起到良好的阴极保护作用。Dokoohak等[30]采用阳极氧化法比较了不同的乙二醇基电解液成分 (包含NH4F、Bmim-BF4或Bmim-Cl) 用于TiO2纳米管的制备。结果表明,由于含Bmim-BF4的电解液中离子对效果差,纳米管的生长速度非常缓慢,纳米管的形态不规则,管壁粗糙。因此,寻找一种制备规则形貌TiO2纳米管阵列,同时能显著降低被保护金属电位的方法对光生阴极保护领域意义重大。
本文主要通过二次阳极氧化法在3种不同的含氟电解质 (F-、BF4-、F--BF4-) 中制备TiO2纳米管。对TiO2纳米管进行形貌和结构分析,并在开闭可见光条件下进行了光电化学性能测试,探究在不同电解液中制备的3种TiO2纳米管对304不锈钢的光生阴极保护性能并分析了光生载流子分离和复合机制。
1 实验方法
将工业钛片 (纯度≥99.5%) 裁剪成1 cm×2.5 cm长方形钛片,依次放入50 mL丙酮、乙醇和去离子水中超声清洗15 min,去除表面污垢。把清洗过的钛片放入氢氟酸、浓硝酸和去离子水组成的混合溶液[V(HF)∶V(HNO3)∶V(H2O)=1∶3∶6]中30 s进行化学抛光,用去离子水清洗干净后烘干备用。采用二次阳极氧化法,钛片为阳极,高纯石墨片为阴极,两步均在15 ℃条件 (利用恒温槽进行控温),60 V恒压下通电1 h。第一次阳极氧化选用0.4 wt%氟化铵、2.5% (体积分数) 去离子水和乙二醇组成的电解液,阳极氧化结束后在去离子水中利用超声波清洗仪超声清洗20 min,去除一次阳极氧化生成的TiO2薄膜。第二次阳极氧化的电解液 (质量分数) 分为3种:(1) 0.4%氟化铵、2.5%水和乙二醇电解液;(2) 0.4%氟硼酸铵、2.5%水和乙二醇电解液;(3) 0.2%氟化铵、0.2%氟硼酸铵、2.5%水和乙二醇混合电解液 (所有电解液均使用磁力搅拌器搅拌30 min)。阳极氧化结束后用去离子水洗掉钛片表面残留的电解液,烘干后的样品置于马弗炉中,在空气气氛下从室温以5 ℃/min的速度升温至450 ℃,保温2 h,之后随炉冷却至室温,制得不同电解液条件下的TiO2纳米管 (FTNT、BFTNT、F-BFTNT)。
利用Gemini300 型扫描电子显微镜 (SEM) 观察样品形貌;采用D8 advance型X射线衍射仪 (XRD) 分析样品的晶相结构;采用U-4100紫外可见分光光度计 (UV-vis DRS) 和F-230光致发光分光计测定样品的光学性质;通过CHI660E电化学工作站测定电化学性能。
光电化学性能测试采用双电解槽连用系统,其中光解槽和腐蚀电解槽之间由特制Nafion膜相连接,采用500 W高压氙灯 (GXZ500) 作为可见光光源垂直照射到光阳极上。进行光电流密度-时间测试和开路电位测试时,以3种TiO2纳米管 (FTNT、BFTNT、F-BFTNT) 作为光阳极,置于光解槽中,光解槽中的溶液为含0.25 mol/L Na2S+0.35 mol/L Na2SO3的空穴捕获剂,腐蚀电解槽中为含有3.5% (质量分数) NaCl溶液的三电极体系,304不锈钢与光阳极利用导线耦联作为工作电极 (工作面积为1 cm2),Ag/AgCl为参比电极,Pt电极为对电极。其中光电流密度-时间测试时,每隔50 s进行一次开闭光转换,开路电位测试时,每隔300 s进行一次开闭光转换,开闭光转换均依靠电子快门实现,同时利用多功能电子精密计时器实现自动计时。电化学阻抗测试时,电解质溶液为0.1 mol/L Na2SO4,3种TiO2纳米管分别作为工作电极,Ag/AgCl为参比电极,Pt电极为对电极,利用ZSimDemo 3.30d软件进行拟合。电压为开光下的开路电位,测试频率为105~10-2 Hz,扰动电压为0.01 V。所有测试均进行3次平行实验。
2 结果与讨论
2.1 形貌分析
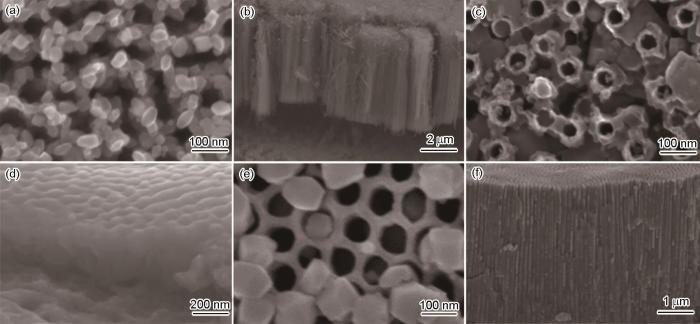
3种所制备的TiO2纳米管阵列形貌如图1所示。由图1a的FTNT表面形貌可以看出,电解液中只有F-时,整个体系反应活性高,对氧化薄膜刻蚀和溶解速率较快,难以控制。上层颗粒是由于过快的刻蚀和溶解作用而碎掉的氧化膜,堵塞管口。由图1b的截面图看出,管长6 μm,相较于其他两种情况管长最长,纳米管之间的排列较为规整有序。由图1c的BFTNT表面形貌可以看出,电解液中只有BF4-时,体系温和,对氧化薄膜刻蚀和溶解速率慢,因此管径最小,上层氧化膜溶解少;由图1d其截面图可以看出,管长300 nm,刻蚀速率慢导致管长最短,纳米管之间的排列极不规整,管不通透。由图1e的F-BFTNT表面形貌可以看出,电解液中F-和BF4-共存时,整个体系介于上述两种情况之间,既有F-进行快速刻蚀,又有BF4-中和了F-的强攻击性,整个纳米管孔径较为均匀,上层氧化膜溶解情况有所改善。由图1f其截面图形貌可以看出,管长4 μm,纳米管排列均匀有序,管最为通透。
图1
图1
3种TiO2纳米管阵列的表面及截面形貌
Fig.1
Surface images (a, c, e) and cross section images (b, d, f) of FTNT (a, b), BFTNT (c, d) and F-BFTNT (e, f)
2.2 结构分析
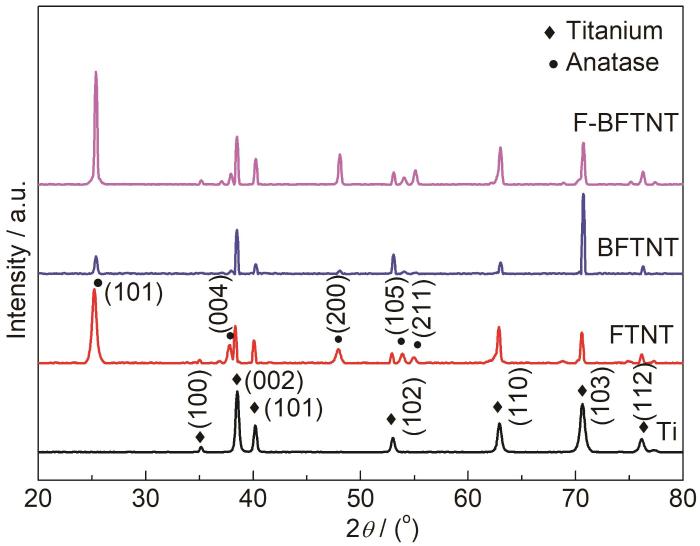
FTNT、BFTNT、F-BFTNT的XRD图谱以及纯Ti片的XRD图谱如图2所示。可以看出,纯Ti片在35.0°、38.4°、40.2°、53.0°、62.9°70.6°76.2°处有7个衍射峰,为基底Ti的衍射峰,分别对应于 (100)、 (002)、(101)、(102)、(110)、(103)、(112) 7个晶面 (PDF#89-2762)。在-3种TiO2纳米管在25.4°、37.8°、48.1°、54.0°、55.2°处有5个衍射峰,分别对应着锐钛矿型TiO2的 (101)、(004)、(200)、(105)、(211) 晶面 (PDF#89-4921)。其中,F-BFTNT中锐钛矿型TiO2的衍射峰最为尖锐,说明此时TiO2结晶度最高,同时基底Ti的衍射峰强度最高,因此对基底Ti的遮挡作用最弱。BFTNT图谱中基底Ti和锐钛矿型TiO2的衍射峰最弱,原因是纳米管没有到达Ti基底,有一层氧化膜遮挡住了Ti基底,管径小,管不通透,同时TiO2结晶度最差。FTNT中锐钛矿型TiO2的衍射峰较强,说明此时TiO2结晶度较好,同时基底Ti的衍射峰强度相较于F-BFTNT有所减弱,原因是FTNT管长最长,管径较小,对基底Ti的遮挡作用变强。
图2
2.3 紫外可见漫反射光谱分析
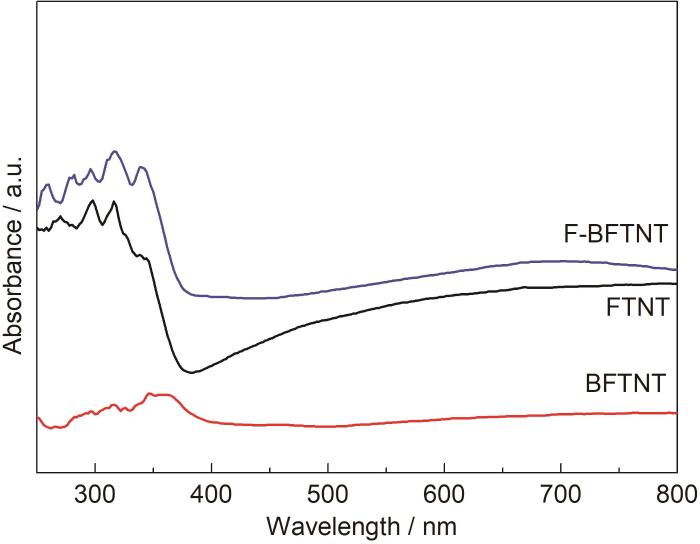
紫外可见漫反射光谱可以分析样品对光的吸收或反射,以此判断样品对光的利用程度。FTNT、BFTNT、F-BFTNT的紫外可见漫反射图谱如图3所示。可以看出,3种TiO2纳米管阵列的光吸收范围主要在紫外光区。其中BFTNT对光的吸收最低,原因是BFTNT管径小且不均匀,不利于光生电子传输;管壁粗糙,加大了光生电子和空穴的复合几率,从而降低了对光的吸收。其次是FTNT,原因是FTNT管长过长,而纳米管作为光生电子-空穴的传输和复合场所,过长的管长不利于光生电子和空穴的分离,因此对光的吸收较低。F-BFTNT对光的吸收最强,原因是F-BFTNT管径均匀,管壁光滑,管长适中,有利于光生电子和空穴的分离以及电子传输,因此对光吸收最强。
图3
图3
FTNT、BFTNT、F-BFTNT紫外-可见光漫反射光谱
Fig.3
UV-vis diffuse reflectance spectra of FTNT, BFTNT and F-BFTNT
2.4 光致发光光谱分析
FTNT、BFTNT、F-BFTNT在270 nm激发光下的光致发光光谱如图4所示。最高的光致发光强度为BFTNT,这说明BFTNT中光生载流子的分离效率最低,原因为BFTNT管径小且不均匀,不利于光生电子传输;管壁粗糙,加大了光生电子和空穴的复合几率。其次具有较高光致发光强度的是FTNT,原因是FTNT管长过长,而纳米管作为光生电子-空穴的传输和复合场所,过长的管长不利于光生电子和空穴的分离。具有最低光致发光强度的是F-BFTNT,F-BFTNT管径均匀,管壁光滑,管长适中,有利于光生电子和空穴的分离以及电子传输。
图4
图4
FTNT、BFTNT、F-BFTNT光致发光光谱
Fig.4
Fluorescence spectra of FTNT, BFTNT and F-BFTNT
2.5 光生阴极保护性能测试
2.5.1 光电流密度-时间曲线分析
光电流密度-时间曲线是评价材料光电化学性能的重要参数,光电流密度越大,光电化学性能越好[31]。在间歇可见光条件下,FTNT、BFTNT、F-BFTNT光电流密度-时间曲线如图5所示。开光瞬间,光阳极被激发生成光生电子和光生空穴,光生电子由价带跃迁至导带,在外电路保护下,导带上的电子转移到被保护金属表面,形成光电流。光电流大小可以衡量光阳极材料的光电转换能力。F-BFTNT具有通透的管道,规整均匀的阵列,适宜的管长和管径,因此具有最高的光电流密度。在开光瞬间,这3种样品都在瞬间产生光电流,说明这3种样品都能在光照下迅速激发出光生电子,具备快速光电响应能力。F-BFTNT的光电流最大,在开光瞬间有很强的光电流响应,经过一段时间后达到稳定状态,此时光生电子生成速率和消耗速率达到动态平衡,光电流密度为45 μA·cm-2,闭光条件下依然有较小的电流存在。BFTNT的光电流最小,原因是BFTNT管径小且不均匀,不利于光生电子传输;管壁粗糙,加大了光生电子和空穴的复合几率;FTNT管长过长,也加大了光生电子和空穴的复合几率。因此,F-BFTNT的光电转换能力最好。
图5
图5
间歇光下FTNT、BFTNT、F-BFTNT光电流密度-时间曲线
Fig.5
Photocurrent density-time curves of FTNT, BFTNT and F-BFTNT in intermittent light
2.5.2 开路电位分析
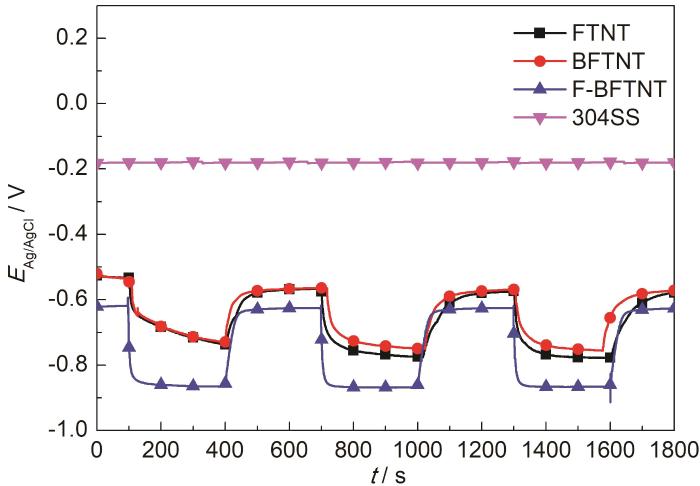
在间歇可见光条件下,3种光阳极耦联304不锈钢的开路电位如图6所示。304不锈钢的自腐蚀电位约-0.19 V (vs. Ag/AgCl),当开路电位低于其自腐蚀电位,304不锈钢被提供阴极保护,开路电位越负,阴极保护效果越好。在开光条件下,BFTNT耦联304不锈钢的开路电位负移至-0.76 V,FTNT耦联304不锈钢的开路电位负移至-0.78 V。F-BFTNT在开光条件下的开路电位最负,为-0.87 V,同时它的开路电位达到稳定状态的速度最快,在闭光条件下依然有较负的开路电位,说明对304SS的阴极保护效果最好。此结果与光电流密度-时间曲线的分析结果相匹配。
图6
图6
间歇可见光条件下,3种光阳极耦联304不锈钢的开路电位
Fig.6
Variations of OCP of FTNT, BFTNT and F-BFTNT coupled with 304SS in intermittent light
2.5.3 电化学阻抗谱图分析
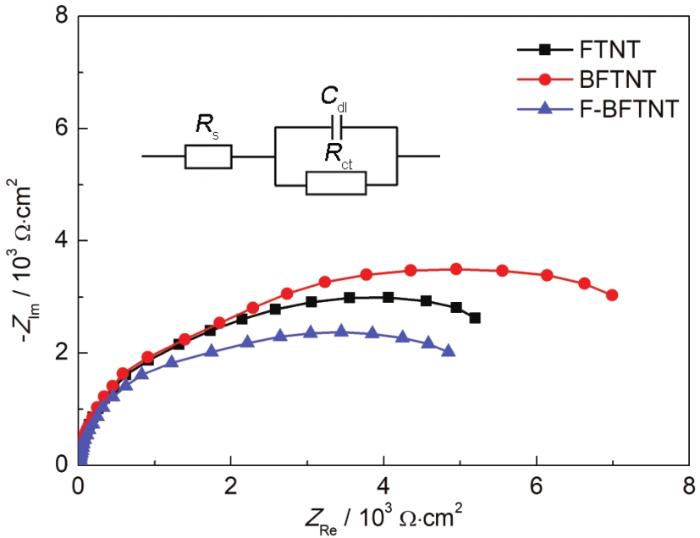
在模拟太阳光条件下,3种电化学阻抗谱的Nyquist图及等效拟合电路图如图7所示。采用R(CR) 等效电路,其中,Rs为电解质溶液电阻;Rct为电荷转移电阻,可以表征304不锈钢的腐蚀速率;Cdl为双电层电容。光阳极阻抗弧半径越小,电子传输电阻越小,电子传输效率更高。由图7a可知,F-BFTNT相比于其他两种样品具有更小的弧半径。表1是模拟太阳光下FTNT、BFTNT、F-BFTNT的电化学阻抗谱拟合参数,F-BFTNT的Rct值最小,为4.958 kΩ·cm-2,Rct值越小,界面电化学反应速率越强,电荷转移能力越强[32],对304不锈钢的保护作用越好。因此,模拟太阳光下F-BFTNT对304不锈钢的保护效果最好。
图7
图7
模拟太阳光下3种电化学阻抗谱的Nyquist图和等效电路图
Fig.7
Nyquist plots of FTNT, BFTNT and F-BFTNT in simulated sunlight and equivalent circuit diagram
表1 模拟太阳光下3种电化学阻抗谱拟合参数
Table 1
| Sample | Rs / Ω·cm2 | Cdl / F·cm-2 | Rct / kΩ·cm2 |
|---|---|---|---|
| FTNT | 12.55 | 8.746×10-4 | 5.896 |
| BFTNT | 10.62 | 5.970×10-4 | 7.156 |
| F-BFTNT | 10.72 | 9.570×10-4 | 4.958 |
2.6 光生载流子分离复合机制
图8
3 结论
(1) 复合电解液中制备的TiO2纳米管孔径较为均匀,管长适中,纳米管排列均匀有序,为光生电子和空穴的分离以及光生电子的传输提供了合适场所。
(2) 复合电解液中制备的纳米管对光的吸收最强,光生电子和空穴的复合率最低,光电流密度最大,耦联304不锈钢的开路电位为-0.87 V,分别比另外两种单氟体系制备的纳米管的开路电位低90和110 mV,且具有更小的电荷转移电阻。
(3) 复合电解液比单氟电解液制备的TiO2纳米管作为光阳极对金属具有更好的光生阴极保护效果。
参考文献
Exploring the origin of enhanced activity and reaction pathway for photocatalytic H2 production on Au/B-TiO2 catalysts
[J].
Improvement in corrosion protection properties of TiO2 coatings by chromium doping
[J].
Fabrication, characterization and photoelectrochemical activity of tungsten-copper co-sensitized TiO2 nanotube composite photoanodes
[J].
Efficient Co-B-codoped TiO2 photocatalyst for degradation of organic water pollutant under visible light
[J].
Tuning the selectivity of photocatalytic synthetic reactions using modified TiO2 nanotubes
[J].Differently modified TiO2 nanotubes were used to achieve a drastic change in the selectivity of a photocatalytic reaction. For the photocatalytic oxidation of toluene, depending on the electronic properties of TiO2 (anatase, rutile, Ru-doped), a strong change in the main reaction product (namely benzoic acid versus benzaldehyde) can be achieved, and certain undesired reaction pathways can be completely shut down. © 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Preparation of TiO2 nanotube arrays with efficient photocatalytic performance and super-hydrophilic properties utilizing anodized voltage method
[J].
Fabrication of Ni3S2/TiO2 photoanode material for 304 stainless steel photocathodic protection under visible light
[J].
Sb2S3/Sb2O3 modified TiO2 photoanode for photocathodic protection of 304 stainless steel under visible light
[J].
Effect of preparation and surface modification of TiO2 on its photoelectrochemical cathodic protection performance
[J].
TiO2的制备及表面修饰工艺对其光电化学阴极保护性能的影响
[J].
Influence of DMF modified TiO2 film on the photogenerated cathodic protection behavior
[J].
添加DMF对TiO2薄膜光生阴极保护性能影响研究
[J].
Structural transformation, photocatalytic, and field-emission properties of ridged TiO2 nanotubes
[J].
Titania nanotube arrays for local drug delivery: recent advances and perspectives
[J].
The design, fabrication, and photocatalytic utility of nanostructured semiconductors: focus on TiO2-based nanostructures
[J].Recent advances in basic fabrication techniques of TiO2-based nanomaterials such as nanoparticles, nanowires, nanoplatelets, and both physical- and solution-based techniques have been adopted by various research groups around the world. Our research focus has been mainly on various deposition parameters used for fabricating nanostructured materials, including TiO2-organic/inorganic nanocomposite materials. Technically, TiO2 shows relatively high reactivity under ultraviolet light, the energy of which exceeds the band gap of TiO2. The development of photocatalysts exhibiting high reactivity under visible light allows the main part of the solar spectrum to be used. Visible light-activated TiO2 could be prepared by doping or sensitizing. As far as doping of TiO2 is concerned, in obtaining tailored material with improved properties, metal and nonmetal doping has been performed in the context of improved photoactivity. Nonmetal doping seems to be more promising than metal doping. TiO2 represents an effective photocatalyst for water and air purification and for self-cleaning surfaces. Additionally, it can be used as an antibacterial agent because of its strong oxidation activity and superhydrophilicity. Therefore, applications of TiO2 in terms of photocatalytic activities are discussed here. The basic mechanisms of the photoactivities of TiO2 and nanostructures are considered alongside band structure engineering and surface modification in nanostructured TiO2 in the context of doping. The article reviews the basic structural, optical, and electrical properties of TiO2, followed by detailed fabrication techniques of 0-, 1-, and quasi-2-dimensional TiO2 nanomaterials. Applications and future directions of nanostructured TiO2 are considered in the context of various photoinduced phenomena such as hydrogen production, electricity generation via dye-sensitized solar cells, photokilling and self-cleaning effect, photo-oxidation of organic pollutant, wastewater management, and organic synthesis.
Structural, electrical and optical properties of multilayer TiO2 thin films deposited by sol-gel spin coating
[J].
Pt-coated TiO2 nanorods for photoelectrochemical water splitting applications
[J].
Preparation of ZnO/TiO2 composite film on 304 stainless steel and its photo-cathodic protection properties
[J].
304不锈钢表面ZnO/TiO2复合薄膜的制备与光生阴极防腐蚀性能研究
[J].
Hydrothermal synthesis of TiO2/carbon composites and their application for removal of organic pollutants
[J].
Easy and fast preparation of TiO2- based nanostructures using microwave assisted hydrothermal synthesis
[J].
Photocathodic protection of 304 stainless steel by MnS/TiO2 nanotube films under simulated solar light
[J].
Observation of optical band-gap narrowing and enhanced magnetic moment in Co-doped sol-gel-derived anatase TiO2 nanocrystals
[J].
Non-uniform doping outperforms uniform doping for enhancing the photocatalytic efficiency of Au-doped TiO2 nanotubes in organic dye degradation
[J].
Truncated titanium/semiconductor cones for wide-band solar absorbers
[J].
Titanium dioxide nanotubes (TNT) in energy and environmental applications: an overview
[J].
Recent advance on engineering titanium dioxide nanotubes for photochemical and photoelectrochemical water splitting
[J].
AgInS2 and graphene co-sensitized TiO2 photoanodes for photocathodic protection of Q235 carbon steel under visible light
[J].
Enhanced photocathodic protection performance of Ag/graphene/TiO2 composite for 304SS under visible light
[J].
Manipulating morphology, pore geometry and ordering degree of TiO2 nanotube arrays by anodic oxidation
[J].
Enhanced photoelectrochemical cathodic protection performance of MoS2/TiO2 nanocomposites for 304 stainless steel under visible light
[J].
Effect of the seawater flow rate and static pressure on the cathode protection
[D].
模拟海水流速、静压力对阴极保护的影响
[D].
New insight into electrosynthesis of ordered TiO2 nanotubes in EG-based electrolyte solutions: combined experimental and computational assessment
[J].To obtain a better understanding of TiO2 nanotube (TiO2-NT) synthesis in different ethylene glycol (EG)-based electrolyte solutions by electrochemical anodization, the primary steps of TiO2-NT formation were studied by experimental techniques. In this regard, three different EG-based electrolyte solutions were used for anodic oxidation of titanium foil. The first electrolyte solution contains conventional ammonium fluoride (NH4F) dissolved in EG/water (98 : 2 v/v). In the second one, Ti foil anodization is performed in an electrolyte solution containing the 1-butyl-3-methyl-imidazolium tetrafluoroborate (Bmim-BF4) ionic liquid. Finally, the fluorine-containing species was replaced by the 1-butyl-3-methyl-imidazolium chloride (Bmim-Cl) ionic liquid. The results indicate that the TiO2-NTs did not form by anodization in the EG/H2O/Bmim-Cl electrolyte solution at 60 V. Interestingly, this electrolyte solution is less viscous than the fluorine-containing electrolyte solutions. In addition, we report a detailed study on the structural arrangement of electrolyte solution components near the solid surfaces using molecular dynamics (MD) simulation methods to reveal the factors governing the difference of the ionic species distribution. The MD results elucidate the role of the ionic constituents in the length of the nanotube arrays at a certain anodization condition. Furthermore, as reported herein for the first time, the lifetimes of ion-ion contacts and the interactions of ionic species with TiO2 walls have a substantial effect on the resulting nanotubes. These characteristics are analyzed by using radial distribution functions, density profiles, distance analysis, time correlation functions, and mean-square-displacements, complemented by DFT calculations.
Light-generated cathodic protection properties of Fe2O3/TiO2 nanocomposites for 304 stainless steel
[J].
Fe2O3/TiO2纳米复合材料对304不锈钢的光生阴极保护性能
[J].
Facile post-growth doping of nanostructured hematite photoanodes for enhanced photoelectrochemical water oxidation
[J].
Photocathodic protection of 304 stainless steel by Bi2S3/TiO2 nanotube films under visible light
[J].